Abstract
Bleeding disorders, including von Willebrand disease (VWD), hemophilia, other coagulation factor deficiencies, platelet disorders, defects of fibrinolysis, and connective tissue disorders, have both maternal and fetal implications. Successful management of bleeding disorders in pregnant women requires not only an understanding of bleeding disorders but also an understanding of when and how bleeding occurs in pregnancy. Bleeding does not occur during a normal pregnancy with a healthy placenta. Bleeding occurs during pregnancy when there is an interruption of the normal utero-placental interface, during miscarriage, during an ectopic pregnancy, or at the time of placental separation at the conclusion of pregnancy. Although mild platelet defects may be more prevalent, the most commonly diagnosed bleeding disorder among women is VWD. Other bleeding disorders are less common, but hemophilia carriers are unique in that they are at risk of bleeding themselves and of giving birth to an affected male infant. General guidance for maternal management of a woman who is moderately or severely affected includes obtaining coagulation factor levels at a minimum in the third trimester; planning for delivery at a center with hemostasis expertise; and anticipating the need for hemostatic agents. General guidance for fetal management includes pre-pregnancy counseling; the option of preimplantation genetic testing for hemophilia; delivery at a tertiary care center with pediatric hematology and newborn intensive care; consideration of cesarean delivery of a potentially severely affected infant; and avoidance of invasive procedures such as scalp electrodes and operative vaginal delivery in any potentially affected infant.
Learning Objectives
Understand when and how bleeding occurs in pregnancy
Be able to provide general guidance for the maternal and fetal management of a woman with a bleeding disorder during pregnancy
Bleeding disorders
Bleeding disorders that the hematologist may encounter during pregnancy include von Willebrand disease (VWD), hemophilia carrier status with factor VIII or factor IX deficiency, thrombocytopenia, platelet dysfunction, defects of fibrinolysis, connective tissue disorders, and other coagulation factor deficiencies. The most commonly encountered inherited bleeding disorder during pregnancy, besides, perhaps, a mild undiagnosed platelet disorder, is VWD. Based on enrollment of symptomatic patients in hemostasis centers1 and a Danish national registry,2 the prevalence of VWD is only 1 in 10 000, and based on a population study of childbearing-age women, the prevalence of VWD is 1 in 4000.3 In a study of patients with a history of bleeding or bruising, however, the prevalence is 1 in 1000,4 and based on 2 population studies of personal bleeding symptoms, low von Willebrand factor levels, and family history of VWD, the prevalence of VWD is approximately 1%,5,6 suggesting that the hematologist will encounter undiagnosed cases as well as diagnosed ones. As for the prevalence of other inherited bleeding disorders, the prevalence of hemophilia carrier status is approximately 1 in 3000,7 and the prevalence of rare, severe inherited bleeding disorders is 1 in 500 000 to 2 million.8 Although the range of bleeding disorders seen in specialized treatment centers may be skewed to more severe cases and therefore may not represent what would be encountered by the practicing hematologist, surveillance of females in the Centers for Disease Control and Prevention's network of hemophilia treatment centers from 2012 to 2021 revealed that approximately 62% of enrollees had VWD, 7% were carriers of hemophilia with factor VIII (FVIII) or factor IX (FIX) deficiency, 11% had various other coagulation factor deficiencies, 19% had platelet disorders, and 2% had connective tissue disorders or disorders of fibrinolysis.9 Although this review focuses primarily on inherited bleeding disorders, general principles in the management of bleeding disorders in pregnancy often apply to both inherited and acquired conditions.
