Abstract
Venous thromboembolism (VTE) is a multifactorial disease, and its risk depends on exposure to risk factors and predisposing conditions. Based on their strength of association with a VTE episode, risk factors are classified as major or minor and determined using a temporal pattern to be transient or persistent. All patients with VTE should receive anticoagulant treatment for at least 3 months in the absence of an absolute contraindication. Beyond this period, selected patients may be candidates for an extended phase of anticoagulation aimed at secondary VTE prevention. The risk of recurrent VTE if anticoagulation is discontinued is probably the main driver of decision-making regarding extended treatment. The risk of recurrence after VTE associated with major risk factors is low if the risk factor is no longer present. In this case, treatment can be discontinued. If the major risk factor is persistent, anticoagulation should be continued. After VTE occurring in the absence of risk factors, anticoagulation should probably be continued indefinitely if the risk for bleeding is low and preferably with minimal effective doses of anticoagulants. VTE occurring after exposure to minor risk factors is probably the most challenging situation, especially if the clinical manifestation was acute pulmonary embolism. Understanding the actual role of minor risk factors in the occurrence of VTE helps in estimating the risk of recurrence and avoiding the dangers associated with unnecessary anticoagulation. The availability of safer strategies for anticoagulation could allow personalized strategies for secondary prevention of VTE.
Learning Objectives
Understand the strength of different risk factors for VTE that support the classification in provoked, minimally provoked, or unprovoked VTE
Detail the risk of recurrence after the discontinuation of anticoagulant treatment for index VTE
CLINICAL CASE
A 33-year-old woman presents to the outpatient clinic. Three months before she had visited the emergency department for left lower limb edema. A complete compression ultrasonography was performed, and a deep vein thrombosis (DVT) had been diagnosed at the popliteal and trifurcation levels. Two weeks earlier, she had experienced a left ankle sprain and was prescribed brace immobilization without surgery. She has now completed 3 months of oral anticoagulant treatment. She has no relevant medical history, and her body mass index is 23; she was on a combined oral contraceptive for birth control at the time of the lower leg injury. Now she is asking for advice on the need to continue anticoagulant treatment. In fact, she would like to have a second baby. She also asks whether she should have further testing to assess a potential predisposition to thrombosis.
Introduction
Venous thromboembolism (VTE) includes DVT and pulmonary embolism (PE) that may occur as separate events or in combination. In the United States, the average annual rate of hospitalization in the adult population due to VTE was 239 per 100 000 during 2007 to 2009.1 In Europe the incidence of VTE has been recently reported to be 131 per 100 000 person-years.2 PE, either alone or in combination with DVT, accounts for 30% to 40% of VTE events.3
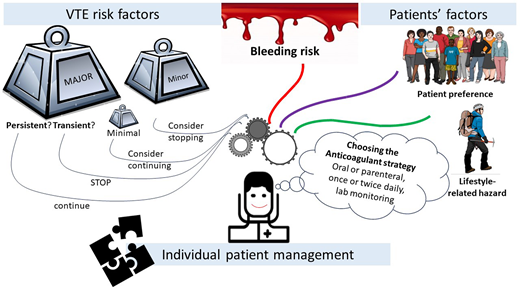
The clinical course of major VTE, which includes proximal DVT and/or PE, is characterized by the risk for recurrence and death in the acute phase and by the risk of recurrence and long-term sequelae such as chronic thromboembolic pulmonary hypertension or postthrombotic syndrome thereafter; these events account for a substantial burden of illness in terms of quality of life.4,5 The risk of recurrence is reduced by over 90% by appropriate anticoagulation. However, anticoagulant treatment has the counterbalance of increased risk for bleeding events. The duration of anticoagulant treatment after index VTE should be accurately evaluated by taking into account the risk for recurrence, the risk for bleeding, and the implications in lifestyle and occupational hazards for each individual patient.6
For all patients with a diagnosis of acute major VTE, anticoagulant treatment is composed of an initial and a long-term phase (primary treatment) and for selected patients by an extended phase for secondary VTE prevention.7,8 The identification of candidates for extended anticoagulation should be based on the estimated risk for recurrent VTE once anticoagulant treatment is withdrawn. This risk is strongly related to the features of index VTE.
