Abstract
Venous thromboembolism (VTE) is a leading cause of maternal morbidity and mortality worldwide. Despite the impact of VTE on pregnant and postpartum people and on society, guidelines addressing prevention, diagnosis, and management of VTE in pregnant and postpartum people frequently are based on recommendations from expert opinion and are extrapolated from data in nonpregnant populations. Pregnant individuals are frequently excluded from clinical trials, which is a barrier to providing safe, effective care. Anchoring to a case discussion, this review provides an update on recently published and ongoing randomized clinical trials (RCTs), prospective clinical management studies, and other research in this area. It highlights, in particular, the results of the Highlow RCT, which addresses optimal prevention of recurrence during pregnancy in people with prior VTE. Finally, we raise awareness of the impact of national and international clinical trial networks on the conduct of RCTs in pregnancy. We conclude, based on these data, that academic VTE clinical trials in pregnant women can and must be done.
Learning Objectives
To understand the impact of VTE in pregnancy and the crucial importance of excellent prevention and diagnostic and management pathways
To review the most recently published data and guidelines addressing optimal prevention, diagnosis, and management of VTE in pregnancy
CLINICAL CASE
It was February 2018. Aries was a 27-year-old clinical nurse specialist at 28 weeks' gestation in their first pregnancy. They were being cared for in the emergency department with suspected pulmonary embolism. They complained of left-sided pleuritic chest pain without breathlessness. Their respiratory rate was 16 breaths per minute, blood pressure was 111/70 mm Hg, heart rate is 84 beats per minute, and oxygen saturations were 99% on room air. They had no lower limb symptoms. I met them, and we discussed their suspected diagnosis.
The impact of venous thromboembolism (VTE) in pregnancy
VTE is a leading cause of death of pregnant and postpartum people.1,2 Those who survive can have lifelong disability. VTE risk is higher during pregnancy than in the nonpregnant state and peaks postpartum: pooled incidence rates of 1.2 (95% confidence interval [CI]: 1.0-1.4) and 4.2 (95% CI: 2.4-7.6) per 1000 person-years have been reported during the antenatal and postpartum periods, respectively.3
I explained to Aries that we suspect pulmonary embolism. On one hand, Aries could appreciate the importance of not missing a pulmonary embolism diagnosis in pregnancy. However, they were worried about being exposed to radiation through diagnostic imaging. We had a discussion.
Radiation exposure during imaging for pulmonary embolism in pregnancy (Table 1)
A normal perfusion scan and a negative computed tomography pulmonary angiogram (CTPA) are considered effective for ruling out pulmonary embolism in pregnancy.2 Sensitivity and negative predictive value of lung scintigraphy and CTPA are reported to be high; however, large, adequately powered studies comparing methodologies are lacking.4,5 Both maternal and fetal radiation exposure are low when modern imaging methods are used.2 Low-dose perfusion scanning (estimated fetal radiation dose 0.02-0.20 mGy) and CTPA (estimated fetal radiation dose 0.05-0.5 mGy) expose baby to doses far below the threshold for fetal radiation complications (which is accepted to be 50-100 mGy).2,6 Moreover, advances in CT technology have reduced radiation exposure through methods that include reduced kilovoltage, contrast- monitoring component, and anatomic coverage of the scan, and using iterative reconstructive techniques.2,7,8 We recently reported that additional breast radiation dose reduction can be achieved by combining low-dose CTPA with breast shields in pregnancy without impacting image quality: shielding reduced surface breast radiation dose by 66% (to 0.5 ± 0.3 mGy) in an anthropomorphic phantom and by 48% (to 0.7 ± 0.2 mGy) in study participants.8
| Test . | Fetal radiation dose (mGy) . | Maternal breast dose (mGy) . |
|---|---|---|
| Chest X-ray | <0.01 | <0.1 |
| Perfusion lung scan: | ||
| Low dose: ~40 MBq | 0.02-0.20 | 0.16-0.5 |
| High dose: ~200 MBq | 0.20-0.60 | 1.2 |
| Ventilation lung scan | 0.10-0.30 | <0.01 |
| CT pulmonary angiography | 0.05-0.5 | ~1-10a (lower with modern CTPA techniques) |
| Test . | Fetal radiation dose (mGy) . | Maternal breast dose (mGy) . |
|---|---|---|
| Chest X-ray | <0.01 | <0.1 |
| Perfusion lung scan: | ||
| Low dose: ~40 MBq | 0.02-0.20 | 0.16-0.5 |
| High dose: ~200 MBq | 0.20-0.60 | 1.2 |
| Ventilation lung scan | 0.10-0.30 | <0.01 |
| CT pulmonary angiography | 0.05-0.5 | ~1-10a (lower with modern CTPA techniques) |
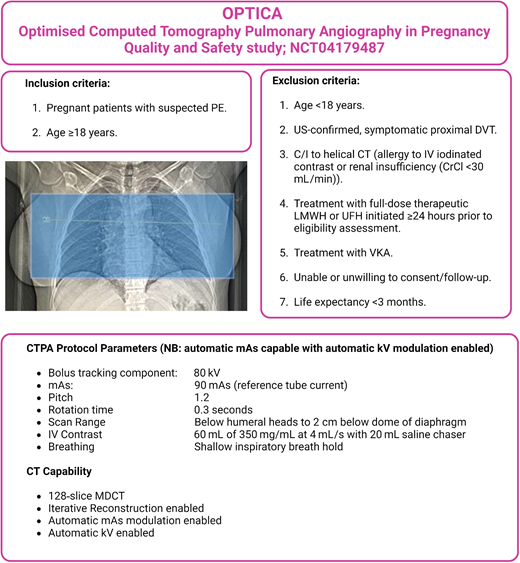
A prospective clinical management study, “OPTICA” (Optimised Computed Tomography Pulmonary Angiography [CTPA] in Pregnancy, Quality and Safety; NCT04179487) aims to validate the safety of such an optimized low-dose CTPA protocol as part of local algorithms for evaluation of suspected pulmonary embolism in pregnancy. The primary outcome is the incidence of VTE at 3 months in people in whom the baseline CTPA excluded pulmonary embolism6 (Figure 1).