Bleeding in pregnancy
Successful management of bleeding disorders in pregnant women requires not only an understanding of bleeding disorders but also an understanding of when and how bleeding occurs in pregnancy. Bleeding does not occur during a normal pregnancy with a healthy placenta. Bleeding occurs during pregnancy when there is an interruption of the normal utero-placental interface, during miscarriage, during an ectopic pregnancy, or at the time of placental separation at the conclusion of pregnancy, whenever that is. Almost all bleeding that occurs during pregnancy or after delivery arises from the small blood vessels within the uterus and can be referred to as obstetric bleeding. Obstetric bleeding is controlled by emptying the uterus and promoting uterine contraction. Most of the rest of the bleeding that occurs during pregnancy or after delivery arises from incisions, lacerations, ruptured vessels, or ruptured viscus, including the bleeding that accompanies birth trauma, cesarean delivery, or a ruptured ectopic pregnancy and can be referred to as surgical bleeding. Surgical bleeding is controlled by ligatures and sometimes embolization. Less than 1% of bleeding that occurs during pregnancy or after delivery is related to a coagulation defect.10
Identifying the pregnant woman at risk of bleeding
Every pregnant woman is at risk of bleeding during pregnancy and at the time of delivery. Almost always bleeding is due to obstetrical or surgical factors, but without a normal number of platelets, normal platelet function, normal levels of VWF, normal levels of coagulation factors, normal fibrinolytic activity, and normal collagen, a woman may be at risk for excessive bleeding. A patient may be referred to the hematologist for care with a known bleeding disorder or may be referred for evaluation of a possible bleeding disorder. A personal history that suggests an underlying bleeding disorder is well understood and includes:11
Heavy menstrual bleeding especially since menarche
Iron deficiency anemia requiring treatment or transfusion
Prolonged bleeding from trivial cuts
Nose bleeds
Notable bruising without injury
Bleeding from the oral cavity or gastrointestinal tract without an anatomic lesion
Prolonged or excessive bleeding following dental extractions and/or procedures
Unexpected bleeding during or following surgery
Hemorrhage that required blood transfusion
It also includes other reproductive tract bleeding that may have been overlooked, such as:
Hemorrhagic ovarian cysts
Excessive bleeding at the time of miscarriage
Delayed postpartum hemorrhage (PPH), occurring 24 hours or more after delivery
Immediate PPH, occurring less than 24 hours after delivery, is rarely associated with an underlying bleeding disorder. The coagulopathy that accompanies PPH is usually acquired and acute resulting from massive hemorrhage due to obstetrical or surgical factors.12 Patients may be referred to the hematologist for evaluation of a possible bleeding disorder after a massive PPH, but when an underlying bleeding disorder is discovered, the patient is likely to have had other signs or symptoms of a bleeding disorder preceding the pregnancy.13 So while patients with an underlying bleeding disorder are at risk of excessive bleeding during pregnancy or after delivery, isolated PPH, which accompanies 3% of births,14 rarely indicates an underlying bleeding disorder.
Diagnosis of an underlying bleeding disorder during pregnancy is complicated by the fact that pregnancy is a hypercoagulable state. Not every aspect of this hypercoagulable state is understood, but during pregnancy and the early postpartum period, there in an increase in VWF,15,16 FVIII,15,16 fibrinogen,17,18 and certain other coagulation factors,18 a decrease in the natural anticoagulant protein S,18 a decrease in fibrinolytic activity,18 and a decrease in platelet number,19 but an increase in mean platelet volume20 and platelet reactivity.21 Because of this hypercoagulable state, the final diagnosis of an underlying bleeding disorder should be made during baseline health conditions and not during pregnancy.22
Von Willebrand disease (VWD)
VWD, deficiency of normal von Willebrand factor (VWF), is characterized by mucocutaneous bleeding (nose bleeds, heavy menstrual bleeding, or easy bruising) as opposed to deep tissue bleeding (muscle or joint bleeds), although the latter may be seen in patients with VWD, particularly those with more severe disease. While women and men are equally likely to inherit VWD, women are disproportionately affected due to the bleeding challenges of menstruation, miscarriage, and childbirth and, as noted by Erik von Willebrand in his original paper,23 are twice as likely to be diagnosed with the disease. Type 1 VWD, which is usually mild to moderate in severity, accounts for 80% of diagnosed VWD and is due to a reduced level of normal VWF (quantitative deficiency) with VWF antigen and VWF activity levels proportionally decreased. The threshold for diagnosis according to the latest American Society of Hematology (ASH), International Society on Thrombosis and Haemostasis (ISTH), National Hemophilia Foundation (NHF), and World Federation of Hemophilia (WFH) guidelines on the diagnosis of von Willebrand disease22 is a VWF level ≤0.30 IU/mL regardless of bleeding history, and for patients with abnormal bleeding (or during pregnancy, we would add), a VWF level ≤0.50 IU/mL. Inheritance is autosomal dominant. Type 2 VWD, which is usually moderate in severity, accounts for most of the rest of diagnosed VWD cases and is due to a low level of functional VWF manifest by a reduced ratio of VWF activity relative to VWF antigen of less than 0.7.22 Type 2 VWD is further subdivided into 4 different subclasses depending on the mechanism leading to the qualitative dysfunction. Inheritance is usually autosomal dominant. Type 3 VWD, which is severe and extremely rare, affects approximately 1 in 1 000 000 persons and is due to undetectable levels of VWF with very low levels of FVIII.22 Inheritance is autosomal recessive or codominant (as occurs in compound heterozygotes).24