Epidemiology of index VTE
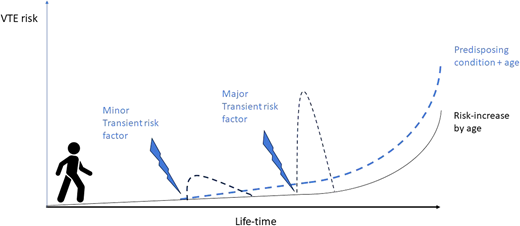
VTE is a multifactorial disease, and its risk depends on exposure to risk factors and predisposing conditions (Figure 1).3,9,10 The attributable risk and the strength of association with VTE varies among individual risk factors; in addition, more than 1 risk factor or predisposing condition can coexist in each individual patient.
Lifetime course of VTE risk based on presence/absence of predisposing conditions and on exposure to major or minor risk factors.
Lifetime course of VTE risk based on presence/absence of predisposing conditions and on exposure to major or minor risk factors.
Based on exposure to risk factors or underlying predisposing conditions, VTE can be classified as associated or not associated with identifiable risk factors. Risk factors can be classified as major or minor based on the strength of association with VTE and as persistent or transient based on duration of exposure (Table 1).11
Risk factors and predisposing conditions for VTE
| Risk factors . | Surgical . | Nonsurgical . |
|---|---|---|
| Major transient risk factors | Orthopedic, general, urologic, or gynecologic surgery (duration >45 minutes) | Immobilization with/without paresis |
| Trauma | Bedridden because of acute disease | |
| Critical illness | ||
| Major persistent | — | Cancer |
| Neurologic disease with paresis | ||
| Minor transient risk factors | Orthopedic, general, urologic, or gynecologic surgery (duration ≤45 minutes) | Limb trauma with/without plaster cast |
| Limb trauma requiring minor surgery | Pregnancy or postpartum | |
| Estrogen use for contraception or hormone therapy | ||
| Long-haul air travel | ||
| Acute infections | ||
| Minor persistent | — | Inflammatory bowel disease |
| Autoimmune disease | ||
| Predisposing conditions | — | Increasing age |
| Obesity | ||
| Heart failure | ||
| Prior VTE |
| Risk factors . | Surgical . | Nonsurgical . |
|---|---|---|
| Major transient risk factors | Orthopedic, general, urologic, or gynecologic surgery (duration >45 minutes) | Immobilization with/without paresis |
| Trauma | Bedridden because of acute disease | |
| Critical illness | ||
| Major persistent | — | Cancer |
| Neurologic disease with paresis | ||
| Minor transient risk factors | Orthopedic, general, urologic, or gynecologic surgery (duration ≤45 minutes) | Limb trauma with/without plaster cast |
| Limb trauma requiring minor surgery | Pregnancy or postpartum | |
| Estrogen use for contraception or hormone therapy | ||
| Long-haul air travel | ||
| Acute infections | ||
| Minor persistent | — | Inflammatory bowel disease |
| Autoimmune disease | ||
| Predisposing conditions | — | Increasing age |
| Obesity | ||
| Heart failure | ||
| Prior VTE |
Trauma, surgery, and VTE
All major trauma and surgeries associated with extensive tissue damage, blood stasis due to immobilization, pneumoperitoneum or the use of tourniquets, and, potentially, the release of procoagulant factors are major risk factors for VTE. Landmark studies have reported rates of asymptomatic VTE as high as 50% after major trauma or major orthopedic surgery in the absence of antithrombotic prophylaxis.