OPTICA study (NCT 04179487) overview, outlining inclusion and exclusion criteria, CTPA protocol parameters, and settings.Inset: The scan range for the OPTICA study extends from below the humeral heads to approximately 2 cm below the lowest dome of diaphragm. C/I, contraindication; CrCl, creatinine clearance (calculated by Cockroft-Gault equation); CT, computed tomography; PE, pulmonary embolism; UFH, unfractionated heparin; US, ultrasound; VKA, vitamin K antagonist.
OPTICA study (NCT 04179487) overview, outlining inclusion and exclusion criteria, CTPA protocol parameters, and settings.Inset: The scan range for the OPTICA study extends from below the humeral heads to approximately 2 cm below the lowest dome of diaphragm. C/I, contraindication; CrCl, creatinine clearance (calculated by Cockroft-Gault equation); CT, computed tomography; PE, pulmonary embolism; UFH, unfractionated heparin; US, ultrasound; VKA, vitamin K antagonist.
Diagnosis of pulmonary embolism in pregnancy
Aries asked, “Do I really need to have a scan?” They knew that diagnostic algorithms that combine pretest probability scores with D-dimers can rule out pulmonary embolism in nonpregnant patients.2 At the time of their assessment, these algorithms were not validated in pregnancy; however, studies were ongoing, which have since been completed and published.
First, a UK prospective cohort study augmented with additional cases of confirmed pulmonary embolism did not demonstrate diagnostic utility for D-dimers or clinical decision rules in people with suspected pulmonary embolism during pregnancy.9,10 In this study, objective pulmonary embolism diagnostic imaging and clinical diagnosis were permitted and there was no fixed diagnostic algorithm. Subsequently, 2 multicenter prospective diagnostic management outcome studies were published. In the first, the “CT-PE-Pregnancy” study (ClinicalTrials.gov: NCT00740454), pulmonary embolism was excluded without CTPA imaging in pregnant people with non-high revised Geneva pretest probability score and a negative D-dimer (defined as <500 ng/L).11 The primary outcome, symptomatic VTE at 3 months, occurred in 0.0% (95% CI: 0.0%-1.0%) of untreated people; 11.7% did not require diagnostic imaging. Bilateral compression ultrasound (CUS) was mandated in people qualifying for CTPA but had a low diagnostic yield.
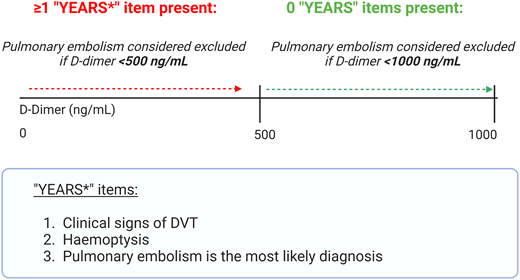
A second multicenter prospective management study with a similar design (the Artemis study12 ) evaluated an algorithm termed “YEARS” (Figure 2), adapted for pregnancy. Pulmonary embolism was excluded in people with no “YEARS” items and a D-dimer level <1000 ng/mL, or ≥1 “YEARS” item and D-dimer <500 ng/mL. CUS was performed if there were clinical signs of deep vein thrombosis (DVT). At 3-month follow-up, only 1 participant developed a popliteal DVT (0.21%; 95% CI: 0.04%-1.2%). Exposure to diagnostic imaging could be avoided in 39% (95% CI: 35%-44%) of patients. The diagnostic yield of targeted CUS was 7%.
The “YEARS” items, which were included in the Artemis study diagnostic algorithm (Netherlands Trial Register number, NL5726).12 In this study, pulmonary embolism was excluded in people with no YEARS items and a D-dimer level <1000 ng/mL, or ≥1 YEARS item and D-dimer <500 ng/mL. CUS was performed if there were clinical signs of DVT.
The “YEARS” items, which were included in the Artemis study diagnostic algorithm (Netherlands Trial Register number, NL5726).12 In this study, pulmonary embolism was excluded in people with no YEARS items and a D-dimer level <1000 ng/mL, or ≥1 YEARS item and D-dimer <500 ng/mL. CUS was performed if there were clinical signs of DVT.
The hospital in which Aries was a patient was a recruiting site for the Artemis12 study. Had Aries been a participant in this trial, it would have been noted that they had one “YEARS” item (pulmonary embolism most likely diagnosis) at the time of recruitment, meaning that a CTPA would be required (rather than rule out without diagnostic imaging) with a D-dimer test >500 ng/mL. Their D-dimer subsequently returned as 1200 ng/mL. A CTPA revealed a left lower lobe pulmonary embolism.
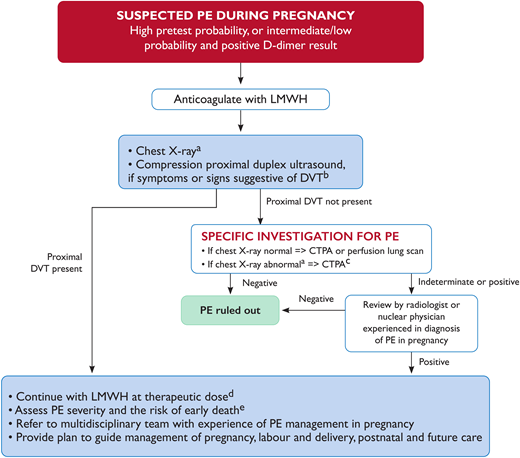
The studies described previously impacted the 2019 European Society of Cardiology (ESC) Guidelines on Diagnosis and Management of Acute Pulmonary Embolism,2 which now state that D-dimer measurement in conjunction with clinical prediction rules “should be considered” during investigation of suspected pulmonary embolism in pregnancy (as summarized by the algorithm in figure 3). The ESC guidelines define the statement “should be considered” (which indicates a Class IIa recommendation) as “weight of evidence/opinion is in favour of usefulness/efficacy.”