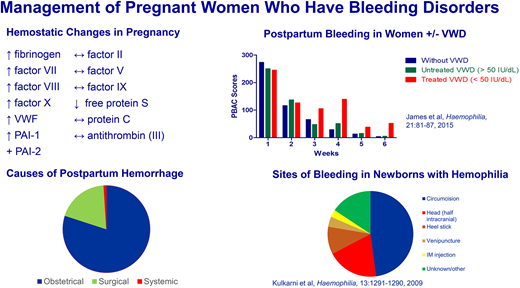
While VWF and FVIII levels rise during pregnancy, in women with VWD, median levels are lower than levels in women without VWD and fall rapidly after delivery, approaching baseline by 1 week postpartum and reaching baseline by 3 weeks postpartum.15 Overall, the risk of bleeding during pregnancy (antepartum bleeding, immediate PPH, severe PPH, perineal hematoma, and delayed PPH) is increased by 2- to 10-fold in women with VWD.3,25 In a systematic review of 87 studies (71 case reports or case series and 16 cohort studies) of maternal bleeding complications in 811 deliveries in women with VWD, the primary PPH rate was 32% and the secondary PPH rate was 13% among the cohort studies, but only 3 of the studies were prospective and only 1 included a comparison group of unaffected women.26 In this latter prospective study of women whose levels were at least 50 precent of normal or higher (≥0.5 IU/mL) at 36 weeks gestation, the risk of PPH was not significantly increased compared with unaffected women.15
Hemophilia
Hemophilia A is due to deficiency of FVIII and hemophilia B to deficiency of FIX. Hemophilia is characterized classically by deep tissue (muscle and joint) bleeding, although an increased risk of mucosal bleeding can also be seen. The inheritance of hemophilia is X-linked. Males with hemophilia have an abnormal gene for FVIII or FIX on their single X chromosome. The prevalence among males is about 1 in 5000. Females with an abnormal gene for FVIII or FIX on 1 of their 2 X chromosomes are considered carriers. Table 1 summarizes obligate versus possible carrier status in hemophilia. In a study of the pedigrees of families with hemophilia, there were 156 female carriers for every 100 males with hemophilia.7 Female carriers are very rarely affected with severe hemophilia, but it is possible in the case of monosomy X (the presence of a single X chromosome), homozygosity or compound heterozygosity for two abnormal X chromosomes, or nonrandom X inactivation (the most common of these 3 mechanisms). Women who are heterozygous for an abnormal gene for FVIII or FIX on 1 of their 2 X chromosomes and have factor levels below the hemostatic range also meet the criteria for hemophilia.27 Approximately 30% of carriers have levels in the hemophilia range.23 The ISTH recently published a new nomenclature for hemophilia carriers based on factor levels and symptoms. For women and girls with FVIII or FIX >0.05 and <0.40 IU/mL, the classification would be mild; with FVIII or FIX 0.01-0.05 IU/mL, the classification would be moderate; and with FVIII or FIX <0.01 IU/mL, the classification would be severe hemophilia. If FVIII or FIX were ≥0.40 IU/mL, the classification would be symptomatic (with a bleeding phenotype) or asymptomatic (without a bleeding phenotype).28
Obligate versus possible carrier status in hemophilia
| . | Obligate carrier . | Possible carrier . |
|---|---|---|
| Mother | • Of a son with hemophilia if there is another affected male relative • Of more than 1 son with hemophilia | Of only 1 son with hemophilia |
| Sister | • Of a male with hemophilia • Of a female carrier | |
| Daughter | Of a man with hemophilia | Of a female carrier |
| Other relation | • Of a male with hemophilia • Of a female carrier |
| . | Obligate carrier . | Possible carrier . |
|---|---|---|
| Mother | • Of a son with hemophilia if there is another affected male relative • Of more than 1 son with hemophilia | Of only 1 son with hemophilia |
| Sister | • Of a male with hemophilia • Of a female carrier | |
| Daughter | Of a man with hemophilia | Of a female carrier |
| Other relation | • Of a male with hemophilia • Of a female carrier |
The risk of PPH in carriers is hard to estimate, but in case series and in 1 cohort study, the rate of PPH ranged from 13 to 22%.29 Most of these reports were retrospective or based on patient recall, which is inherently inaccurate, but the suggestion is that the risk of PPH is increased.29 In a systematic review of 17 case reports or case series and 11 cohort studies of maternal bleeding complications in 502 deliveries in hemophilia carriers, there was a PPH rate of 20% among the cohort studies.30
Unlike for patients with VWD, in whom genetic testing is less commonly performed and results will generally not affect the management of pregnancy or delivery, in carriers or potential carriers of hemophilia, identifying the underlying genetic variant prior to pregnancy is very valuable.29 Once a likely causative variant is identified, carriers of hemophilia have the option of undergoing in vitro fertilization for preimplantation genetic testing of embryos; or during pregnancy of undergoing prenatal diagnosis of hemophilia by chorionic villous sampling (placental biopsy performed during the first trimester of pregnancy) or amniocentesis (amniotic fluid sampling performed during the second or third trimester of pregnancy). Regardless of previous genetic testing of a carrier or potential carrier of hemophilia, determination of the fetal sex should be performed by either ultrasound, serum cell free DNA, or invasive genetic testing (chorionic villus sampling or amniocentesis). If the fetus is female, there is a 50% chance she will be a carrier. If the fetus is male, there is a 50% chance he will have hemophilia.
