Abstract
Antiphospholipid antibodies (aPL) are autoimmune antibodies directed toward phospholipids or phospholipid-protein complexes, particularly those containing β2-glycoprotein I (β2GPI). Persistently positive aPL accompanied by arterial or venous thrombosis, or recurrent pregnancy loss, constitutes the antiphospholipid syndrome (APS). Several types of aPL with different specificities have been defined and may be detected in the clinical lab, including lupus anticoagulants (detected using clotting assays) and anticardiolipin, anti-β2GPI and anti-prothrombin/phosphatidylserine antibodies (detected by ELISA); each of the last 3 aPL may be either IgG, IgM, or IgA, though IgA antibodies are not included in criteria for APS. Due to the relative rarity of APS and the heterogeneity of aPL, thrombosis risk stratification is challenging, and randomized clinical trials for thrombosis treatment and prevention have been limited. This lack of high-quality data has made the clinical management of APS difficult, and existing guidelines are few and could not possibly cover many of the scenarios encountered in managing patients with APS. In this review, we present 3 patients with aPL and/or APS who highlight treatment dilemmas, and we discuss background information that may help guide clinical judgment in developing individualized treatment plans for patients with these enigmatic antibodies.
Learning Objectives
Evaluate the significance of a mild, transient risk factor in defining APS treatment in a patient with IgM antiphospholipid antibodies
Assess the need for transitioning a patient with APS from a DOAC to warfarin
Assess the role of anticoagulation in asymptomatic aPL
Introduction
Antiphospholipid syndrome (APS) is among the most common causes of acquired thrombophilia and may be found in >10% of patients presenting with new thromboembolic events (TE).1 Unlike most inherited thrombophilias, APS is associated with both venous and arterial thromboembolism (VTE and ATE, respectively). Moreover, the rate of thrombotic recurrence in APS is sufficiently high to lead to recommendations that patients with APS be placed on indefinite anticoagulation.2,3 Antiphospholipid antibodies (aPL) occur in 20% to 30% of patients with systemic lupus erythematosus as well as in other autoimmune disorders, but most patients seen by hematologists have primary APS. The particular importance of APS is not only the risk of developing thrombotic disease but also its association with increased mortality and shortened survival. Moreover, there are currently no widely accepted biomarkers or risk stratification strategies to identify patients with high precision who may be able to discontinue antithrombotic medication.
Risk stratification approaches in patients with APS are limited primarily to assessment of aPL levels and whether one or more aPL tests are positive. While such assessments are useful, the hazard ratios derived from them are relatively small and the confidence intervals wide; this limits applicability to individual patients with aPL/APS and leads to uncertainty as to optimal management. Moreover, questions unique to patients with different clinical histories and aPL patterns are often not addressed by broad guidelines. In this review, we discuss 3 cases seen in our clinics that raise management questions and discuss the underlying rationale for our clinical decisions. While there is often no “right or wrong” approach to APS, some of the background data discussed here will provide information useful for approaching other APS patients with diverse presentations.
CLINICAL CASE 1
A 77-year-old female physician presents for an opinion concerning duration of anticoagulation. She had undergone cervical discectomy and fusion 6 months previously. She was not on prophylactic anticoagulation during surgery but was able to sit in a chair the day of surgery and was ambulatory upon discharge the following day. One week after discharge she developed pain in her right calf and chest discomfort and was diagnosed with acute deep vein thrombosis in the right peroneal and soleal vein and extensive pulmonary embolism involving lobar, segmental, and subsegmental arteries. She was treated with unfractionated heparin and transitioned to apixaban. When seen by a hematologist in follow-up, an evaluation for hypercoagulability revealed anti-β2GPI IgM antibodies of 87 standard IgM units (SMU) and anticardiolipin (aCL) IgM antibodies of 56 IgM phospholipid units (MPL). Lupus anticoagulant could not be determined due to apixaban. Based on these results, her apixaban was changed to warfarin. Studies repeated 3 months later showed β2GPI IgM antibodies of 105 SMU and aCL IgM antibodies of 63 MPL. She saw another hematologist who advised her that since she had a provoked thrombotic event, she had received an adequate course of anticoagulation (6 months) and could discontinue. She presents now for another opinion as to whether warfarin should be discontinued.