European Society of Cardiology (ESC) algorithm for diagnostic workup and management of suspected pulmonary embolism during pregnancy and up to 6 weeks postpartum. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). https://doi.org/10.1093/eurheartj/ehz405. (a) If chest X-ray is abnormal, consider also alternative cause of chest symptoms. (b) DVT in pelvic veins may not be ruled out by CUS. If the entire leg is swollen, or there is buttock pain or other symptoms suggestive of pelvic thrombosis, consider magnetic resonance venography to rule out DVT. (c) CTPA technique must ensure very low fetal radiation exposure (see Table 1). (d) Perform full blood count (to measure hemoglobin and platelet count) and calculate creatinine clearance before administration. Assess bleeding risk and ensure absence of contraindications. (e) See Konstantinides and Meyer.2 High, intermediate, and low PE pretest probability as defined in Konstantinides and Meyer.2 CTPA, computed tomography pulmonary angiography; CUS, compression ultrasonography; PE, pulmonary embolism. Reproduced with permission from Konstantinides and Meyer.2
European Society of Cardiology (ESC) algorithm for diagnostic workup and management of suspected pulmonary embolism during pregnancy and up to 6 weeks postpartum. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). https://doi.org/10.1093/eurheartj/ehz405. (a) If chest X-ray is abnormal, consider also alternative cause of chest symptoms. (b) DVT in pelvic veins may not be ruled out by CUS. If the entire leg is swollen, or there is buttock pain or other symptoms suggestive of pelvic thrombosis, consider magnetic resonance venography to rule out DVT. (c) CTPA technique must ensure very low fetal radiation exposure (see Table 1). (d) Perform full blood count (to measure hemoglobin and platelet count) and calculate creatinine clearance before administration. Assess bleeding risk and ensure absence of contraindications. (e) See Konstantinides and Meyer.2 High, intermediate, and low PE pretest probability as defined in Konstantinides and Meyer.2 CTPA, computed tomography pulmonary angiography; CUS, compression ultrasonography; PE, pulmonary embolism. Reproduced with permission from Konstantinides and Meyer.2
The pregnancy-adapted “YEARS” algorithm was subsequently externally validated using data from the “CTPE-pregnancy” study in a post hoc analysis.13 The pulmonary embolism prevalence was 6.5%; 91 people had no “YEARS” items, and 280 had one or more items. Of 371 people, 77 met criteria for pulmonary embolism exclusion and would not have undergone CTPA according to the “YEARS” algorithm (which includes risk-adapted D-dimer assessment, as discussed previously). The failure rate was 0%, although this is an imprecise estimate (0.77; 95% CI 0.0-3.9%).
Moreover, a recent individual patient data meta-analysis including data from 893 patients from the CT-PE-pregnancy11 and Artemis12 studies supported the use of noninvasive diagnostic strategies in pregnant people with suspected pulmonary embolism, as pulmonary embolism could be ruled out based on non-high clinical probability and a normal D-dimer in up to 40% of people.14 Point estimates of the failure rates were acceptably low, applying a safety threshold dependent on pulmonary embolism prevalence at baseline. For the YEARS algorithm, the sensitivity, failure rates, and efficiency (number of CTPA scans avoided) were 98% (95% CI 88%-100%), 1.4% (95% CI 0.49%-3.3%), and 43% (95% CI 40%-46%), respectively. The efficiency of CUS in patients without DVT symptoms was low, at 0.79% (95% CI 0.16%-2.4%), but 10-fold higher in those with DVT symptoms, at 7.9% (95% CI 3.9%-15%). The baseline pulmonary embolism prevalence was 5.4%.
Efforts to further improve pulmonary embolism diagnosis in pregnancy are ongoing, including the recent derivation of a novel pretest probability score: the pregnancy-adapted Geneva (PAG) score.15 In contrast to previous rules, the PAG score includes only objective items that are relevant to pregnant people, excluding items such as age >65 years or cancer. The authors derived the PAG score using data from the CT-PE-Pregnancy study.11 The area under the curve of the PAG and the original Geneva pretest probability scores were 0.795 (95% CI 0.690-0.899), and 0.684 (95% CI 0.563-0.805), respectively.
CLINICAL CASE (continued)
As a nurse, Aries was interested to know whether similar studies were evaluating algorithms for suspected DVT in pregnancy.
Diagnosis of DVT during pregnancy
D-dimers and clinical prediction rules are not currently validated for DVT exclusion in pregnancy, and diagnostic imaging is essential. The LEFt clinical decision rule shows promise in evaluating pregnant people with suspected DVT. Points are given for Left leg symptoms (1 point), Extremity swelling (≥2 cm difference in calf circumference; 1 point) and First-trimester symptom onset (1 point). People with 0 or 1 point have an “unlikely” clinical probability, and those with >1 point a “likely” clinical probability. In retrospective analyses of 2 cohort studies, the diagnostic failure rate of the LEFt rule was 3.1% (95% CI: 0.8%-7.7%)16 and 0% (95% CI: 0%-8.2%),17 respectively, highlighting the need for more data. The ongoing LEaD study (Safely Ruling Out Deep Vein Thrombosis in Pregnancy With the LEFt Clinical Decision Rule and D-Dimer: A Prospective Cohort Study; NCT02507180) aims to prospectively evaluate the performance of the diagnostic algorithm.