The risk of postoperative VTE after major surgery (interventions longer than 30 minutes) varies also by type of surgery (abdominal, orthopedic, neurosurgery, etc), type of anesthesia, patient features (age and male sex), underlying conditions (obesity, active cancer, malnutrition), and occurrence of postoperative complications.12
The epidemiology of VTE after trauma is also multifactorial and depends on patient features, type of bone fracture (lower vs upper limbs, long-bone fractures, surgery for bone fractures), need for immobilization, and whether surgery is required. Isolated lower limb trauma requiring immobilization is associated with an 18.0% risk of asymptomatic VTE (95% CI, 12.9-23.1) and a 2.0% risk of symptomatic VTE (95% CI, 1.3-2.7).13,14 The risk increases with patient-related risk factors, including coexisting medical conditions, age, obesity, previous VTEs, medications, pregnancy or the postpartum state, and procoagulant changes after surgery.
In adults requiring temporary immobilization (eg, a leg cast or brace in an ambulatory setting) for an isolated lower limb injury, the rate of major VTE without pharmacological thromboprophylaxis ranged from 0% to 11.7%, symptomatic VTE from 0% to 2.1%, and PE from 0% to 2.1%.15 The role of minor injuries as triggers for VTE is debated. In fact, the relationship between these events is difficult to study systematically due to recall bias in retrospective studies and because most of these minor injuries may not require medical care. Overall, minor injuries were reported to be associated with the risk of VTE, particularly if located in the leg and in factor V Leiden carriers.
Medical risk factors for VTE
In nonsurgical settings, immobilization and cancer are probably the risk factors with a stronger association with VTE.11,16 Immobility, defined as confinement to bed for more than 72 hours or more than 7 days or bedridden or nonambulatory status, was associated with a 3-fold increased risk for VTE (odds ratio [OR], 3.17; 95% CI, 2.18-4.62); a similar risk was shown for paresis (OR, 2.97; 95% CI, 1.20-7.36).16
Active cancer is a strong risk factor for VTE, for either out- or inpatients, mainly in those receiving chemotherapy; in fact, up to 15% to 20% of cancer patients experience VTE during the course of their treatment.17
Critical illness, defined as requiring an intensive or coronary care unit or a need for resuscitation (7 observational studies; OR, 1.65; 95% CI, 1.39-1.95) and acute infections including cellulitis, pneumonia, and sepsis (OR, 1.48; 95% CI, 1.16-1.89) were associated with an increased risk of VTE.16 Overall, hospitalization in general medical units is associated with an increased VTE incidence that varies as a function of both underlying medical conditions and immobility.
A number of conditions not related to hospitalization are also associated with an increased risk for VTE, but their association is probably weaker in comparison to those mentioned above. Inflammatory diseases, mainly rheumatoid arthritis, systemic lupus erythematosus, and inflammatory bowel disease, are associated with VTE, with low certainty of the evidence (OR, 2.33; 95% CI, 1.13-4.83).16 Another intriguing association is that between long-haul air travel—a duration of at least 4 hours—and VTE, the so called economy class syndrome. By studying 8755 employees of international organizations accounting for a total time of exposure to long-haul flights of 6872 patient-years, the incidence rate of symptomatic VTE was shown to be 3.2 per 1000 patient-years, as compared to 1.0 per 1000 patient-years in individuals not exposed to air travel (incidence rate ratio, 3.2; 95% CI, 1.8-5.6).18
Elevated body mass index has been identified as a risk factor for VTE in most observational population-based studies.19 Combined oral contraceptive use increases the risk of VTE by approximately 2-4-fold, but the absolute risk is still lower than 0.1%.20 Hormone replacement therapy is associated with a slightly increased risk of VTE compared to no hormone exposure (OR, approximately 1.5-2).21
Classification of VTE
Based on the epidemiology of the index event, VTE is usually categorized as occurring in the absence of any identifiable risk factor (unprovoked or idiopathic VTE) or in association with major transient or minor transient or persistent risk factors (provoked VTE).7,8,22 However, classification of VTE is still controversial, mainly due to a paucity of evidence for the risks associated with some specific conditions.23 In fact, when the strength of association between a risk factor and VTE decreases, as is the case for minor or even minimal risk factors, the sample size required to demonstrate the association dramatically increases, and high-quality studies are scarce. Overall, exposure to a risk factor like major trauma, major surgery, or active cancer may be a sufficient cause for VTE. However, exposure to minor risk factors may only provide a partial explanation for the index VTE (Figure 1). The threshold of attributable VTE risk to consider exposure to a risk factor a sufficient cause of VTE is undefined. According to expert consensus, a major risk factor is associated with a greater than 10-fold increase in the risk of first VTE, while a minor risk factor is associated with a 3- to 10-fold increase.22
The classification of VTE may have implications on the risk of recurrence (Table 2). In fact, an inverse correlation exists between the attributable risk of each individual transient risk factor for an index VTE and the risk of recurrence after discontinuation of anticoagulant treatment. A direct correlation exists between the attributable risk of persistent risk factors at index VTE and the risk of recurrence, at least while the risk factor is present. In this view, the definition of the specific attributable VTE risk for each condition could facilitate the prediction of recurrence.