Other inherited coagulation factor deficiencies
Factor XI (FXI) deficiency, while not as common as hemophilia A or B, is more common than the other inherited coagulation factor deficiencies. It is also more common among persons of Ashkenazi Jewish ancestry where heterozygosity approaches 1 in 11 individuals and homozygosity or compound heterozygosity approaches 1 in 450 individuals.31 FXI is part of the intrinsic pathway and the kallikrein-kinin system. FXI deficiency is characterized by variable mucocutaneous bleeding that does not always correlate with the FXI level. Inheritance of FXI deficiency is autosomal dominant with severe forms following a recessive or compound heterozygous pattern. Heterozygotes typically have an FXI activity level of 20% to 60%.31 Severe FXI deficiency is defined as an FXI activity level of less than 20%.31 In a retrospective study of obstetric and perioperative management of patients with FXI deficiency, FXI levels did not change among 81 women whose levels were measured at least twice during pregnancy at different time points.32 While the risk of bleeding in FXI deficiency is variable, the rate of PPH appears to be increased. The rate of PPH was found to be 11% in a cohort that included 143 vaginal and 63 cesarean deliveries,32 and 18% in a systematic review of 498 deliveries.33
Factor VII (FVII) deficiency is considered the most common autosomal recessive coagulation factor deficiency. Like FXI deficiency, FVII deficiency is also characterized by variable mucocutaneous bleeding that does not always correlate with the FVII level. The ISTH classifies FVII deficiency as severe (FVII <10% with greatest risk for major spontaneous bleeding); moderate (FVII 10%-20% with risk for mild spontaneous or provoked bleeding); and mild (FVII 20%-50% with mild or no bleeding).34 Patients with severe FVII deficiency, which has a prevalence of 1 in 500 000, are usually homozygotes or compound heterozygotes.
Congenital fibrinogen deficiency with either a reduction in the quantity of fibrinogen (afibrinogenemia, hypofibrinogenemia) or the quality of fibrinogen (dysfibrinogenemia) is very rare, affecting less than 1 in 1 000 000 individuals.35 There is a strong correlation between fibrinogen levels and maternal bleeding and, unlike other coagulation factor deficiencies, a strong correlation between fibrinogen levels and bleeding at the utero-placental interface. Patients with afibrinogenemia experience spontaneous bleeds into muscles and joints and are at significant risk of intracranial hemorrhage. Patients with hypofibrinogenemia are usually asymptomatic but are vulnerable to bleeding after trauma. Patients with dysfibrinogenemia can have both spontaneous bleeding and spontaneous thromboses.35 Levels that might not result in systemic bleeding, however, can still result in bleeding at the utero-placental interface, resulting in miscarriage or placental abruption as well as PPH.35
Factor XIII (FXIII) deficiency is very rare, affecting 1 to 2 per million individuals with the prevalence varying by country and the frequency of consanguinity. In 2 different European registries, FXIII deficiency accounted for 6%-7% of patients with rare bleeding disorders.36,37 Severe FXIII deficiency (FXIII <10%) is associated with provoked bleeding, delayed wound healing, and repeated miscarriages.37 In a systematic review of the literature, the rate of miscarriage was 66% and the rate of PPH 25%.38 Successful pregnancies have been achieved with FXIII replacement.37
Platelet disorders
Platelet disorders are disorders of platelet number and function and range in severity from mild to very severe. Severe platelet disorders include Bernard-Soulier syndrome, a deficiency of the platelet glycoprotein receptor Ib/IX, and Glanzmann's thrombasthenia, a deficiency of the platelet glycoprotein receptor IIb/IIIa. Patients with both Glanzmann's and Bernard-Soulier are at high risk for PPH,39,40 but they can also make alloantibodies to various platelet antigens, further complicating pregnancies with fetal/neonatal alloimmunization. In a review of patients with Glanzmann's from a single center, 3 of 9 neonates had severe thrombocytopenia, all of whom were born to mothers who were positive for antiplatelet antibodies,41 and in a systematic review of patients with Bernard-Soulier, 6 neonates from 30 pregnancies were diagnosed with neonatal alloimmune thrombocytopenia, all of whom were born to mothers who were positive for antiplatelet antibodies.40 Prevention of fetal/neonatal alloimmunization involves the use of antepartum intravenous immunoglobulin with or without the addition of steroids.42
Management of pregnant women with any bleeding disorder