Back to the case; management of acute VTE in pregnancy
In line with 2019 ESC guidelines,2 Aries's antenatal and peripartum care was guided by a multidisciplinary team with experience in pulmonary embolism management in pregnancy. In the Rotunda Hospital, Dublin, Ireland, where Aries was being cared for, written plans are jointly agreed on by a multidisciplinary team and discussed with the patient themselves. This approach is now also endorsed by the authors of a recent expert consensus toolkit from the Foundation for Women and Girls with Blood Disorders Thrombosis Subcommittee on the multidisciplinary care of pregnant people with VTE or at risk of VTE,18 with recommendations being provided on the roles of individual team members in the care of pregnant people with VTE. Low-molecular-weight heparin (LMWH) is recommended by international guidelines and by these consensus recommendations for the treatment of VTE during pregnancy, and direct oral anticoagulants are contraindicated.2,18 Importantly, the Foundation for Women and Girls with Blood Disorders Thrombosis Subcommittee expert consensus toolkit18 makes recommendations on the specific contents of a delivery plan, which should include the timing, route, and location of delivery; an intrapartum anticoagulation plan (with guidance on the time to discontinue anticoagulation in the event of a planned or unplanned delivery and whether bridging anticoagulation is required); the timelines required for eligibility for neuraxial anesthesia; and, where relevant, a postpartum anticoagulation plan (with advice on options based on the infant feeding plan).
Management of therapeutic LMWH in the peripartum period for pregnant people lacks high-quality supporting data.18 Guidelines consider competing risks and benefits when making recommendations on the timing of peripartum regional analgesia.2,19 These guidelines suggest that regional analgesia should be avoided unless LMWH has been discontinued at least 24 hours before delivery (assuming normal renal function and including risk assessment at extremes of body weight). ESC guidelines recommend that “LMWH should not be given for at least 4 hours after removal of the epidural catheter; the decision on timing and dose should consider whether the epidural insertion was traumatic and take into account the risk profile of the (pregnant person).”2 For example, if a shorter time interval between the removal of the epidural catheter and commencement of the first LMWH is selected following this risk assessment, the first dose could initially be a prophylactic one. Indeed, UK guidelines20 make the following suggestion: “A thromboprophylactic dose of LMWH . . . should be given 4 hours postoperatively (at least 4 hours after removal of the epidural catheter, if appropriate) and the treatment dose recommenced 8 to 12 hours later.”1 Importantly, LMWH can be given to breastfeeding people.
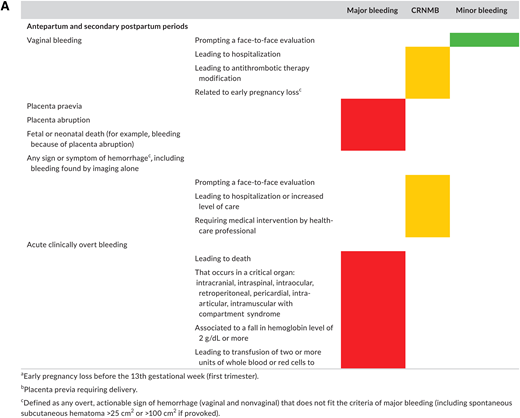
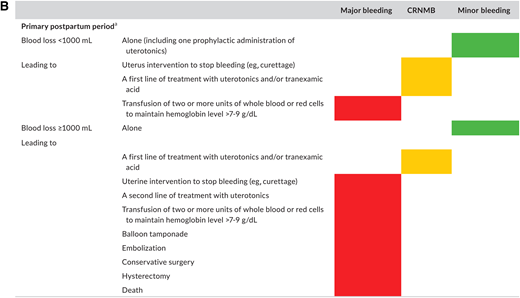
Postpartum hemorrhage (PPH) is also a leading cause of maternal death.21 As we manage acute VTE during pregnancy with anticoagulation, the potential increased bleeding risk is therefore highly relevant.2,22 A recent systematic review sought to characterize the risk of bleeding in pregnant people managed with therapeutic LMWH.23 The authors noted variability in bleeding definitions used in individual studies. Because of this limitation, only a descriptive report of outcomes was possible. The authors reported major bleeding in 2.9%-5.0% and PPH risk of 12%-30% in people receiving therapeutic anticoagulation. Importantly, the authors highlighted the lack of high-quality data despite this critical fact: both VTE and bleeding are global health priorities that kill thousands of pregnant people every year.21,24 In a 2019 systematic review only 34% of pregnant people included in an LMWH trial had bleeding events prospectively recorded using a standardized definition.25 Arising from this unmet clinical need, a new classification of bleeding during and after pregnancy for use in clinical trials has been proposed by the Scientific and Standardization Subcommittee on Control of Anticoagulation of the International Society on Thrombosis and Haemostasis (ISTH)25 (Figure 4).
Proposed definition of bleeding events in studies evaluating antithrombotic therapy in pregnant (individuals) from ISTH Scientific and Standardization Subcommittee on Control of Anticoagulation (reproduced from45 with permission from Elsevier. License no. 5518820689792; License date 30/03/2023. Colors correspond to the criteria selected for each class of bleeding: red for major bleeding, orange for clinically relevant nonmajor bleeding, and green for minor bleeding, respectively. (A) Proposed classification for antepartum and secondary postpartum (24 h to 6 weeks after delivery) periods. (B) Proposed classification for primary postpartum (first 24 h of delivery) period.
