X-linked sideroblastic anemia (XLSA) is caused by mutations in the erythroid-specific 5-aminolevulinic acid synthase (ALAS2)gene. Hemizygous males have microcytic anemia and iron overload. A 38-year-old male presented with this phenotype (hemoglobin [Hb] 7.6 g/dL, mean corpuscular volume [MCV] 64 fL, serum ferritin 859 μg/L), and molecular analysis of ALAS2 showed a mutation 1731G>A predicting an Arg560His amino acid change. A 36-year-old brother was hemizygous for this mutation and expressed the mutated ALAS2 mRNA in his reticulocytes, but showed almost no phenotypic expression. All 5 heterozygous females from this family, including the 3 daughters of the nonanemic hemizygous male, showed marginally increased red-cell distribution width (RDW). Although variable penetrance for XLSA in males has been previously described, this is the first report showing that phenotypic expression can be absent in hemizygous males. This observation is relevant to genetic counseling, emphasizing the importance of gene-based diagnosis.
Introduction
X-linked sideroblastic anemia (XLSA) is caused by mutations (primarily missense) in the erythroid-specific 5-aminolevulinic acid synthase (ALAS2) gene.1,2Affected males usually present in the first 2 decades of life with symptoms of anemia, and in middle age with manifestations of secondary iron overload. Phenotypic expression of XLSA varies considerably in males, and is partly related to the type of ALAS2 mutation, with more than 25 mutations described so far in more than 30 kindreds.3,4 In addition, modifying genes such as those for genetic hemochromatosis may significantly exacerbate XLSA in hemizygous males.3 On the other hand, genetic and acquired factors responsible for skewed X-chromosome inactivation may lead to late-onset XLSA in heterozygous females.5 6
Whereas the existence of modifying genes capable of worsening XLSA is well established, little information is available on genetic factors that may attenuate or suppress its phenotypic expression.1By studying a family with a new ALAS2 mutation, we provide evidence that phenotypic expression of XLSA may be absent in hemizygous males.
Study design
The proband (subject II-2 in Figure1A) was found to have microcytic anemia (hemoglobin [Hb] level 7.6 g/dL; mean corpuscular volume [MCV] 64 fL) with high red-cell distribution width (RDW) (30.9%) at the age of 38. Serum ferritin was raised, transferrin was 82% saturated, and 30% of the patient's bone marrow erythroblasts were ring sideroblasts. A diagnosis of XLSA was made. On treatment with 300 mg/d pyridoxine, hemoglobin level averaged around 10 g/dL. Iron chelation therapy with deferoxamine (DFO) normalized serum ferritin.
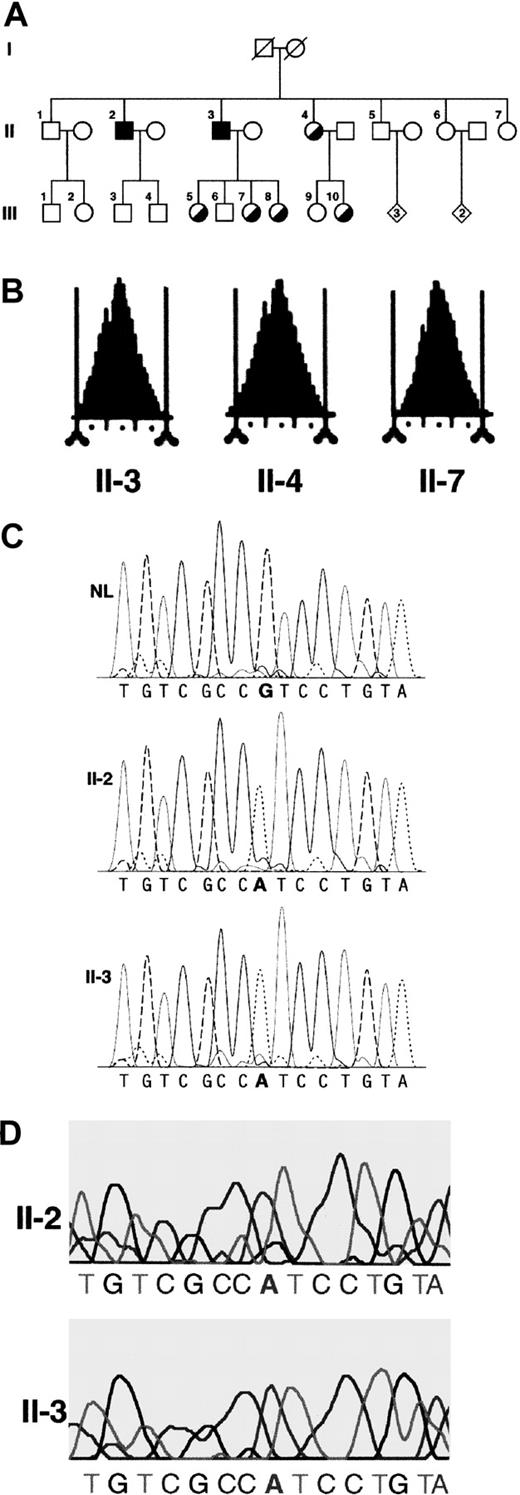
Most relevant clinical and molecular findings in the family studied.