Current guidelines on VTE: recommendation on secondary prevention
| Risk factor at index VTE . | ESC 201930 . | ASH 20207 . | NICE31 . | CHEST 20218 . |
|---|---|---|---|---|
| Unprovoked | Extended oral anticoagulation of indefinite duration should be considered. | Suggests indefinite antithrombotic therapy over stopping anticoagulation, except for high-risk of bleeding. In certain circumstances . . . clinicians and patients may use prognostic scores, or tests . . . to aid in reaching a final decision. | Consider continuing anticoagulation, taking bleeding risk, risk of recurrence, and patient preference into account. In low bleeding risk patient the benefits of continuing anticoagulation treatment are likely to outweigh the risks. | We recommend offering extended-phase anticoagulation. Patient preference and predicted risk of recurrent VTE or bleeding should influence the decision. |
| Transient risk factor | Major transient risk factor, discontinuation of oral anticoagulation is recommended after 3 months. Extended oral anticoagulation of indefinite duration should be considered after a first PE associated with a minor transient risk factor. | Temporary risk factors discontinue anticoagulant therapy after completion of the primary treatment. Chronic risk factorsa suggests indefinite antithrombotic therapy over stopping anticoagulation. | Consider stopping anticoagulation treatment at 3 months following a provoked DVT or PE if the provoking factor is no longer present and the clinical course has been uncomplicated. | Major transient risk factor, we recommend against offering extended-phase anticoagulation. Minor transient risk factor, we suggest against offering extended-phase anticoagulation. |
| Persistent risk factor | Extended oral anticoagulation of indefinite duration should be considered for patients with a first episode of PE associated with a persistent risk factor. | Chronic risk factors may continue anticoagulant therapy indefinitely for secondary prevention after completion of the primary treatment. | We recommend offering extended-phase anticoagulation. |
| Risk factor at index VTE . | ESC 201930 . | ASH 20207 . | NICE31 . | CHEST 20218 . |
|---|---|---|---|---|
| Unprovoked | Extended oral anticoagulation of indefinite duration should be considered. | Suggests indefinite antithrombotic therapy over stopping anticoagulation, except for high-risk of bleeding. In certain circumstances . . . clinicians and patients may use prognostic scores, or tests . . . to aid in reaching a final decision. | Consider continuing anticoagulation, taking bleeding risk, risk of recurrence, and patient preference into account. In low bleeding risk patient the benefits of continuing anticoagulation treatment are likely to outweigh the risks. | We recommend offering extended-phase anticoagulation. Patient preference and predicted risk of recurrent VTE or bleeding should influence the decision. |
| Transient risk factor | Major transient risk factor, discontinuation of oral anticoagulation is recommended after 3 months. Extended oral anticoagulation of indefinite duration should be considered after a first PE associated with a minor transient risk factor. | Temporary risk factors discontinue anticoagulant therapy after completion of the primary treatment. Chronic risk factorsa suggests indefinite antithrombotic therapy over stopping anticoagulation. | Consider stopping anticoagulation treatment at 3 months following a provoked DVT or PE if the provoking factor is no longer present and the clinical course has been uncomplicated. | Major transient risk factor, we recommend against offering extended-phase anticoagulation. Minor transient risk factor, we suggest against offering extended-phase anticoagulation. |
| Persistent risk factor | Extended oral anticoagulation of indefinite duration should be considered for patients with a first episode of PE associated with a persistent risk factor. | Chronic risk factors may continue anticoagulant therapy indefinitely for secondary prevention after completion of the primary treatment. | We recommend offering extended-phase anticoagulation. |
Cancer patients are excluded from this recommendation.
