Abstract
Anemia is common during pregnancy, and while most anemia is physiologic, the most common pathologic cause is iron deficiency. The American College of Obstetricians and Gynecologists (ACOG) recommends confirmation of iron deficiency anemia with iron studies when anemia is diagnosed during pregnancy but acknowledges that presumptive treatment for suspected iron deficiency anemia is common in practice. Currently ACOG does not recommend treating iron deficiency without anemia during pregnancy. Though the benefits of treating iron deficiency anemia during pregnancy are clear, the optimal route of iron repletion remains uncertain. Results of ongoing large, randomized trials will help define the optimal route of iron treatment for pregnant patients diagnosed with iron deficiency anemia.
Learning Objectives
Overview physiologic versus pathologic anemia during pregnancy
Discuss screening and treatment guidelines for iron deficiency anemia in pregnancy
Review current data on optimal iron treatment method (oral versus intravenous) for iron deficiency anemia in pregnancy
CLINICAL CASE
A patient has had an uncomplicated pregnancy and undergoes routine third-trimester prenatal laboratory screening for gestational diabetes and anemia. The hemoglobin results at 10.2 g/dL. The obstetrician prescribes oral iron with instructions to take 1 tablet once daily in the evenings with orange juice. The patient initially follows these instructions but stops taking the iron tablet within a few weeks due to feeling noticeably more bloated and constipated. On routine laboratory evaluation upon admission for delivery, the hemoglobin is 9.0 g/dL. The labor course is notable for fetal distress requiring cesarean delivery, which was complicated by postpartum hemorrhage due to uterine atony. Total estimated blood loss was 1500 mL. The hemoglobin on postoperative day 1 is 7.2 g/dL. The patient receives a blood transfusion for symptomatic anemia.
Anemia in pregnancy
According to the World Health Organization (WHO), nearly 40% of pregnancies are complicated by anemia.1 Despite its high prevalence, there is no standard definition of anemia during pregnancy: the WHO defines anemia as a hemoglobin <11 g/dL or hematocrit <33% at any time during pregnancy,2 whereas the Centers for Disease Control and Prevention (CDC) as well as the American College of Obstetricians and Gynecologists (ACOG) propose a trimester-based definition. Specifically, ACOG and the CDC define anemia in pregnancy as hemoglobin <11 g/dL (hematocrit <33%) during the first or third trimesters or hemoglobin <10.5 g/dL (hematocrit <32%) during the second trimester (Table 1).3 Historically, there were different diagnostic thresholds for anemia during pregnancy that were based on maternal race.4 To reduce racial inequities in screening and treating anemia in perinatal populations, the same standard is now applied universally to diagnose anemia during pregnancy.
Contrasting definitions of anemia and iron deficiency during pregnancy
| . | World Health Organization2,11 . | American College of Obstetricians & Gynecologists3 . |
|---|---|---|
| Ferritin | <15 ng/L * | <30 ng/L ** |
| Hemoglobin | ||
| First trimester | <11 g/dL | <11 g/dL |
| Second trimester | <11 g/dL | <10.5 g/dL |
| Third trimester | <11 g/dL | <11 g/dL |
| . | World Health Organization2,11 . | American College of Obstetricians & Gynecologists3 . |
|---|---|---|
| Ferritin | <15 ng/L * | <30 ng/L ** |
| Hemoglobin | ||
| First trimester | <11 g/dL | <11 g/dL |
| Second trimester | <11 g/dL | <10.5 g/dL |
| Third trimester | <11 g/dL | <11 g/dL |
WHO definition for iron deficiency during first trimester of pregnancy;
ACOG definition for iron deficiency during any trimester of pregnancy.
Differentiating between physiologic and pathologic causes of anemia in pregnancy
The high prevalence of perinatal anemia is driven by physiological changes that occur during pregnancy. Because plasma volume expands by 40% to 50% but erythrocyte mass expansion is only 15% to 25%, a physiological dilutional anemia commonly develops as pregnancy progresses.3 Though physiologic dilutional anemia typically results in mild anemia (hemoglobin of 10 to 11 g/dL), it is impossible to differentiate between physiologic dilutional anemia and pathological causes of anemia during pregnancy without a laboratory workup. This workup targets the more common pathologic causes of anemia, which include, but are not limited to, iron deficiency anemia, anemia of chronic disease, folic acid deficiency anemia, anemia associated with vitamin B12 deficiency, or inherited hemoglobinopathies such as thalassemia or sickle cell anemia.3 Specifically, ACOG guidelines recommend screening all pregnant people for anemia with a complete blood count twice during routine prenatal care: once in the first trimester and again between 24 weeks and 0 days gestation and 28 weeks and 6 days gestation.3 Though ACOG guidelines do not recommend routine repeat anemia screening in the third trimester or at term, this is commonly done in the United States.