This case presents several questions that should be considered when developing a treatment plan, including:
Did she experience a provoked or unprovoked thrombotic event?
Has her course of anticoagulation been adequate?
Do the presence of IgM antiphospholipid antibodies influence the decision on whether to continue or discontinue anticoagulation?
The definition of an unprovoked versus a provoked VTE is subjective, but a framework for classification has been provided by Kearon et al.4 This patient's event was chronologically related to surgery. The exact duration of anesthesia was uncertain, but the patient was ambulatory upon hospital discharge the day after the procedure. We therefore considered this event likely to be provoked but by a minor, transient risk factor.
Delineation of a transient risk factor as a provocation for VTE supports the position that a relatively short-term course of anticoagulation therapy may be appropriate. Though this approach is advocated in guidelines,5 recent opinion has suggested that the risk for rethrombosis should be based more on individual patient characteristics and risk factors rather than solely whether the event seemed provoked or nonprovoked.6 This is supported by data from the Garfield-VTE study, a registry of treatment patterns and recurrent thrombosis in patients with VTE.7 In a report from that study that included more than 10000 patients, the risk of recurrent VTE was similar in patients with unprovoked VTE and those with transient provoking factors (hazard ratio [HR] 0.84; 95% CI 0.62% to 1.14%), suggesting that the relationship between this patient's thrombotic event and her limited surgical procedure should not be the only factor influencing decisions regarding anticoagulation duration.
Important in this patient's history is the presence of persistently positive IgM anticardiolipin and anti-β2GPI antibodies; how these antibodies are perceived to have contributed (or not) to the patient's thrombotic event will change her risk assessment from that of a transient minor provoking factor (surgery) to one of a persistent risk factor. Although positivity for IgM antiphospholipid antibodies is included in the criteria for APS,8 there remains disagreement about their significance (Table 1). In an observational study of 255 stroke patients under 55 years of age, Rodriguez-Sanz et al. observed a significant correlation between levels of IgM aPL measured within 48 hours of stroke and stroke severity.9 Del Ross et al. reported on 106 patients with thrombotic APS, finding that 13 patients had isolated, persistently positive IgM aPL of medium to high levels.10 In a large study of patients with neurological disorders, IgM aPL were the most common persistently positive aPL subtype in patients with cerebrovascular accidents.11 Urbanski et al. reported that 14.3% of a series of 168 patients with APS had isolated IgM antibodies; these patients were older and had a higher incidence of stroke after adjusting for other cardiovascular risk factors.12 They were more frequently treated with aspirin alone, though patients treated in this manner had an increased incidence of recurrent events. In contrast, Chayoua et al. analyzed anticardiolipin and anti-β2GPI antibodies in 1008 consecutive APS patients and controls.13 They reported that isolated IgM aPL were present in only 3.5% to 5.4% of patients with thrombotic APS, compared with 5.7% to 12.3% of patients with obstetric APS. Combined positivity for lupus anticoagulant and IgG and IgM aPL was strongly associated with thrombosis, however. The lower frequency of isolated IgM aPL in this study may reflect greater discrepancies in solid phase assays for IgM antibodies.14