Epidemiology of recurrent VTE
As for index VTE, the risk for recurrent VTE is also multifactorial, based on patient features, epidemiology of the index event, and the exposure to upcoming risk factors or predisposing conditions.24
Based on evidence from landmark studies, VTE not associated with identifiable risk factors has a high risk for recurrence in the first 2 years after discontinuation of anticoagulant treatment.25 The risk declines in the following 3 years, then reaches a plateau of about 3% per year and never falls to 0. The risk of recurrence after an initial event of major VTE provoked by a temporary risk factor is expected to be about half that of unprovoked VTE, with no evidence that this effect can be modified by the length of anticoagulant treatment or the type of VTE (DVT vs PE). VTE associated with surgical risk factors seems to have a lower risk for recurrence in comparison with VTE associated with medical risk factors.
Of note, limited data are currently available on the risk for recurrence after exposure to specific risk factors, except for cancer.17,26-29 Concerning the risk for recurrence after oral contraceptive–associated VTE, a meta-analysis of 19 studies including 1537 women found that the incidence of recurrence after the discontinuation of anticoagulation was 1.22% per person-year (95% CI, 0.92-1.62; I2 , 6%) during 5828 person-years of follow-up.27 Similarly, the risk for recurrent VTE after an index pregnancy–associated VTE seems to be low and mainly related to subsequent pregnancy.
The recurrence risk in healthy patients with travel-associated VTE in the absence of other risk factors is unknown. There is controversy as to whether travel-associated VTE should be regarded as provoked vs unprovoked (especially since it is quite transient in duration). This controversy exists for other minor risk factors such as short immobilizations or mild trauma and suggests the potential for a further categorization of minor risk factors into minor and minimal. However, while awaiting further evidence on the risk for recurrence, this further categorization would have no or minimal clinical implications.
Making decisions about anticoagulation
Secondary prevention of recurrences should be tailored based on the estimated risk for recurrent VTE after discontinuation of anticoagulation and the estimated risk for bleeding if anticoagulation is continued.7,8,30,31 The risk of recurrence decreases over time after discontinuation of anticoagulation for the index event, while the risk of bleeding during anticoagulation remains constant over time. Of note, case-fatality rates of recurrent VTE and major bleeding events are expected to be similar during the initial period of VTE treatment.32 Over time, the case-fatality rate of recurrent VTE declines, while the case-fatality rate of major bleeding remains stable. Case-fatality rates of recurrent VTE have been reported to be higher in patients initially presenting with PE than with DVT.32
Direct anticoagulants are associated with a reduced risk of bleeding in comparison with vitamin K antagonists. The risk of major bleeding was estimated to be 1.92% (95% CI, 1.57-2.33) per year with vitamin K antagonists, with about one-fourth of events caused by intracerebral hemorrhage.33 DOACs are associated with a reduced risk of major and intracerebral bleeding in comparison to vitamin K antagonists, though the risk of nonmajor clinically relevant bleeding is not negligible. The safety of these agents may encourage physicians to reduce the threshold of recurrence risk in patients who are candidates for extended anticoagulation. In fact, time-limited prolongation of anticoagulant treatment beyond 3 months delays recurrences without reducing the absolute risk of recurrence after discontinuation of treatment. This evidence, mainly derived from patients suffering unprovoked VTE, should discourage the prolongation of anticoagulation for time-limited periods.