(A) Pedigree of the family studied. Circles denote female family members; squares, male family members; and diamonds, additional members of either sex (the number of additional members is shown in the diamonds). Symbols with diagonal lines indicate deceased members. Filled squares indicate hemizygous males; half-filled circles indicate heterozygous women. (B) Red cell volume histograms obtained with a Bayer Technicon H3 in 3 family members. The vertical tick marks correspond to 10 fL increments. Subject II-7 shows a typical normal picture. Subject II-4 is a typical heterozygous woman with a small proportion of microcytic red cells (tail on the left side of the histogram). The nonanemic hemizygous brother (subject II-3) shows a red cell volume histogram that is almost normal. (C) Dideoxy dye terminator sequence analysis of polymerase chain reaction–amplified, exon 11 genomic DNA from a healthy individual (NL), the proband (II-2), and his brother (II-3). (D) Sequence analysis of reverse transcribedALAS2 mRNA expressed in reticulocytes from the anemic (II-2) and nonanemic (II-3) hemizygous brothers.
Most relevant clinical and molecular findings in the family studied.
(A) Pedigree of the family studied. Circles denote female family members; squares, male family members; and diamonds, additional members of either sex (the number of additional members is shown in the diamonds). Symbols with diagonal lines indicate deceased members. Filled squares indicate hemizygous males; half-filled circles indicate heterozygous women. (B) Red cell volume histograms obtained with a Bayer Technicon H3 in 3 family members. The vertical tick marks correspond to 10 fL increments. Subject II-7 shows a typical normal picture. Subject II-4 is a typical heterozygous woman with a small proportion of microcytic red cells (tail on the left side of the histogram). The nonanemic hemizygous brother (subject II-3) shows a red cell volume histogram that is almost normal. (C) Dideoxy dye terminator sequence analysis of polymerase chain reaction–amplified, exon 11 genomic DNA from a healthy individual (NL), the proband (II-2), and his brother (II-3). (D) Sequence analysis of reverse transcribedALAS2 mRNA expressed in reticulocytes from the anemic (II-2) and nonanemic (II-3) hemizygous brothers.
The procedures followed were in accordance with the ethical standards of the institutional committee on human experimentation and with the Helsinki Declaration of 1975, as revised in 1983. Routine hematologic measurements were obtained using a Bayer Technicon (Milan, Italy) H3 automatic cell analyzer. For evaluation of potential thalassemia defects, standard procedures were used.7 Molecular analyses of the HFE and ALAS2 genes were performed as previously described.3 The expression of mutantALAS2 messenger RNA (mRNA) was evaluated in peripheral blood reticulocytes from the proband and his brother, as previously described.6 8
Results and discussion
The pedigree of the proband's family is shown in Figure 1A, while hematologic and iron status data for the family members are reported in Table 1.
A single point mutation in exon 11 of the ALAS2 gene was found in the proband (Table 1 and Figure 1C), after all exons, intron-exon boundaries, and the 5′ and 3′ flanking regions were sequenced in both directions.3 The only mutation found was a transition from G to A at nucleotide 1731 that predicts an amino acid change of a conserved arginine to the more compact histidine at position 560 (Arg560His). There were 2 males (including the proband) who were hemizygous, and 5 females, including the 3 obligate heterozygote daughters of the nonanemic hemizygous male (II-3), who were heterozygous for this mutation (Table 1). These results were independently obtained in 2 different laboratories and the mutation was confirmed in a third. In addition, sequence analysis of cDNA derived from reticulocyte RNA revealed that both the proband and his brother expressed mRNA from the mutated ALAS2 allele in erythroid cells (Figure 1D). The 2 brothers' DNA samples that were used for sequencing were shown to be nonidentical and derived from the same parents by genotyping of informative short tandem repeat polymorphisms at 15 loci (data not shown).