Selected studies of isolated IgM antibodies in APS
| . | Type of study . | Patient selection . | N . | Findings . |
|---|---|---|---|---|
| Rodriguez-Sanz9 | Observational, cross-sectional | >55 years Acute brain infarct | 255 (161 male) | • 22 APS (4 before IS, 18 after) • IgM aCL within 48 h of admission correlate with admission NIHSS by MVA • IgG aCL within 48 h of admission correlate with 3-month mRS by MVA |
| Del Ross10 | Retrospective | APS/thrombosis | 106 (81 female) | • VTE = 55, ATE = 48, small vessel = 3 • 13 single positive for IgM (12.3%) • Single IgM stable over mean of 10.4 yr • Single IgM were significantly older and had increased retinal vein thrombosis |
| Sahebari11 | Cross-sectional | Neurological disorder and ≥1 aPL | 100 | • IgM anti-β2GPI positive in 100% of optic neuritis • IgM aCL was most common antibody in stroke patients |
| Urbanski12 | Retrospective | Single-positive IgM aCL or anti-β2GPI vs IgM aPL with ≥1 other aPL | 168 | • 24 patients with isolated IgM APS (9 isolated aCL, 2 isolated anti-β2GPI) • IgM only were older • Isolated IgM more common in stroke (OR 3.1; 95% CI 1.2-1.9; P = 0.18) • Single IgM positivity persisted in 70% |
| Chayoua13 | Retrospective | Multicenter study of APS samples | 1008 (763 female) | By MVA • LAC: OR 2.3 (95% CI 1.6-3.3) to 2.4 (95% CI 1.7-3.4) • IgG aCL or anti-β2GPI: OR 2.3 (95% CI 1.6-3.5) to 3.2 (95% CI 2.0-5.0) • IgM aCL or anti-β2GPI: NS • Isolated IgM aCL or anti-β2GPI in 3.5%-5.4% |
| . | Type of study . | Patient selection . | N . | Findings . |
|---|---|---|---|---|
| Rodriguez-Sanz9 | Observational, cross-sectional | >55 years Acute brain infarct | 255 (161 male) | • 22 APS (4 before IS, 18 after) • IgM aCL within 48 h of admission correlate with admission NIHSS by MVA • IgG aCL within 48 h of admission correlate with 3-month mRS by MVA |
| Del Ross10 | Retrospective | APS/thrombosis | 106 (81 female) | • VTE = 55, ATE = 48, small vessel = 3 • 13 single positive for IgM (12.3%) • Single IgM stable over mean of 10.4 yr • Single IgM were significantly older and had increased retinal vein thrombosis |
| Sahebari11 | Cross-sectional | Neurological disorder and ≥1 aPL | 100 | • IgM anti-β2GPI positive in 100% of optic neuritis • IgM aCL was most common antibody in stroke patients |
| Urbanski12 | Retrospective | Single-positive IgM aCL or anti-β2GPI vs IgM aPL with ≥1 other aPL | 168 | • 24 patients with isolated IgM APS (9 isolated aCL, 2 isolated anti-β2GPI) • IgM only were older • Isolated IgM more common in stroke (OR 3.1; 95% CI 1.2-1.9; P = 0.18) • Single IgM positivity persisted in 70% |
| Chayoua13 | Retrospective | Multicenter study of APS samples | 1008 (763 female) | By MVA • LAC: OR 2.3 (95% CI 1.6-3.3) to 2.4 (95% CI 1.7-3.4) • IgG aCL or anti-β2GPI: OR 2.3 (95% CI 1.6-3.5) to 3.2 (95% CI 2.0-5.0) • IgM aCL or anti-β2GPI: NS • Isolated IgM aCL or anti-β2GPI in 3.5%-5.4% |
aCL, anticardiolipin antibodies; aPL, antiphospholipid antibodies; APS, antiphospholipid syndrome; ATE, arterial thromboembolism; β2GPI, β2-glycoprotein I; h, hours; IS, ischemic stroke; LAC, lupus anticoagulant; mRS, modified Rankin scale; MVA, multivariate analysis; NIHSS, NIH stroke scale; NS, not significant; OR, odds ratio; VTE, venous thromboembolism.