Recent studies have shown that a consistent proportion of patients who experienced VTE associated with transient risk factors receive extended anticoagulation for prevention of recurrences. In a large international registry, 36.7% of patients with transient provoking risk factors were still receiving anticoagulant treatment 12 months following the index VTE.34,35 However, reducing the threshold for extended anticoagulation potentially exposes a consistent proportion of patients to an unnecessary risk of bleeding. In addition, extending anticoagulation may limit everyday life activities and sports.36
As a further option for secondary prevention of VTE, rivaroxaban and apixaban also have shown a favorable efficacy to safety profile when used in prophylactic regimens.37,38 In patients treated for an index VTE for whom there was clinical equipoise regarding their need for continued anticoagulation, prophylactic doses of apixaban reduced the risk of recurrent VTE by an extent similar to therapeutic dose, with a promising safety profile.37 This study, in which 90% of patients were treated for a first unprovoked VTE, was not powered for safety outcomes, but its results changed international guidelines and clinical practice. In the Einstein Choice study, the risk of recurrent VTE was significantly lower with rivaroxaban at either a treatment or a prophylactic dose than with aspirin, without a significant increase in bleeding rates.38 About 60% of the patients entered the Einstein Choice study after an index VTE associated with risk factors. Overall, these studies paved the way for extended prevention of VTE with potentially safer anticoagulant regimens than the therapeutic regimens of vitamin K antagonists or DOACs. Reduced doses of rivaroxaban and apixaban represent the regimen of choice for secondary prevention of VTE in the majority of noncancer patients for their efficacy to safety profile. Unfortunately, patients with a high risk for recurrence were not included in these studies, and specific data are required before the reduced-dose regimen can be used in this setting.
In conclusion, secondary prevention of VTE is a challenging management issue; fatal recurrences may occur, mainly after an index PE, major bleeding continues to be associated with long-term anticoagulation, and clinically relevant nonmajor bleeding may impact quality of life as well as compromise everyday life activities.36 Though the risk of recurrence is definitely higher after VTE occurring in the absence of risk factors, the cumulative incidence of recurrent VTE after an index episode associated with risk factors has been described to be about 15% at 10 years.39
Several scores and models have been proposed to support decisions on the duration of anticoagulation. However, despite extensive efforts, the accuracy of proposed models and scores continues to be suboptimal.40 Clinicians should be aware that prediction tools can be used to support decision-making, but the final decision on treatment duration cannot skip holistic medical assessment.
CLINICAL CASE (continued)
All the above information is essential for providing our patient advice after the initial 3 months of anticoagulant treatment. The patient was on combined oral contraceptive therapy at the time of the index VTE. This makes the risk for recurrent VTE low after discontinuation of both anticoagulants and hormone therapies. In addition, the patient suffered VTE after nonsurgical ankle trauma. As this is not a major trauma, doubts remain on whether the VTE should be considered provoked after the exposure to this sole risk factor. However, the association of minor trauma and estro-progestin therapy is probably strong enough to convey a sufficient risk for VTE. In addition, as the patient suffered DVT, recurrence, if any, will probably occur as DVT, thus reducing the risk of fatal events. In this patient extended treatment is not required. After discontinuation of anticoagulation, an antithrombotic prophylaxis could be considered in the event of pregnancy, also based on patient features. Tests for thrombophilia could be considered, but their clinical implications are limited due to the low level of evidence supporting thrombophilia-guided anticoagulation strategy.41
Future perspectives
A randomized clinical trial is ongoing in patients who experienced VTE associated with a major provoking factor, including major surgery or major trauma, and have at least 1 persistent risk factor for VTE (such as persistent immobility, obesity, heart failure, or inflammatory/autoimmune disorders).42 After completion of at least 3 months of standard-dose therapeutic anticoagulation, patients will be randomized to apixaban at 2.5 mg twice daily or placebo for 12 months.
In addition, potential advances could come from clinical studies of factor XI inhibitors.43 Should these agents turn out to be as effective and safer than currently available anticoagulants in reducing VTE, a new paradigm for extended treatment could emerge.
Conflict-of-interest disclosure
Cecilia Becattini: honoraria: Bayer HealthCare, Bristol Myers Squibb, Daiichi Sankyo.
Ludovica Anna Cimini: no competing financial interests to declare.
Off-label drug use
Cecilia Becattini: nothing to disclose.
Ludovica Anna Cimini: nothing to disclose.