Learning Objectives
Explain the age-related challenges involved in considering anticoagulation for acute VTE
Identify the best treatment strategy for acute VTE in older patients with chronic renal disease and low weight
CLINICAL CASE
A 76-year-old woman with a past medical history of coronary artery disease and on chronic therapy with aspirin presents to the emergency room with right lower extremity pain and swelling. A venous duplex ultrasound showed an acute thrombus involving the right common femoral vein. Her hemoglobin is 11.5 mg/dL and platelet count is 158,000/uL. Her weight is 58 kg, and her serum creatinine is 1.3 mg/dL (estimated creatinine clearance [CrCl] of 36 mL/min). What anticoagulant and dosing are appropriate for this patient?
Introduction
A delicate balance exists between preventing morbidity and mortality from venous thromboembolism (VTE) and preventing associated bleeding complications in older patients. While older age is associated with increased risk of venous thrombosis,1 it is also associated with increased risk of bleeding and ambiguity around the appropriate dosing of anticoagulation.2 The evidence to support anticoagulation selection and dosing in older patients is limited since they are not well represented in clinical trials for the approval of direct oral anticoagulants (DOACs) in VTE.3 Furthermore, common conditions in this population such as stage 4-5 chronic kidney disease (CKD), anemia, liver disease, hypertension, or concomitant use of dual antiplatelet therapy were excluded from registration trials.4-7
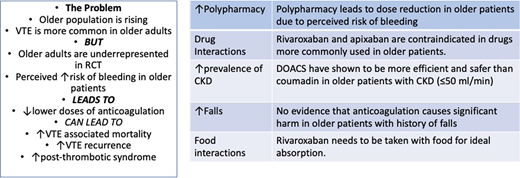
The problem
The incidence rate of VTE increases with age from 1 per 1000 person-years in the general population to 6-8 per 1000 person-years in those 80 and older.1,8 There are physiologic and acquired risk factors that are responsible for this high incidence. Aging is associated with increasing levels of procoagulant factors, as well as impairment in the fibrinolytic pathway.3 At the same time, cancer, immobility, cardiovascular disease, and chronic inflammatory diseases contributing to VTE risk are also more common with aging.9
The treatment of VTE in older patients is challenging. Studies have shown that adherence to treatment is lower in older patients across different diseases such as atrial fibrillation, leading to poor clinical outcomes and increase risk of VTE relapse.10,11 Falls are also more common in this age group.9 This has been a very well described concern of physicians when prescribing therapeutic anticoagulation,2 even though our ability to predict future bleeding due to falls is very limited. Interactions with other drugs may also affect the absorption of DOACs12 in the setting of frequent polypharmacy in this population.9 For example, carbamazepine, a strong P-glycoprotein and CYP3A4 inducer sometimes prescribed for neuropathic pain, can decrease serum concentrations of most DOACs.
The case for full dose anticoagulation in older patients
The five landmark studies that led to the approval of apixaban, dabigatran, edoxaban, and rivaroxaban in VTE treatment4-7,13 have a median age of 55-60 years, even though the majority of VTE occur in patients over 70 years1 (Table 1). Albeit for low numbers or participants, the efficacy and safety reported on those trials remained favorable for DOAC vs vitamin K antagonists (VKA) in patients 75 and older.14
Evidence on use of therapeutic anticoagulation for treatment of VTE in elderly patients3
| TRIAL . | Drug and dose . | Number of patients . | Patients ≥75 . | Patients with CrCl ≤50 mL/min . | Pertinent results for elderly population . |
|---|---|---|---|---|---|
| EINSTEIN-DVT/PE4,5 | Rivaroxaban 15 mg twice daily for 3 weeks followed by 20 mg daily | 8281 Mean age = 57 years | 1283 (18%) | 664 (8%) | No differences in primary and safety outcome in different age, weight, and CrCl groups Excluded patients with “high risk of bleeding” (not defined) |
| RE-COVER6 | Dabigatran 150 mg twice daily | 2564 Mean age = 55 years | 290 (11.3%) | 133 (5.2%) | No differences in primary and safety outcome in different age, weight, and CrCl groups Excluded patients with “high risk of bleeding” (not defined) 100 mg or less of daily aspirin was acceptable |
| AMPLIFY7 | Apixaban 10 mg twice daily for 1 week, followed by 5 mg twice daily | 5395 Mean age = 57 years | 768 (14%) | 327 (6.2%) | No differences in primary outcome in different age, CrCl, and weight groups. Safety outcome favored apixaban for age subgroups 65-75 and >75 Safety outcome favored apixaban on weight >60 kg Excluded patients with “high risk of bleeding” (not defined), Hb <9 g/dL, platelets <100 000/m3, or patients on dual antiplatelet therapy Aspirin at a dose of 165 mg or less was accepted |
| HOKUSAI-VTE8 | Heparin lead-in followed by edoxaban 60 mg, or 30 mg in patients with CrCl 30-50 mL/min or weight <60 kg | n = 8292 Mean age = 55.8 years | 1004 (12%) | 541 (6.6%) | No differences in primary outcome and safety outcomes in different age and weight groups Excluded patients with “high risk of bleeding” (not defined) and CrCl <30 mg/dL 100 mg or less of daily aspirin was acceptable |
| TRIAL . | Drug and dose . | Number of patients . | Patients ≥75 . | Patients with CrCl ≤50 mL/min . | Pertinent results for elderly population . |