Proposed definition of bleeding events in studies evaluating antithrombotic therapy in pregnant (individuals) from ISTH Scientific and Standardization Subcommittee on Control of Anticoagulation (reproduced from45 with permission from Elsevier. License no. 5518820689792; License date 30/03/2023. Colors correspond to the criteria selected for each class of bleeding: red for major bleeding, orange for clinically relevant nonmajor bleeding, and green for minor bleeding, respectively. (A) Proposed classification for antepartum and secondary postpartum (24 h to 6 weeks after delivery) periods. (B) Proposed classification for primary postpartum (first 24 h of delivery) period.
High-quality data are eagerly anticipated from ongoing cohort studies. For example, the prospective, multicenter “PREP and GO” (PRospective Evaluation of Peripartum Anticoagulation manaGement for thromboembolism; NCT05756244) study will evaluate peripartum anticoagulation management among pregnant people with VTE and its impact on patient outcomes using standardized definitions and adjudicated outcomes. The primary objective is to estimate the combined incidence of major and clinically relevant nonmajor bleeding up to 6 weeks postpartum for the most common 6 antepartum strategies (Table 2).
Anticipated common antepartum management strategies based on intention, used in the “PREP and GO” (PRospective Evaluation of Peripartum Anticoagulation manaGement for thromboembolism; NCT05756244) multicenter prospective cohort study
| Prophylactic-dose LMWH strategies . | More-than-prophylactic-dose (intermediate/therapeutic dose) LMWH strategies . |
|---|---|
| Prophylactic-dose LMWH with expectant management (held with contractions) | More-than-prophylactic-dose LMWH with expectant management (held with contractions) |
| Prophylactic-dose LMWH and IOL (held for 12 hours) | More-than-prophylactic-dose LMWH and IOL (held for 24 hours) |
| Prophylactic-dose LMWH and cesarean delivery (held for 12 hours) | More-than-prophylactic-dose LMWH and caesarean delivery (held for 24 hours) |
| Switched to prophylactic-dose UFHa | Switched to intermediate/therapeutic-dose UFH |
| Prophylactic-dose LMWH strategies . | More-than-prophylactic-dose (intermediate/therapeutic dose) LMWH strategies . |
|---|---|
| Prophylactic-dose LMWH with expectant management (held with contractions) | More-than-prophylactic-dose LMWH with expectant management (held with contractions) |
| Prophylactic-dose LMWH and IOL (held for 12 hours) | More-than-prophylactic-dose LMWH and IOL (held for 24 hours) |
| Prophylactic-dose LMWH and cesarean delivery (held for 12 hours) | More-than-prophylactic-dose LMWH and caesarean delivery (held for 24 hours) |
| Switched to prophylactic-dose UFHa | Switched to intermediate/therapeutic-dose UFH |
Typically switched between 37-38 weeks' gestation.
The primary objective is to estimate the combined incidence of major and clinically relevant nonmajor bleeding up to 6 weeks postpartum for the most common 6 antepartum strategies. Of the 8 strategies listed above, the investigators expect 6 predominant strategies. If other possible strategies are used other than those listed above (eg, continuing anticoagulation throughout labor intentionally or stopping anticoagulation early at 37-38 weeks' gestation), they will also be recorded. Prophylactic-dose LMWH: enoxaparin 40 mg daily, dalteparin 5000 IU daily, tinzaparin 4500 IU daily, nadroparin 2850 IU daily; more-than-prophylactic-dose LMWH: Anything higher in dose than what is listed above, including intermediate-dose and therapeutic-dose LMWH.
IOL, induction of labor; UFH, unfractionated heparin.
Furthermore, the ongoing Pregnancy AND Anticoagulation (PANDA) study, being conducted via the “Venous thromboEmbolism Network U.S.” (VENUS) national VTE research network is a prospective observational cohort of 250 pregnant people who require anticoagulation. The primary objective is to compare the incidence of pregnancy complications associated with anticoagulation around the time of delivery between pregnant people treated with either unfractionated heparin or LMWH around the time of delivery. The Pregnancy AND Anticoagulation study has a composite endpoint of cesarean delivery, labor induction, inability to give epidural or spinal anesthesia, postpartum hemorrhage, and venous thrombosis from 36 weeks to 6 weeks postpartum.
Back to the case; postpartum management
Aries recovered well and continued therapeutic LMWH until 6 weeks postpartum, in line with guideline and consensus statement recommendations favoring limited duration anticoagulation (no fewer than 3 months in total and usually continuing for at least 6 weeks postpartum) for a pregnancy-provoked VTE event.2,26
CLINICAL CASE (continued)
It is now January 2023, and Aries presents to the outpatient clinic, 6 weeks into their second pregnancy. They are keen to understand how they can optimally protect themselves from experiencing VTE recurrence. We discuss this and the results of the recently published landmark Highlow randomized controlled trial (RCT).27
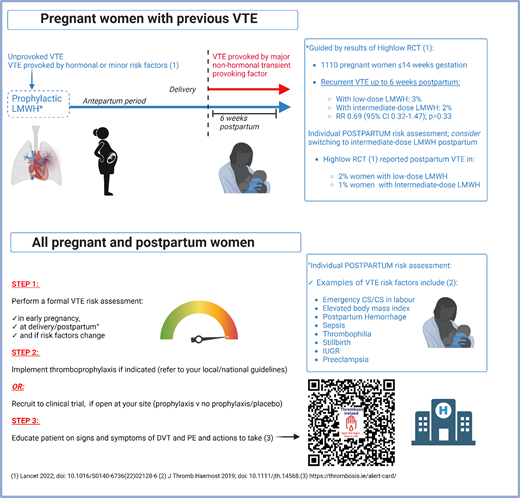
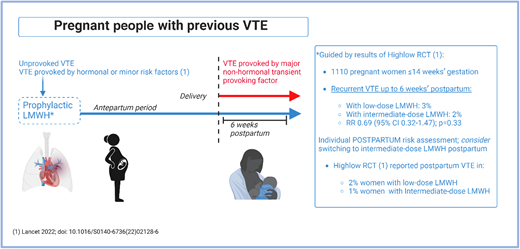
Aries is aware that people with previous VTE, particularly an unprovoked or hormone-provoked event, are at higher risk of recurrence during pregnancy than outside pregnancy.1,11,28-30 In contrast, pregnancy-associated VTE recurrence risk is lower (1.0%; 95% CI: 1.9%-5.7%) in people with a previous VTE provoked by a major nonhormonal transient risk factor.31 Consequently, there had been, prior to 2022, a consistent recommendation in international and society guidelines for pharmacologic thromboprophylaxis during pregnancy and for 6 weeks postpartum in individuals in these higher-risk categories32-34 (Figure 5). However, the optimal LMWH dose for recurrent VTE prevention was not known.