In order to demonstrate that the above mutation was not a polymorphism, we studied 100 DNA samples from unrelated females, none of whom had this change. This indicates that the transition from G to A at nucleotide 1731 is not a polymorphism, since it was found in less than 1% of the population. In addition, significant difference was found between the mean RDW values (a phenotypic marker of XLSA3) for 5 healthy and 5 heterozygous females carrying the 1731G>A mutation. As detailed in Table 1 (footnote on RDW), these latter had markedly higher RDW values (F = 50, P = .0001), indicating that all of them had the typical hematologic phenotype of female carriers.
By contrast, the 2 hemizygous brothers had markedly different hematologic phenotypes. The proband (II-2) showed a classical microcytic anemia with high RDW and secondary iron overload. In comparison, his brother (II-3) had normal red cell counts and no evidence of iron overload. However, he showed marginally low MCV and marginally elevated RDW on some occasions. A feature of XLSA is the presence of microcytic red cells. Figure 1B shows red cell volume histograms of 3 representative family members: the nonanemic hemizygous brother shows an almost normal pattern.
Although unlikely, we could not exclude that the mutantALAS2 in the nonanemic healthy brother was silenced through a mechanism of intercistronic suppression.9 In order to establish whether other inherited disorders could explain the different phenotypes, additional studies were performed. Molecular analysis of HFE excluded the Cys282Tyr or His63Asp mutations, while investigations for beta or alpha thalassemia defects revealed no imbalance in globin chain synthesis in both brothers. No potentially causative nongenetic factors were identified.
Variable penetrance of pyridoxine-responsive XLSA has been previously reported. In a family with the mutation 1215C>G predicting an Ser388Thr amino acid change,10 3 hemizygous males were studied. Their hemoglobin levels at clinical onset ranged from 5.0 g/dL to 14.4 g/dL, but all of them showed microcytosis (60-73 fL). There were 4 hemizygous males who were studied in a family with the mutation 923G>A predicting a Gly291Ser amino acid change,11 and variable hemoglobin levels at clinical onset were found. All patients, however, were anemic (hemoglobin from 9.2 g/dL to 12.5 g/dL) and showed reduced MCV values (60-74 fL). Therefore, all these patients clearly had the XLSA phenotype, and modifying genes or acquired factors capable of worsening or ameliorating XLSA were likely playing a role in various individuals.
The present family is unique because the severe hematologic phenotype of the proband was almost completely absent in his brother. The fact that all 3 of obligate heterozygote daughters of this nonanemic hemizygote had mild phenotypes (marginally elevated RDW), argues against worsening factors in these family members, and would be consistent with the presence of a silencing factor(s) in the nearly normal hemizygote, as in the case of complete suppression of congenital dyserythropoietic anemia type II in a homozygous male.12On the other hand, different genetic backgrounds may be responsible for the expression of XLSA in the proband, similar to the variable clinical expression in heterozygous gene carriers for mutations in the autosomal dominant porphyrias.13
Hemizygous males with ALAS2 mutations and absent XLSA phenotype may be less rare than thought. In fact, these individuals are likely to go unnoticed, since only males with abnormal phenotype are usually studied in families with X-linked inherited disorders. This emphasizes the importance of gene-based diagnosis in families with XLSA. Identification of the ALAS2 mutation in the subject II-3 has led to the recognition of a heterozygous state in his 3 daughters, who will now benefit from genetic counseling. In the future, they will be advised that their offspring might be at risk for anemia and iron overload14 but could benefit from a simple oral administration of pyridoxine that might prevent development of XLSA. We believe that all at-risk individuals in families with XLSA who request testing should have their DNA examined for ALAS2 mutations, regardless of normal hematologic findings, sex, and age.
The authors thank Joyce Hoy, Barrie Francis, and Maria Ramirez for technical help with DNA sequencing and genotype analyses.
Prepublished online as Blood First Edition Paper, August 8, 2002; DOI 10.1182/blood-2002-03-0685.
Supported by grants from MIUR, Rome, Italy, from AIRC, Milan, Italy, from IRCCS Policlinico S. Matteo and from Fondazione Ferrata Storti, Pavia, Italy (M.C.); grants R01 DK40895 and R01 DK26824 from the National Institutes of Health, Bethesda, MD, and grant 584 from the March of Dimes Birth Defects Foundation (D.F.B.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Mario Cazzola, Division of Hematology, IRCCS Policlinico S. Matteo, 27100 Pavia, Italy; e-mail:mario.cazzola@unipv.it.