There is some general guidance that applies to the management of pregnant women with any bleeding disorder. Women should be informed about what they can expect during pregnancy and childbirth, including the risk of increased bleeding complications at delivery and the chance that their infant will be affected. Ideally women will have been diagnosed before pregnancy, have had the opportunity for molecular testing if that is available, and have had the opportunity for preconception counseling with their hematologist and obstetric provider. When appropriate, a woman and her partner should be referred for genetic counseling. During pregnancy, at a minimum, women should have their coagulation factor levels checked at registration for prenatal care and again at 36 weeks' gestation. Additional levels in the second and early third trimester are of value in managing any bleeding during pregnancy and in anticipation of an early delivery. Women should refrain from taking low-dose aspirin for preeclampsia prevention. Women with a moderate or severe bleeding disorder, or those at risk of having a severely affected infant, should deliver in a tertiary care center with the requisite specialists and services—hemostasis expertise, maternal-fetal medicine, anesthesia, laboratory, pharmacy, blood bank, pediatric hematology, and newborn intensive care. For hospitals that have the infrastructure, formal multidisciplinary team review of cases can be helpful. Women with a moderate or severe bleeding disorder should have the opportunity to meet with a member of the anesthesia team prior to delivery to establish the option of neuraxial anesthesia or plan for an alternative. Those expecting a potentially severely affected infant should have the opportunity to meet with pediatric hematology prior to delivery. Anticipation of a mildly or moderately affected infant is not an indication for cesarean delivery, but while there is a risk of nonsevere fetal bleeding (as in the case of a woman with VWD or a hemophilia carrier), invasive procedures such as fetal scalp electrodes, and if possible, operative vaginal deliveries should be avoided. If a severely affected infant is anticipated, a cesarean delivery should be performed. (See the section “Management at the time of delivery: hemophilia” below.) The usual measures to prevent PPH (ie, uterotonic medication and active management of the third stage of labor) should be used. Obstetric and surgical bleeding should be managed aggressively. Intravenous tranexamic acid (TXA) can be safely used immediately after delivery (1 g repeated in 30 minutes if necessary).43 Oral TXA can be used for prevention or treatment of delayed postpartum bleeding at a dose of 1 to 1.3 g every 8 hours. Regardless of the outcome of any testing during pregnancy, nonsteroidal anti-inflammatory drugs should be avoided postpartum. Umbilical cord blood should be obtained to test the infant. Circumcision of a male infant should be postponed until a bleeding disorder is ruled out or established and a suitable treatment plan made. Patients should have contact with a provider in the first week or two after hospital discharge.
Breastfeeding should be permitted in infants whose mothers require treatment with TXA, especially since TXA is given for only a short duration. In a prospective study of women receiving TXA at the time of cesarean delivery, the concentration in breast milk was only 1% of the maternal plasma concentration,44 which is consistent with unpublished data from the manufacturer.45 In the 20 060 subject Woman Trial, no adverse effects were noted in exposed infants.43 In a small study of the long-term effects of infants exposed to TXA during lactation, no adverse effects were noted.46
Management at the time of delivery: VWD
Management at the time of delivery may include the cautious use of 1-deamino-8-D-arginine vasopressin (DDAVP or desmopressin) to induce endothelial secretion of VWF and FVIII, VWF concentrates (plasma-derived or recombinant), and antifibrinolytics, most commonly TXA. Specific therapy by VWD subtype is summarized in Table 2. In a multicenter prospective study of the postpartum management of VWD, 32 women with VWD were enrolled during 35 pregnancies. By the time of admission for delivery, approximately half of the women (17 women during 18 pregnancies) had VWF levels greater than 50% (0.5 IU/dL) and therefore were not treated. All these women had type 1 VWD. The other 15 women (during 17 pregnancies) who did not achieve VWF levels greater than 50% (0.5 IU/dL) by the time of admission for delivery (30% of those with type 1 VWD and all of those with type 2 VWD) were treated. Except for 2 women who received desmopressin and 1 woman who received no treatment after delivery, all the women who were treated received VWF concentrate before and after delivery.15
Specific therapy at the time of delivery for VWD subtypes
| Subtype . | Specific therapy for VWD . |
|---|---|
| 1 | • Most will not require treatment. • If VWF levels <50% or 0.5 IU/mL at 36 weeks' gestation, treatment is required at the time of delivery. A higher threshold for treatment such as 0.8 IU/dL or 1.0 IU/mL has been adopted by some experts and may be considered. • VWF and FVIII levels should be followed to guide dosing. • Consider cautious use of desmopressin if a desmopressin trial was performed outside of pregnancy and efficacy was documented. |