How we approach VTE prevention in pregnant people with a prior VTE history. “Unprovoked VTE; VTE provoked by hormonal or minor risk factors” is an abbreviated reference to those patients who should receive both antepartum and postpartum LMWH. This group is described in the inclusion criteria for the Highlow study as “Patients with previous objectively confirmed VTE, either unprovoked, in the presence of use of oral contraceptives or estrogen/progestagen use, or related to pregnancy or the postpartum period, or minor risk factors (e.g., long distance travel, minor trauma).”27
How we approach VTE prevention in pregnant people with a prior VTE history. “Unprovoked VTE; VTE provoked by hormonal or minor risk factors” is an abbreviated reference to those patients who should receive both antepartum and postpartum LMWH. This group is described in the inclusion criteria for the Highlow study as “Patients with previous objectively confirmed VTE, either unprovoked, in the presence of use of oral contraceptives or estrogen/progestagen use, or related to pregnancy or the postpartum period, or minor risk factors (e.g., long distance travel, minor trauma).”27
This situation was rectified by the publication in late 2022 of the multicenter, multinational academic Highlow RCT.27 This RCT recruited 1110 pregnant individuals aged ≥18 years and ≤14 weeks' gestation who had experienced prior objectively confirmed VTE that was either unprovoked or provoked by a hormonal (or pregnancy-related) risk factor; 70 hospitals from 9 countries participated. Individuals were randomized to either weight-adjusted intermediate-dose or fixed low-dose LMWH. There was no significant difference between the 2 groups in the primary efficacy outcome (recurrent, objectively confirmed, centrally adjudicated VTE up to 6 weeks postpartum), which occurred in 3% and 2% in the low- and intermediate-dose groups, respectively (relative risk [RR] 0.69 [95% CI 0.32-1.47]; P = 0.33). The primary safety outcome (major bleeding) occurred in 4% of each of the intermediate-dose and low-dose groups (RR 1.16 [95% CI 0.65-2.09]), demonstrating that low-dose LMWH is the appropriate dose for prevention of pregnancy-related recurrent VTE. Interestingly, postpartum VTE recurrence occurred more frequently in people receiving low-dose than intermediate-dose LMWH (2% and 1%, respectively). Although it is important to point out that this was a post hoc analysis, for which the study was not powered, it suggests a potentially interesting hypothesis that an intermediate postpartum LMWH dose could result in reduced VTE rates in the postpartum period. However, to definitively answer this question, an adequately powered study for this outcome is required.
Primary VTE prevention
We know that prior VTE is an important risk factor for VTE during pregnancy and postpartum (and that people with prior VTE should receive postpartum pharmacologic thromboprophylaxis [Figure 5]), but that this risk factor is identified in only 1%-2% of pregnant individuals.35 How should VTE prevention be optimized postpartum in individuals with other, more commonly occurring risk factors and combinations of these risk factors?
Identifying people at increased risk of developing postpartum VTE may allow for targeted intervention.32 In postpartum individuals with high VTE risk, the benefits of thromboprophylaxis may outweigh the risks.1 VTE risk factors are common: in an Irish study including 21,019 postpartum VTE risk assessments,36 we reported that 78% of pregnant people had at least one VTE risk factor and that one-fifth of people developed new VTE risk factors in the peripartum period that would not have been identified antenatally,35 highlighting the crucial importance of VTE risk assessment not only during pregnancy but also postpartum.