A recent study of relevance to this patient analyzed aPL profiles in patients >65 years with APS, finding that APS was more frequent in elderly males and more often associated with arterial events, including myocardial infarction.15 The older cohort also had a significantly higher incidence of single positivity, particularly for IgM aCL, and increased mortality. While this report does not confirm a pathogenic role for IgM aCL, the implications of this study and others demonstrating a high incidence of arterial events in elderly APS patients are concerning.
Our recommendation for this patient was to continue warfarin, with periodic monitoring of antiphospholipid antibody levels.
CLINICAL CASE 2
A 53-year-old man was referred for evaluation of triple-positive antiphospholipid antibodies. He was diagnosed with medically intractable epilepsy six years ago and had a strong family history of coronary artery disease. Imaging studies to evaluate the site of his epileptic foci suggested an origin in the orbital/anterior- mesial temporal regions. He gave a history of a distant diagnosis of antiphospholipid antibodies, though he had never experienced a TE. A previous echocardiogram revealed no valvular thrombi, and several previous magnetic resonance imaging studies revealed no evidence of cerebral ischemia. Antiphospholipid testing revealed a lupus anticoagulant (positive dilute Russel's viper venom time, hexagonal phospholipid assay), anticardiolipin IgG and IgM each >150 GPL/MPL units, anticardiolipin IgA of 21.2 APL, and anti-β2GPI IgG and IgM each >150 standard IgG units (SGU)/SMU. He was referred for evaluation of the need for prophylactic anticoagulation.
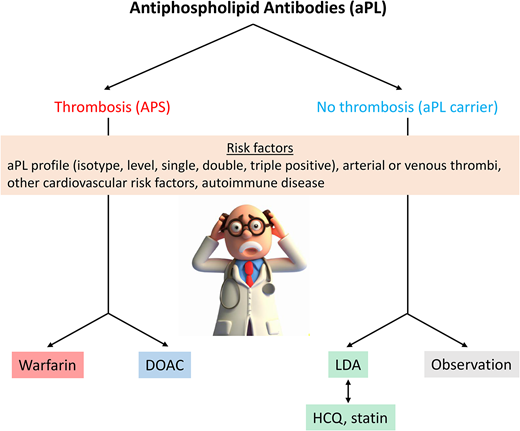
This patient presents a common dilemma as to whether anticoagulation is indicated in a patient with aPL who has not experienced a prior TE (Figure 1).
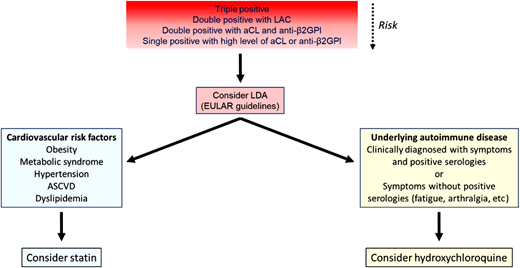
Decision tree for management of patients with asymptomatic aPL. Initial risk assessment is based primarily on the antibody profile, with triple-positive patients considered highest risk. Progressively less risk is depicted by less intense shading in the red box, though grading of this risk is imprecise. Patients with significant cardiovascular risk factors might also benefit from statins (blue box) and patients with rheumatologic symptoms, even without diagnostic serologies, from hydroxychloroquine. ASCVD, atherosclerotic cardiovascular disease; EULAR, European Alliance of Associations for Rheumatology; LAC, lupus anticoagulant; LDA, low dose aspirin.
Decision tree for management of patients with asymptomatic aPL. Initial risk assessment is based primarily on the antibody profile, with triple-positive patients considered highest risk. Progressively less risk is depicted by less intense shading in the red box, though grading of this risk is imprecise. Patients with significant cardiovascular risk factors might also benefit from statins (blue box) and patients with rheumatologic symptoms, even without diagnostic serologies, from hydroxychloroquine. ASCVD, atherosclerotic cardiovascular disease; EULAR, European Alliance of Associations for Rheumatology; LAC, lupus anticoagulant; LDA, low dose aspirin.