| 2 | Expect that treatment will be required with VWF concentrates. |
| 2A | • Most will require VWF concentrates. • Some may respond to desmopressin (requires previous desmopressin trial with documented efficacy). |
| 2B | • Treat with VWF concentrates. • Desmopressin is contraindicated as it may worsen thrombocytopenia. |
| 2M | • Most will require VWF concentrates. • Some may respond to desmopressin (requires previous desmopressin trial with documented efficacy). |
| 2N | • Most will require VWF concentrates. • Some may respond to desmopressin (requires previous desmopressin trial with documented efficacy). |
| 3 | • Requires VWF concentrates. |
| Subtype . | Specific therapy for VWD . |
|---|---|
| 1 | • Most will not require treatment. • If VWF levels <50% or 0.5 IU/mL at 36 weeks' gestation, treatment is required at the time of delivery. A higher threshold for treatment such as 0.8 IU/dL or 1.0 IU/mL has been adopted by some experts and may be considered. • VWF and FVIII levels should be followed to guide dosing. • Consider cautious use of desmopressin if a desmopressin trial was performed outside of pregnancy and efficacy was documented. |
| 2 | Expect that treatment will be required with VWF concentrates. |
| 2A | • Most will require VWF concentrates. • Some may respond to desmopressin (requires previous desmopressin trial with documented efficacy). |
| 2B | • Treat with VWF concentrates. • Desmopressin is contraindicated as it may worsen thrombocytopenia. |
| 2M | • Most will require VWF concentrates. • Some may respond to desmopressin (requires previous desmopressin trial with documented efficacy). |
| 2N | • Most will require VWF concentrates. • Some may respond to desmopressin (requires previous desmopressin trial with documented efficacy). |
| 3 | • Requires VWF concentrates. |
Desmopressin, if used at all at the time of delivery, must be used with caution. It is a synthetic analog of vasopressin (antidiuretic hormone), administered at delivery intravenously at a dose of 0.3 µg/kg, with a maximum dose of 25-30 µg, over 25 to 30 minutes, or intranasally 300 µg.47 (Of note, intranasal desmopressin has limited availability in the US.) If additional treatment is required, VWF concentrates are recommended instead. Desmopressin is used in patients with type 1 VWD and some patients with type 2 (A, M, N) VWD who have a history of mild bleeding. Desmopressin is contraindicated in type 2B, as it may worsen thrombocytopenia, and in type 3 due to lack of response.48 If desmopressin is to be used, a desmopressin trial should be performed prior to pregnancy to document efficacy. If a trial has not been performed prior to pregnancy, VWF concentrates and TXA should be used instead. A desmopressin trial should not be performed during pregnancy.48 Since life-threatening hyponatremia, seizures, and neurological injury have occurred with the use of desmopressin,47,49 the use of hypotonic fluids should be avoided (normal saline is favored), oral intake of water should be limited, and serial serum sodium measurements should be obtained. Oxytocin, administered to induce labor and administered postpartum, also has some antidiuretic activity. Furthermore, it is almost impossible to limit fluids during labor or at the time of delivery, when women routinely receive a minimum of 2 to 3 liters. Desmopressin is specifically contraindicated in patients with preeclampsia and those with active coronary artery disease, cerebrovascular disease, peripheral vascular disease, or increased risk of thrombosis.48
Alternatively, VWF concentrates may be safely used in any patient with VWD who requires treatment. VWF concentrates are either plasma derived or recombinant. The plasma-derived concentrates also contain FVIII. The usual initial dose is 40-80 VWF:RCo activity units/kg with maintenance doses of 20-40 VWF:RCo activity units/kg every 12 hours as needed for at least 3 days following vaginal delivery and at least 5 days following cesarean delivery.15,50 While maintenance doses are often administered in bolus fashion, they can also be administered continuously at 2 VWF:RCo activity units/kg per hour, which allows for more constant VWF levels and facilitates the option of neuraxial anesthesia. In women with VWD for whom neuraxial anesthesia during labor is deemed suitable, the recent ASH ISTH NHF WFH guidelines suggest targeting a minimum VWF activity level of 0.50 IU/mL.48 In women receiving repeated dosing of factor replacement it is important that VWF and FVIII activity be measured in real time so that adjustments can be made to avoid under- and over-dosing. In the systematic review by Punt et al., there were two cases of venous thromboembolism in women receiving factor replacement.26 TXA can be used postpartum to help prevent delayed onset bleeding. The recent ASH ISTH NHF WFH guidelines suggest the use of oral TXA after delivery in all types of VWD.48