When anemia is identified on the screening complete blood count, ACOG recommends additional evaluation, which “may include a medical history, physical examination, and measurements of the complete blood count, red blood cell indices, serum iron levels, and ferritin levels” with consideration for peripheral smear, hemoglobin electrophoresis, or genetic testing based on personal or family history.3 However, ACOG also endorses empirical treatment with iron and additional investigations if there is no response.3 Perhaps due to ACOG's inconsistency, there is a wide variation in practice patterns concerning the diagnosis of anemia during pregnancy. For example, some obstetricians screen for hemoglobinopathies universally during the first trimester, while others obtain hemoglobin electrophoresis only in the setting of anemia and family history or geographic location. Moreover, serum iron or ferritin are not routinely performed for anemic pregnant patients. Rather, the presumptive diagnosis for anemia during pregnancy is iron deficiency anemia, and iron repletion therapy is often initiated without confirming the diagnosis for most patients.
Ongoing trials comparing IV to oral iron for iron deficiency anemia during pregnancy36–40
| Trial . | Location/funding . | Inclusion . | IV iron (1) . | IV iron (2) . | Oral iron . | Masking . | Primary outcome . | N . |
|---|---|---|---|---|---|---|---|---|
| EDIVA39 | Bangladesh Gates | Hb <10 at 13-32 weeks | Ferric carboxymaltose, 1000 mg | - | Ferrous sulfate, 60 mg bid | No | Maternal anemia (Hb <11) at 36 weeks | 900 |
| IVIDA236 | US NIH | Hb <10 & ferritin <30 at 24-28 weeks | Ferric derisomaltose, 1000 mg | - | Ferrous sulfate, 65 mg qd-tid | Yes | Maternal peripartum blood transfusion | 746 |
| IVON37 | Nigeria Gates | Hb <10 at 20-32 weeks | Ferric carboxymaltose, 1000 mg | - | Ferrous sulfate, 65 mg tid | No | Maternal anemia (Hb <11) at 36 weeks | 1056 |
| RAPID IRON38 | India Children Investment Fund Foundation (UK) | Hb <10 at 13-26 weeks | Ferric carboxymaltose, 1000 mg | Ferric derisomaltose, 1000 mg | Ferrous sulfate, 60 mg bid | No | Low birth weight Maternal anemia (Hb <11) at 30-34 weeks | 4320 |
| REVAMP-TT40 | Malawi Gates | Hb <10 at 27-35 weeks | Ferric carboxymaltose, 1000 mg | - | Ferrous sulfate, 60 mg bid | No | Maternal anemia (Hb <11) at 36 weeks | 590 |
| Trial . | Location/funding . | Inclusion . | IV iron (1) . | IV iron (2) . | Oral iron . | Masking . | Primary outcome . | N . |
|---|---|---|---|---|---|---|---|---|
| EDIVA39 | Bangladesh Gates | Hb <10 at 13-32 weeks | Ferric carboxymaltose, 1000 mg | - | Ferrous sulfate, 60 mg bid | No | Maternal anemia (Hb <11) at 36 weeks | 900 |
| IVIDA236 | US NIH | Hb <10 & ferritin <30 at 24-28 weeks | Ferric derisomaltose, 1000 mg | - | Ferrous sulfate, 65 mg qd-tid | Yes | Maternal peripartum blood transfusion | 746 |
| IVON37 | Nigeria Gates | Hb <10 at 20-32 weeks | Ferric carboxymaltose, 1000 mg | - | Ferrous sulfate, 65 mg tid | No | Maternal anemia (Hb <11) at 36 weeks | 1056 |
| RAPID IRON38 | India Children Investment Fund Foundation (UK) | Hb <10 at 13-26 weeks | Ferric carboxymaltose, 1000 mg | Ferric derisomaltose, 1000 mg | Ferrous sulfate, 60 mg bid | No | Low birth weight Maternal anemia (Hb <11) at 30-34 weeks | 4320 |
| REVAMP-TT40 | Malawi Gates | Hb <10 at 27-35 weeks | Ferric carboxymaltose, 1000 mg | - | Ferrous sulfate, 60 mg bid | No | Maternal anemia (Hb <11) at 36 weeks | 590 |
Prevalence of iron deficiency and iron deficiency anemia during pregnancy
Iron deficiency anemia is the most common pathologic cause of anemia, affecting nearly 1 in every 5 pregnant persons in the United States.5 Each pregnancy requires approximately 1000 milligrams (mg) of total iron to support increased erythrocyte production, normal placental and fetal development, and anticipated blood loss at delivery.3 Many pregnant people are unable to ingest or absorb sufficient dietary iron,6 leaving them vulnerable to developing iron deficiency or iron deficiency anemia. Indeed, according to data from the United States National Health and Nutrition Examination Survey from 1999 to 2006, 25% of all pregnant people in the United States have iron deficiency, with rates of 7%, 24%, and 39% in the first, second, and third trimester, respectively.5 Rates of iron deficiency during pregnancy differ by demographic factors: compared with those who are non-Hispanic White or have fewer than 3 children, the prevalence of iron deficiency is markedly higher among non-Hispanic Black and Mexican-American pregnant people and those who have 3 or more children.5
To reduce iron deficiency and the risk of subsequently developing iron deficiency anemia, the CDC recommends that all pregnant patients begin low-dose iron supplementation (27 mg of elemental iron daily) at the first prenatal visit.7 Most nonchewable prenatal vitamins include 27 mg of elemental iron. The United States Preventive Task Force does not recommend for or against routine iron supplementation during pregnancy (beyond low-dose supplementation), as it is unclear whether iron supplementation in pregnant people without anemia affects perinatal outcomes.8 Thus, providing more than 27 mg of elemental iron daily for pregnant people without anemia is not currently the standard of care.