It should first be considered whether aPL levels in this patient are persistently positive. Levels of aPL as high as seen here, however, remain positive in >95% of cases, so repeat studies may not be necessary.16
A prospective study by Pengo et al. of 104 patients with triple- positive aPL who had not experienced a prior TE reported 25 first TEs over 4.5 years, for an annual incidence of 5.3%.17 Male sex and risk factors for venous thrombosis (oral estrogen/progesterone, pregnancy, family history, other thrombophilias) imparted relative risks of TE of 4.4% and 3.3%, respectively. In another prospective study, Ruffati et al. assessed the development of a first TE in a cohort of 248 patients with aPL and no prior TE history, with a mean follow-up interval of 39 months.17 The frequency of different types of aPL were not reported and patients were not classified by single, double or triple positivity. The annual incidence of new TE in this population was 1.86% per patient year. The only significant TE risk factors were the presence of a lupus anticoagulant and hypertension. Taken together, these studies suggest that consideration of prophylactic anticoagulation for a triple-positive aPL patient is quite reasonable.
Aspirin provides one option for TE prophylaxis in asymptomatic aPL carriers.18 However, in both studies mentioned above, chronic prophylaxis, almost always with aspirin, was not found to decrease the incidence of TE.17,19 In a randomized study of low-dose aspirin in asymptomatic patients with aPL, outcomes were compromised by the small sample size and lower than expected event rate (2.75/100 patient-years in aspirin-treated patients, 0/100 patient-years in the placebo arm), resulting in an HR for thrombosis of 1.04 (95% CI 0.69%, 1.06%; P = 0.83) in aspirin-treated patients.20 However, a meta-analysis by Arnaud et al. found a decreased incidence of primary thrombosis in patients treated with aspirin (odds ratio 0.50; 95% CI 0.27%, 0.93%), though 10 of the 11 series in the meta-analysis were observational.21 The use of aspirin as primary prophylaxis in asymptomatic patients with high-risk aPL profiles (lupus anticoagulant, double or triple positivity for aPL, or the presence of persistently high positive aPL levels) is endorsed by guidelines of the European Alliance of Associations for Rheumatology (EULAR).22 Proceedings of the 16th International Congress on Antiphospholipid Antibodies also suggest that low-dose aspirin be considered in such patients, while recognizing the need for a randomized trial.23
Statins provide another alternative for primary prophylaxis in high-risk aPL patients. In a mechanistic study, 1 month of treatment with fluvastatin reduced the expression of monocyte procoagulant proteins, including tissue factor, protease-activated receptors 1 and 2, and flt-1, due to inhibition of p38 mitogen-activated protein kinase and reduction in NF-κB/Rel DNA binding activity.24 In another study, fluvastatin significantly reduced inflammatory cytokines levels in patients with aPL, including IL-6, IL-8, Il-1β, and TNFα.25 A retrospective analysis of statins in patients with aPL with or without SLE showed a modest reduction in thrombosis in the primary aPL group (HR 0.12; 95% CI 0.01, 0.98).26 Additional studies are needed to confirm utility of statins in patients with aPL, but they might be considered in patients with risk factors for cardiovascular disease.