Given that VWF levels increase with pregnancy, use of non-pregnant normal levels to determine targets is likely not optimal. While there are ongoing studies in this area, target levels of 1.0 to 1.5 IU/dL have been suggested.50,51 Levels should be maintained at >0.50 IU/dL for at least 3 days after vaginal delivery and at least 5 days following cesarean delivery.50 While VWD in the fetus is not an indication for cesarean delivery, there is a risk of nonsevere fetal bleeding, so invasive procedures such as fetal scalp electrodes and, if possible, operative vaginal deliveries should be avoided.50 Neuraxial anesthesia is considered safe with VWF and FVIII activity levels ≥0.5 IU/mL. Levels ≥0.5 IU/mL should be maintained while the catheter is in place and for 6 hours after removal.48
Management at the time of delivery: hemophilia
For hemophilia carriers, management at the time of delivery requires attention not only to the risk of maternal bleeding but also to the risk of fetal bleeding. FIX levels do not increase significantly during pregnancy, and although FVIII levels do,15,16 they likely do not increase to the same extent as FVIII levels do in women who are not hemophilia A carriers. While we have less data for hemophilia carriers than for patients with VWD, there are similar concerns regarding optimal target levels in carriers of hemophilia A, given that FVIII also increases during normal pregnancy. If a carrier's factor levels are less than 50% (<0.5 IU/mL) (or higher depending on evolving data and local practice) as delivery approaches (at 36 weeks' gestation, for instance), she would be at a greater risk of bleeding at delivery and postpartum. As is true in any bleeding disorder, most bleeding at the time of delivery is due to failure of the uterus to contract (obstetric bleeding) or to birth trauma (surgical bleeding), and while the risk of bleeding at delivery can be mitigated by routine obstetric measures, experts agree that women with factor levels less than 50% (0.5 IU/mL) (or higher depending on evolving data and local practice) should receive treatment with FVIII or FIX replacement at the time of delivery.52 As is also true for women with VWD and other bleeding disorders, there are no randomized controlled trials to guide treatment; when required, treatment is recommended with virally inactivated plasma-derived or recombinant coagulation factor concentrates rather than cryoprecipitate or fresh frozen plasma,52 and any recommendations regarding treatment threshold, product choice, dosing, or therapy duration are based on observational studies or expert opinion. Following delivery, concentrates should be administered to maintain factor levels above 0.5 IU/mL for at least 3 days following vaginal delivery and at least 5 days following cesarean delivery.52 Factor VIII levels should be followed in real time to ensure adequate dosing and avoid overtreatment. Usual doses are 20-50 IU/kg.
An affected male infant has approximately a 3% risk of fetal or neonatal intracranial hemorrhage (ICH) if delivered vaginally and a 0.4% risk if delivered by cesarean.53 We recommend that a woman expecting an affected or potentially affected male be delivered by cesarean before labor. While cesarean delivery does not completely eliminate the risk of ICH, the risk is reduced when cesarean is performed before labor compared with the risk when cesarean is performed after labor.54 Also, although cesarean delivery generally increases risk of maternal bleeding and requires a longer hospital compared with vaginal delivery, this applies to the aggregate of cesarean deliveries— both planned and unplanned. A planned vaginal delivery includes the risk of an unplanned emergency cesarean delivery, which confers a greater risk of maternal bleeding and longer stay compared with a planned cesarean. In the 2 randomized trials of planned cesarean delivery versus planned vaginal delivery, planned cesarean delivery did not confer a greater risk of maternal bleeding or longer stay than planned vaginal delivery.55,56 Various guidelines state that operative vaginal delivery (forceps or vacuum extraction), which greatly increases the risk of ICH,54 should be avoided in the delivery of an affected male, but if the fetus is deep in the pelvis and delivery must be expedited, operative vaginal delivery may be unavoidable. In a large observational study of newborns with hemophilia, 4% of the 466 known obligate or possible carriers still had to be delivered by forceps or vacuum extraction.54 The only certain way to avoid an operative vaginal delivery is to plan for a cesarean delivery. Following delivery, umbilical cord blood should be obtained and sent for factor levels. If the parents desire the infant to be circumcised, the procedure should be postponed until a diagnosis of hemophilia is ruled out or established and an appropriate treatment plan made.