|---|---|---|---|---|---|
| EINSTEIN-DVT/PE4,5 | Rivaroxaban 15 mg twice daily for 3 weeks followed by 20 mg daily | 8281 Mean age = 57 years | 1283 (18%) | 664 (8%) | No differences in primary and safety outcome in different age, weight, and CrCl groups Excluded patients with “high risk of bleeding” (not defined) |
| RE-COVER6 | Dabigatran 150 mg twice daily | 2564 Mean age = 55 years | 290 (11.3%) | 133 (5.2%) | No differences in primary and safety outcome in different age, weight, and CrCl groups Excluded patients with “high risk of bleeding” (not defined) 100 mg or less of daily aspirin was acceptable |
| AMPLIFY7 | Apixaban 10 mg twice daily for 1 week, followed by 5 mg twice daily | 5395 Mean age = 57 years | 768 (14%) | 327 (6.2%) | No differences in primary outcome in different age, CrCl, and weight groups. Safety outcome favored apixaban for age subgroups 65-75 and >75 Safety outcome favored apixaban on weight >60 kg Excluded patients with “high risk of bleeding” (not defined), Hb <9 g/dL, platelets <100 000/m3, or patients on dual antiplatelet therapy Aspirin at a dose of 165 mg or less was accepted |
| HOKUSAI-VTE8 | Heparin lead-in followed by edoxaban 60 mg, or 30 mg in patients with CrCl 30-50 mL/min or weight <60 kg | n = 8292 Mean age = 55.8 years | 1004 (12%) | 541 (6.6%) | No differences in primary outcome and safety outcomes in different age and weight groups Excluded patients with “high risk of bleeding” (not defined) and CrCl <30 mg/dL 100 mg or less of daily aspirin was acceptable |
The prescriber's perception of high risk of bleeding attributed to frailty in older patients often leads to off- label use of lower doses of DOACs. One particular concern is low body weight, which can be seen in up to 20% of patients above age 85.9 A recent survey showed that hematologists frequently reduced the dose of apixaban and rivaroxaban in older patients for the treatment of acute VTE, with only 50%, 35%, and 25% of responders using the label doses in patients between 65 and 74, 75 and 84, and >85, respectively.15 This was also evidenced in a multicenter registry enrolling 3027 consecutive patients with acute, symptomatic VTE (COMMAND VTE),16 in which patients 80 and older received less anticoagulation after the first 10 days (93%, 93%, and 90% for patients <65, 65-80, and >80, respectively, P = 0.04). This study also provided reassuring data on bleeding; even though patients older than 80 had significantly more anemia and lower body weight and were more commonly on antiplatelet agents and nonsteroidal antiinflammatory drugs compared to younger patients, they did not show a statistically higher risk of major bleeding.
Another important challenge in older patients is the high incidence of CKD occurring in up to 35% of patients above age 66.17 Due to the varying level of renal clearance in different DOACs, kidney dysfunction is an important factor when selecting an anticoagulant. On this topic, there is confusion regarding dose reduction recommendations in older patients. While dose adjustment is recommended in patients with a serum creatinine >1.5 mg/dL along with age ≥80 or weight ≤60 kg for stroke prevention in those with atrial fibrillation, it has not been studied for the treatment of acute VTE.18 Pharmacokinetics studies have shown that age alone has a small impact in DOAC exposure.19-22 However, those same studies found that dabigatran clearance was affected by renal function more so than apixaban and rivaroxaban, while edoxaban had a labeled dose reduction for the treatment of VTE if CrCl was 15-50 mL/min, weight was ≤60 kg, or in the setting of a concomitant use of p-glycoprotein inhibitors. In a post-hoc meta-analysis comparing DOAC vs warfarin in registration trials, VTE recurrence and major bleeding were significantly lower in the pooled DOAC treatment arm in the subgroup aged ≥75 years and nonsignficantly lower in the subgroup with CrCl ≤50 mL/min.14
Future research
Due to the small number of older patients in randomized trials, there is an ongoing need for dedicated studies powered for this subgroup. To date, there is no convincing evidence to support a lower dose of DOAC for the treatment of acute VTE for older patients based on age alone. We believe that age, frailty, dependency, and cognitive function should not determine changes in treatment doses of DOAC when there is a clinical indication for anticoagulation for VTE.
Recommendations
We recommend approved dosing of DOAC for the treatment of acute symptomatic VTE per package insert regardless of patient age (Grade 1B).
We suggest dose-reduced edoxaban for patients with CrCl 15-50 mL/min, weight ≤60 kg, or concomitant use of p-glycoprotein inhibitors regardless of patient age, when followed by 5 days of parenteral anticoagulation.
We suggest addressing modifiable bleeding risk factors, including uncontrolled hypertension, avoid antiplatelet therapy when acceptable, nonsteroidal anti-inflammatory drugs or alcohol to reduce the risk of bleeding, rather than using an off-label dose of a DOAC.
Conflict-of-interest disclosure
Nicolas Gallastegui: no competing financial interests to declare.
Camila Masias: no competing financial interests to declare.
Off-label drug use
Nicolas Gallastegui: nothing.
Camila Masias: nothing.