Another potential alternative for prophylaxis is hydroxychloroquine (HCQ). HCQ may reduce aPL-mediated thrombosis risk through several mechanisms, including (1) reducing the binding of aPL-β2GPI complexes to phospholipid bilayers,27 (2) reversing the effects of aPL on annexin V displacement from phospholipid bilayers and cells,28 and 3) inhibiting aPL-induced endothelial cell dysfunction.29 In a prospective nonrandomized study, 20 patients with APS received oral anticoagulation with HCQ, while an additional 20 received oral anticoagulation alone.30 Recurrent thrombosis occurred in 6 patients treated with oral anticoagulants alone and in no patients receiving additional HCQ (P = 0.0086 by time-to-event analysis). Similar results were obtained in another study of primary APS (HR 0.09; 95% CI 0.01% to 1.26% for anticoagulation and HCQ vs anticoagulation alone); this study also suggested that HCQ may be associated with reduction in aPL IgG levels over time. However, the only study to use HCQ in asymptomatic aPL carriers was stopped early due to poor enrollment.31
Finally, a randomized study compared low-dose aspirin in combination with warfarin adjusted to an international normalized ratio (INR) of 1.5 with low-dose aspirin alone in aPL carriers. This study revealed no reduction in thrombosis in the aspirin plus warfarin arm, with subjects in the latter arm having an increased incidence of bleeding.32
CLINiCAL CASE 2 (continued)
Based on these studies, we discussed the pros and cons of each type of intervention with the patient. Given his triple positivity and high antibody levels, we suggested that he consider low-dose aspirin prophylaxis.
CLINICAL CASE 3
A 36-year-old woman presented to the emergency department with an acute ileofemoral deep vein thrombosis. She has no significant past medical history and denied provoking factors. She is not obese and does not smoke. She was started on apixaban upon discharge. At 3-month follow-up, she is found to have β2GPI antibodies of IgG 143 SGU and anticardiolipin IgG 56 GPL. Testing for a lupus anticoagulant could not be completed due to direct oral anticoagulant (DOAC) interference. She is referred to hematology for a discussion of anticoagulation and the need for transitioning to a vitamin K antagonist (VKA). She expresses hesitation about initiating warfarin due to dose monitoring requirements and dietary limitations and asks about continuing on apixaban.
In thrombotic APS, VKAs are the preferred therapy for secondary thrombosis prevention. However, when APS is not diagnosed on initial presentation and patients are initiated on a DOAC, subsequent identification of aPL requires a decision of continuing DOAC or transitioning to warfarin for long-term anticoagulation. DOACs are attractive due to fixed dosing, lack of routine monitoring, minimal drug-to-drug interactions, and generally higher patient satisfaction. Warfarin requires more frequent monitoring, which can be complicated in APS due to artefactual prolongation of point-of-care INRs in patients with lupus anticoagulants.33 However, warfarin therapy does not commonly require significant dietary modification or elimination as often assumed by patients.34 The major concern with DOAC use in APS is efficacy. Four major prospective randomized clinical trials have compared DOACs with warfarin (Table 2).35-38 Collectively, these failed to demonstrate noninferiority of DOACs for secondary prevention of TEs and safety in APS. The first of these was the RAPS trial, which assessed thrombin generation as a global measure of thrombotic risk and anticoagulation effectiveness in APS patients randomized to rivaroxaban or warfarin.35 Endogenous thrombin potential was significantly higher in the rivaroxaban group, thus rivaroxaban did not meet the primary noninferiority end point. However, peak thrombin generation, a secondary end point, was lower with rivaroxaban. These results were interpreted as consistent with a similar overall thrombotic risk in both arms and attributed to differences in anticoagulant mechanisms. However, the trial was not powered to assess clinical end points, and there were no thrombotic episodes in either arm.