This question remains one of the most urgent knowledge gaps in obstetric and VTE practice internationally. Despite its importance, there is a striking lack of data to guide either antepartum and particularly postpartum thromboprophylaxis, at a time when VTE risk is highest and when risk assessment can be challenging. International guideline recommendations vary widely and are based on expert consensus because there are insufficient data to make evidence-based recommendations.1,37 A major issue has been that the use of LMWH injections limits the feasibility of a large RCT, as seen in the experience of the pilot PROSPER trial (LMWH vs placebo among postpartum people with VTE risk factors). Among eligible people refusing consent, 27.2% were uncomfortable with LMWH injections.38
However, there is hope on the horizon (Table 3). Aspirin has shown promise in VTE prevention in nonobstetric populations, notably following hip or knee arthroplasty.39,40 Although these data cannot, at this time, be extrapolated to VTE prevention in pregnancy and postpartum, the use of an oral drug could hypothetically improve patient acceptance of a postpartum trial intervention. Low-dose aspirin (ASA) is considered safe during breastfeeding.32
Ongoing or recently completed (since 01/2023) interventional postpartum pilot RCTs addressing (principally) primary VTE prevention in people with combinations of VTE risk factors in the postpartum period
| Trial . | Pilot PARTUM (NCT04153760) . | PP-HEP (NCT05878899) . | LEAP (NCT05058924) . |
|---|---|---|---|
| Status | Ongoing, recruiting | Closed (March 2023) | Ongoing, recruiting |
| Sponsor | University of Calgary | University Hospital Geneva | Mount Sinai Hospital, Canada |
| Study design | Multicenter, multinational, placebo-controlled, double-blind pilot RCT | Single-center pilot open-label RCT | Single-center pilot open-label RCT |
| Intervention | ASA (81 mg once daily) vs placebo once daily for 6 weeks | Enoxaparin 20-60 mg once daily (according to body weight) for 10 days vs no treatment | 3 weeks of prophylactic LMWHa followed by 3 weeks of ASA (81 mg once daily) vs prophylactic LMWHa for 6 weeks |
| Inclusion criteria (summarized)a | ONE (or more) First Order Criterion: 1. Known inherited thrombophilia prior to enrollment 2. Antepartum immobilization (strict bedrest) for ≥7 days. OR TWO (or more) Second Order Criteria: 1. Prepregnancy BMI ≥30 kg/m2 2. Smoking ≥5 cigarettes/day prepregnancy 3. Previous clinical history of superficial vein thrombosis 4. Pre-eclampsia 5. Current pregnancy ending in stillbirth (>20/40) 6. Emergency cesarean birth 7. Small-for-gestational-age infant at time of delivery 8. Postpartum infection 9. Postpartum hemorrhage (>1000 mL) | Postpartum women within 48 h of delivery, with at least ONE of: 1. Emergency cesarean section 2. Prepregnancy BMI ≥35 kg/m2 3. Known low-risk thrombophilia 4. Preeclampsia 5. Preterm delivery 6. Peripartum systemic infection 7. Intrauterine growth restriction AND/OR at least 2 of: 1. Age ≥35 years 2. Pre-pregnancy BMI 30.0-34.9 kg/m2 3. Current smoking 4. Elective cesarean section 5. Postpartum hemorrhage 6. Antenatal immobility | >18 years of age AND: 1. Personal history of unprovoked VTE prior to pregnancy or hormone associated VTE and not prescribed therapeutic anticoagulation. OR 2. Family history (first-degree relative) of VTE and antithrombin deficiency, protein C or protein S deficiency OR 3. Combined thrombophilia or homozygous for the factor V Leiden mutation or prothrombin gene mutation, and family history of VTE (first-degree relative) |
| Exclusion criteria (summarized)a | 1. >48 hours since delivery of the placenta at randomization. 2. Received >2 doses of LMWH since delivery of the placenta 3. Need for postpartum LMWH. prophylaxis/systemic anticoagulationb 4. Need for postpartum ASAb 5. Contraindication to ASAa 6. <18 years of age 7. Unable or refused consent | 1. Indication for therapeutic anticoagulation 2. High risk of postpartum VTE 3. Increased bleeding risk 4. Contraindication to heparin 5. Age <18 years | 1. Preexisting indication for therapeutic LMWH 2. Contraindication to ASAa 3. Contraindication to LMWHa 4. Active bleeding, excluding physiologic vaginal bleeding 5. Bleeding disorders 6. Known severe hypertension |
| Pilot trial primary objective | Mean recruitment rate per center per month, calculated over 6 months | Recruitment rate (number of study inclusions per month over 6 months) and proportion of participationa | Enrollment rate, consent rate, adherence to prescription, withdrawal of consent rate, rates of contaminationa |
| Target sample size | 384 | 100-200 | 50 |
| Trial . | Pilot PARTUM (NCT04153760) . | PP-HEP (NCT05878899) . | LEAP (NCT05058924) . |
|---|---|---|---|
| Status | Ongoing, recruiting | Closed (March 2023) | Ongoing, recruiting |
| Sponsor | University of Calgary | University Hospital Geneva | Mount Sinai Hospital, Canada |
| Study design | Multicenter, multinational, placebo-controlled, double-blind pilot RCT | Single-center pilot open-label RCT | Single-center pilot open-label RCT |
| Intervention | ASA (81 mg once daily) vs placebo once daily for 6 weeks | Enoxaparin 20-60 mg once daily (according to body weight) for 10 days vs no treatment | 3 weeks of prophylactic LMWHa followed by 3 weeks of ASA (81 mg once daily) vs prophylactic LMWHa for 6 weeks |
| Inclusion criteria (summarized)a | ONE (or more) First Order Criterion: 1. Known inherited thrombophilia prior to enrollment 2. Antepartum immobilization (strict bedrest) for ≥7 days. OR TWO (or more) Second Order Criteria: 1. Prepregnancy BMI ≥30 kg/m2 2. Smoking ≥5 cigarettes/day prepregnancy 3. Previous clinical history of superficial vein thrombosis 4. Pre-eclampsia 5. Current pregnancy ending in stillbirth (>20/40) 6. Emergency cesarean birth 7. Small-for-gestational-age infant at time of delivery 8. Postpartum infection 9. Postpartum hemorrhage (>1000 mL) | Postpartum women within 48 h of delivery, with at least ONE of: 1. Emergency cesarean section 2. Prepregnancy BMI ≥35 kg/m2 3. Known low-risk thrombophilia 4. Preeclampsia 5. Preterm delivery 6. Peripartum systemic infection 7. Intrauterine growth restriction AND/OR at least 2 of: 1. Age ≥35 years 2. Pre-pregnancy BMI 30.0-34.9 kg/m2 3. Current smoking 4. Elective cesarean section 5. Postpartum hemorrhage 6. Antenatal immobility | >18 years of age AND: 1. Personal history of unprovoked VTE prior to pregnancy or hormone associated VTE and not prescribed therapeutic anticoagulation. OR 2. Family history (first-degree relative) of VTE and antithrombin deficiency, protein C or protein S deficiency OR 3. Combined thrombophilia or homozygous for the factor V Leiden mutation or prothrombin gene mutation, and family history of VTE (first-degree relative) |
| Exclusion criteria (summarized)a | 1. >48 hours since delivery of the placenta at randomization. 2. Received >2 doses of LMWH since delivery of the placenta 3. Need for postpartum LMWH. prophylaxis/systemic anticoagulationb 4. Need for postpartum ASAb 5. Contraindication to ASAa 6. <18 years of age 7. Unable or refused consent | 1. Indication for therapeutic anticoagulation 2. High risk of postpartum VTE 3. Increased bleeding risk 4. Contraindication to heparin 5. Age <18 years | 1. Preexisting indication for therapeutic LMWH 2. Contraindication to ASAa 3. Contraindication to LMWHa 4. Active bleeding, excluding physiologic vaginal bleeding 5. Bleeding disorders 6. Known severe hypertension |
| Pilot trial primary objective | Mean recruitment rate per center per month, calculated over 6 months | Recruitment rate (number of study inclusions per month over 6 months) and proportion of participationa | Enrollment rate, consent rate, adherence to prescription, withdrawal of consent rate, rates of contaminationa |
| Target sample size | 384 | 100-200 | 50 |
Full criteria are available for the relevant trials on clinicaltrials.gov.