A woman expecting a female who is potentially a hemophilia carrier does not need a cesarean delivery. As in VWD, there is a risk of nonsevere fetal bleeding, so invasive procedures such as fetal scalp electrodes and, if possible, operative vaginal deliveries should be avoided.
Management of other inherited coagulation factor deficiencies and severe platelet disorders
Women with rare bleeding disorders, even those with mild deficiencies, are at increased risk of PPH.57 The general guidance described in “Management of pregnant women with any bleeding disorder” applies to women with other inherited coagulation factor deficiencies and platelet disorders. Specific background regarding FVII, FXI fibrinogen, and FXIII deficiency is described in the individual sections on these conditions. Their treatment is summarized in Table 3. Bleeding or potential bleeding with severe platelet disorders has been managed with platelet transfusions and recombinant factor VIIa (rFVIIa).
Treatment of other coagulation factor deficiencies
| Deficiency . | Treatment* . |
|---|---|
| Factor VII deficiency | |
| 20%-50%—mild | |
| 10%-20%—moderate; at risk for mild or provoked bleeding | |
| <10%—severe | Low-dose rFVIIa or plasma |
| Factor XI deficiency | |
| 20%-60%—variable risk for bleeding | |
| <20%—still variable risk for bleeding | Plasma or FXI concentrate where available |
| Fibrinogen deficiency | |
| <60mg/dL—at risk for miscarriage | Fibrinogen concentrates where available are the treatment of choice for patients with quantitative or qualitative deficiencies; otherwise, cryoprecipitate for antepartum as well as peripartum prophylaxis. |
| <100mg/dL—at risk for placental abruption | |
| <150mg/dL—at risk for placental abruption in labor and PPH | |
| Factor XIII deficiency | |
| <10%—severe; high risk for miscarriage and PPH | FXIII concentrate where available for antepartum as well as peripartum prophylaxis |
| Deficiency . | Treatment* . |
|---|---|
| Factor VII deficiency | |
| 20%-50%—mild | |
| 10%-20%—moderate; at risk for mild or provoked bleeding | |
| <10%—severe | Low-dose rFVIIa or plasma |
| Factor XI deficiency | |
| 20%-60%—variable risk for bleeding | |
| <20%—still variable risk for bleeding | Plasma or FXI concentrate where available |
| Fibrinogen deficiency | |
| <60mg/dL—at risk for miscarriage | Fibrinogen concentrates where available are the treatment of choice for patients with quantitative or qualitative deficiencies; otherwise, cryoprecipitate for antepartum as well as peripartum prophylaxis. |
| <100mg/dL—at risk for placental abruption | |
| <150mg/dL—at risk for placental abruption in labor and PPH | |
| Factor XIII deficiency | |
| <10%—severe; high risk for miscarriage and PPH | FXIII concentrate where available for antepartum as well as peripartum prophylaxis |
Includes the general guidance described under “Management of pregnant women with any bleeding disorder.”
Conclusions
Bleeding disorders increase the risk of maternal bleeding, particularly at the time of delivery, and in severely affected infants may result in fetal and neonatal ICH. Although mild platelet defects may be more prevalent, the most commonly diagnosed bleeding disorder among women is VWD. Other bleeding disorders are less common, but hemophilia carriers are unique in that they are at risk of giving birth to a severely affected male infant. General guidance for maternal management of an affected woman includes obtaining coagulation factor levels at least in the third trimester; planning for delivery at a center with hemostasis expertise; and anticipating the need for on-site laboratory testing and hemostatic agents. General guidance for fetal management includes prepregnancy counseling; the option of preimplantation genetic testing for hemophilia; delivery at a tertiary care center with pediatric hematology and newborn intensive care; consideration of cesarean delivery of a potentially severely affected infant; and avoidance of invasive procedures such as fetal scalp electrodes and operative vaginal delivery in any potentially affected infant. Future areas of research include determining the optimal factor level target levels for patients with VWD and hemophilia and considering alternative therapies for treatment.
Conflict-of-interest disclosure
Andra H. James: honoraria: Cerus Corporation.
Luis D. Pacheco: no competing financial interests to declare.
Barbara A. Konkle: research funding: CSL Behring, Pfizer, Spark, Takeda, and uniQure; consultancy: BioMarin, Novo Nordisk, Pfizer, Regeneron, and Takeda.
Off-label drug use
Andra H. James: nothing to disclose.
Luis D. Pacheco: nothing to disclose.
Barbara A. Konkle: nothing to disclose.