Randomized control trials of direct oral anticoagulants in APS
| Trial . | Author . | Patient selection . | N . | Triple positive (%) . | Findings . |
|---|---|---|---|---|---|
| RAPS | Cohen | APS, prior ATE excluded | 116 (54 rivaroxaban) | 28 | • Endogenous thrombin potential greater with rivaroxaban (primary end point) • No recurrent thrombosis either arm in 6 months • No difference in MB: 5% DOAC vs 4% VKA |
| TRAPS | Pengo | Triple-positive APS | 120 (59 rivaroxaban) | 100 | • Higher recurrent thrombosis and MB with DOAC • 4 IS, 3 MI (12%) DOAC vs 0 events VKA • MB: 7% DOAC vs 3% VKA |
| EUDRA | Ordi-Ros | APS | 190 (95 rivaroxaban) | 60.5 | • Higher recurrent thrombosis with rivaroxaban • 11 (11.6%) VTE DOAC vs 6 (6.3%) VKA, RR 1.83 [95% CI, 0.71-4.76] • Higher rate of IS with DOAC • Livedo, small vessel disease, cardiac valvular disease associated with increased risk of thrombosis • Triple positivity not associated with increased risk • MB: No difference |
| ASTRO-APS | Woller | APS, protocol modification to exclude prior ATE | 48 (23 apixaban) | 30.4 | • Terminated prematurely • 6 strokes DOAC vs 0 events VKA • Strokes occurred in single-, double-, and triple-positive APS • MB: 0 DOAC vs 1 VKA |
| Trial . | Author . | Patient selection . | N . | Triple positive (%) . | Findings . |
|---|---|---|---|---|---|
| RAPS | Cohen | APS, prior ATE excluded | 116 (54 rivaroxaban) | 28 | • Endogenous thrombin potential greater with rivaroxaban (primary end point) • No recurrent thrombosis either arm in 6 months • No difference in MB: 5% DOAC vs 4% VKA |
| TRAPS | Pengo | Triple-positive APS | 120 (59 rivaroxaban) | 100 | • Higher recurrent thrombosis and MB with DOAC • 4 IS, 3 MI (12%) DOAC vs 0 events VKA • MB: 7% DOAC vs 3% VKA |
| EUDRA | Ordi-Ros | APS | 190 (95 rivaroxaban) | 60.5 | • Higher recurrent thrombosis with rivaroxaban • 11 (11.6%) VTE DOAC vs 6 (6.3%) VKA, RR 1.83 [95% CI, 0.71-4.76] • Higher rate of IS with DOAC • Livedo, small vessel disease, cardiac valvular disease associated with increased risk of thrombosis • Triple positivity not associated with increased risk • MB: No difference |
| ASTRO-APS | Woller | APS, protocol modification to exclude prior ATE | 48 (23 apixaban) | 30.4 | • Terminated prematurely • 6 strokes DOAC vs 0 events VKA • Strokes occurred in single-, double-, and triple-positive APS • MB: 0 DOAC vs 1 VKA |
APS, antiphospholipid syndrome; ATE, arterial thromboembolic event; DOAC, direct oral anticoagulant; IS, ischemic stroke; MB, major bleeding; MI, myocardial infarction; RR, relative risk; VKA, vitamin K antagonist; VTE, venous thromboembolic event.
The TRAPS trial compared rivaroxaban with warfarin for prevention of TEs, major bleeding, and vascular death in high-risk, triple-positive APS patients. This trial was stopped prematurely after an excess of events (4 ischemic strokes, 3 myocardial infarctions) in the rivaroxaban group versus none with warfarin.36 In another prospective, randomized trial by Ordi-Ros, rivaroxaban was associated with nearly twice the incidence of recurrent thrombosis as warfarin, with stroke again more common in rivaroxaban-treated patients. Post hoc analysis, while underpowered, suggested an increased risk in patients with prior arterial thrombosis, livedo racemosa, or APS-related cardiac valvular disease, while a significant association with triple positivity was not identified. Taken together, these trials suggest that rivaroxaban offers inferior protection from thrombosis compared with warfarin in thrombotic APS, especially for individuals with triple-positive disease and prior arterial thrombosis. The fourth trial, ASTRO-APS, compared apixaban with warfarin and was challenged by multiple protocol modifications, dose intensification, and an amendment to exclude patients with prior arterial events, before terminating prematurely.38 The authors reported 6 ischemic strokes in apixaban-treated patients compared with none with warfarin. Strokes occurred in patients with single-, double-, and triple-aPL positivity, though most patients with recurrent events had a history of prior stroke.