As judged by physician and/or local investigator.
ASA, aspirin; × /40, × weeks' gestational age.
The pilot PARTUM multicenter, randomized, double-blind, placebo-controlled trial (ClinicalTrials.gov Identifier: NCT041 53760), now nearly complete, randomizes eligible individuals at elevated VTE risk to low-dose oral aspirin or placebo daily for 6 weeks. The primary outcome of this pilot trial is to determine the feasibility of a full multicenter RCT by determining the mean recruitment rate per center per month, calculated over 6 months.
The single-center “PP-HEP” pilot trial (“Preventing postpartum venous thromboembolism with low-molecular-weight heparin: a feasibility randomized controlled trial”) in Geneva (NCT05878899) has also recently shown that approximately 1 in 4 people deemed to be at intermediate risk of VTE were willing to participate in a pragmatic, open-label trial of a 10-day postpartum LMWH course. Data from this pilot trial were presented at the International Society on Thrombosis and Haemostasis congress 2023. One hundred twenty-two participants were randomized to enoxaparin 40-60 mg per day for 10 days or no treatment. The overall recruitment rate was 12.8 per month,41 providing further evidence that recruitment of people to postpartum LMWH trials is possible, even if projected VTE rates are low, and that regional or country-specific variations in recruitment rates may be important.
Furthermore, an ongoing pilot single-center RCT presented at the American Society of Hematology Meeting 2022 (NCT05058924)42 randomized postpartum individuals deemed to be at elevated VTE risk to either prophylactic LMWH for 3 weeks followed by low-dose aspirin for the following 3 weeks (treatment A) or standard-care prophylactic-intensity LMWH for 6 weeks (treatment B). Recruitment and adherence appear promising, with an enrollment rate reported at American Society of Hematology of 69.2% (18/26) and treatment adherence rates of 98.2% and 94.1% in groups A and B. At 6 weeks quality- of-life scores (measured by the Duke Anticoagulation Satisfaction Scale) improved by 33.3% in group A compared with group B (P = 0.01).
There is a similar dearth of RCT evidence guiding optimal antepartum primary VTE prevention. No VTE risk assessment model has been sufficiently validated. However, the research question has been prioritized. A multicenter study performed by the French STRATHEGE investigators compared VTE and placental vascular complication rates pre- and postimplementation of a risk scoring system (including but not limited to prior VTE events), which was used to determine thromboprophylaxis strategies in 2085 people.43 Vascular events were reported in 190 (19.2%) people before and 140 (13%) after implementation of risk score-driven prophylaxis (RR 0.68 [95% CI 0.55; 0.83]) and the risk of pregnancy-associated VTE was reduced following implementation (RR 0.47 [95% CI 0.27; 0.81]). PPH occurred in 3.2% of people before and 4.5% after implementation (RR 1.38 [95% CI 0.89; 2.13], P = 0.15).
CLINICAL CASE (continued)
Aries was struck by the importance of conducting high-quality studies in pregnancy to improve the care delivered to pregnant people. We had discussed with them the challenges faced by clinicians and patients: despite the very high stakes, pregnant people are often excluded from participation in clinical trials.44
International networks and collaboration are central to the success of RCTs addressing VTE in pregnant people, who have traditionally been excluded. Both the Highlow and PARTUM trials have been endorsed by the International Network of Venous Thromboembolism Clinical Networks (www.invent-vte.com). Participating National VTE networks include CanVECTOR (Canada), INNOVTE (France), INViTE (Ireland), Dutch Thrombosis Network (Netherlands), Center for Thrombosis and Hemostasis (Germany), TRIP (Italy), Norwegian Thrombosis Network, THANZ (Australia and New Zealand), VENUS (United States), CURES (China), and UK-TReN (United Kingdom).
Concluding remarks
Aries elected to commence prophylactic LMWH throughout their pregnancy and chose to increase their dose to an intermediate intensity postpartum, having discussed the remaining data limitations. Their journey demonstrates the crucial importance of prioritization of high-quality RCTs and prospective clinical management studies for the prevention, diagnosis, and management of VTE in pregnant people.
Acknowledgments
The authors would like to thank the patients who contributed to the clinical trials and studies described herein, the principal investigators, and all collaborators.
Conflict-of-interest disclosure
Barry Kevane reports honoraria unrelated to this project from Bayer (advisory board membership) and Leo Pharma.
Fionnuala Ní Áinle reports grants for investigator-initiated studies paid to her institution and unrelated to this project from Sanofi, Daiichi Sankyo, and Bayer and acting as a consultant (membership of a trial executive committee, paid to institution) for Boston Scientific.
Off-label drug use
Barry Kevane: Nothing to disclose.
Fionnuala Ní Áinle: Nothing to disclose.