Whether the findings of these trials represent a class effect or are drug specific remains unknown. Few cohort studies have looked at the use of other DOACs, and insufficient data are available to extrapolate strong conclusions.39,40 Based on trial data, regulatory agencies in the US and Europe changed the labeling of DOACs to advise against their use in patients with APS, especially triple-positive. The 2019 European Society of Cardiology and 2020 American Society of Hematology guidelines similarly recommend against DOAC in APS patients.41,42 However, other societal guidelines, including those of the British Society for Haematology, EULAR, and the International Society on Thrombosis and Hemostasis, offer a conditional approach. While recommending warfarin as the first-choice agent, collectively the guidelines suggest that DOACs may be considered in individuals (1) without high-risk APS features such as triple positivity, arterial thrombosis, small vessel thrombosis, organ involvement, or heart valve disease; (2) already stable on a DOAC; (3) unwilling to undergo INR monitoring; (4) with <60% time in therapeutic range with VKA; or (5) with contraindications to VKA.22,40,43
Limitations in risk stratification in APS and a lack of data pose a challenge to applying these recommendations. Moreover, deficiencies in understanding the driving pathophysiology of APS limit insight into DOAC failure in some patients. VKA and DOACs act upon different aspects of the coagulation system (Figure 2), but what mechanistically accounts for the observed disparity in anticoagulation effectiveness in APS is unknown. Several hypotheses have been raised, including differences in pharmacokinetics with lower trough levels of DOACs, the need for higher anti-Xa activity levels to prevent arterial versus venous events, and higher thrombin generation with DOACs due to more limited blockade of coagulation factors.
Mechanisms of aPL-mediated thrombosis and inhibition by warfarin or DOAC. This figure depicts multiple potential mechanisms underlying APS and the cell types that are affected by aPL. Cellular activation results in cell-specific responses that include releases of neutrophils extracellular traps (NETs), expression of cellular procoagulant activity, and extracellular vesicle release, among others. Warfarin inhibits γ-carboxylation of vitamin K–dependent coagulation factors, thus reducing the catalytic efficiency of coagulation complexes such as the phospholipid-dependent tenase and prothrombinase reactions.
Mechanisms of aPL-mediated thrombosis and inhibition by warfarin or DOAC. This figure depicts multiple potential mechanisms underlying APS and the cell types that are affected by aPL. Cellular activation results in cell-specific responses that include releases of neutrophils extracellular traps (NETs), expression of cellular procoagulant activity, and extracellular vesicle release, among others. Warfarin inhibits γ-carboxylation of vitamin K–dependent coagulation factors, thus reducing the catalytic efficiency of coagulation complexes such as the phospholipid-dependent tenase and prothrombinase reactions.
CLINICAL CASE 3 (continued)
After we discussed the evidence with this patient who has double-positive APS with unknown lupus anticoagulant status and has been doing well on a DOAC, she elected to transition to warfarin.
The management of APS patients is challenging. There are insufficient data concerning APS pathogenesis to formulate a mechanism-based approach. Given the variety of patient characteristics and potential diversity of aPL profiles, it is unlikely that guidelines will cover all situations. Thus, each patient must be assessed individually, with best clinical judgment used in some cases. A better understanding of APS through additional studies will hopefully inform more data-driven treatment approaches in the future.
Acknowledgments
This work is supported by R01 HL164516 (to KRM). AH is supported by TL1DK132770.
Conflict-of-interest disclosure
Anne Hubben: no competing financial interests to declare.
Keith R. McCrae: no competing financial interests to declare.
Off-label drug use
Anne Hubben: Anticoagulant therapy has an approved label for patients with aPL and thrombosis. There are no other medications with an approved label for APS, thus the use of all medications discussed in this review other than warfarin and DOAC should be considered off label.
Keith R. McCrae: Anticoagulant therapy has an approved label for patients with aPL and thrombosis. There are no other medications with an approved label for APS, thus the use of all medications discussed in this review other than warfarin and DOAC should be considered off label.