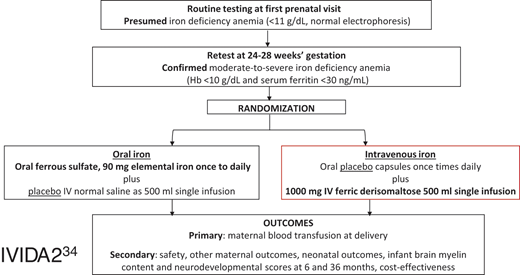
IVIDA2 trial flowsheet.36
If performed, evaluation for iron deficiency anemia during pregnancy by obstetric care providers is usually with serum ferritin only, as it is more easily interpretable compared with the full panel of iron studies,3 despite being an acute phase reactant that may vary during normal pregnancy due to the physiologic rise in hepcidin levels.9 Furthermore, ferritin is considered to be a more sensitive and specific marker for iron deficiency than other markers, including serum iron and transferrin saturation.10,11 However, the WHO and ACOG have different thresholds of serum ferritin required to diagnose iron deficiency during pregnancy (Table 1). The WHO defines iron deficiency during the first trimester of pregnancy as serum ferritin <15 ng/L,12 whereas ACOG defines iron deficiency during pregnancy as a serum ferritin <30 ng/L in any trimester.3 We use the ACOG diagnostic thresholds for iron deficiency during pregnancy as the higher ferritin level has much higher sensitivity (92%) without a significant compromise of specificity (98%).11
Management of iron deficiency without anemia during pregnancy
By applying ACOG definitions of iron deficiency and anemia, we can define iron deficiency without anemia during pregnancy as a serum ferritin <30 ng/L but hemoglobin ≥11.0 g/dL.3 Currently, ACOG does not recommend treatment for iron deficiency without anemia,3 perhaps because there are no compelling data showing that maternal iron deficiency is associated with reduced fetal or neonatal iron stores or adverse childhood neurodevelopmental sequellae.13,14 However, new approaches for evaluating iron homeostasis have prompted a call to change this paradigm.15 Data generated from novel iron testing methods have suggested that neonates born to mothers with lower ferritins have significantly lower ferritins than neonates born to mothers with normal ferritins.16 More research is needed to untangle the potential relationship between maternal iron deficiency without anemia and abnormal neonatal outcomes.
Furthermore, the iron demands of normal pregnancy may increase the risk of iron deficiency anemia later in pregnancy. As untreated iron deficiency anemia increases the risk of perinatal complications,17 waiting for iron deficiency anemia to develop prior to initiating treatment may in fact cause harm. Data on interventions designed to prevent the development of iron deficiency anemia among pregnant people with iron deficiency and normal hemoglobin remain scant. A recent randomized controlled trial conducted in Denmark compared the efficacy of a single-dose (1000 mg) intravenous (IV) iron (ferric derisomaltose) with 100 mg daily oral iron (ferrous fumarate) in preventing anemia among 201 pregnant people at 14 to 21 weeks with iron deficiency (ferritin <30 ng/L).18 Over the 18-week follow-up period, those receiving IV iron had a higher mean hemoglobin increase and were less likely to develop anemia compared with those receiving oral iron (9% vs 27%; 18% difference, 95% CI [10%, 25%]).18 There was no significant difference between the 2 groups in treatment-related adverse events.18 While these results are promising, 11% of the analytic population had iron deficiency anemia at randomization. Thus, these results may not be applicable to pregnant people with iron deficiency but not anemia. More high-quality data are urgently needed to clarify whether treating iron deficiency without anemia during pregnancy improves perinatal outcomes.
Treating iron deficiency anemia in pregnancy
Unlike the clinical conundrum of iron deficiency without anemia during pregnancy, iron deficiency anemia has been associated with increased rates of cesarean delivery, postpartum depression, and perinatal blood transfusion,17,19,20 the major driver of the CDC's severe maternal morbidity composite quality metric.21 Iron deficiency anemia is also associated with increased risk of low birth weight, preterm birth, and small-for-gestational-age neonates.17,19 Moreover, those who are iron deficient during pregnancy are at risk of delivering iron-deficient neonates, who themselves are at risk for delayed growth and development even after postnatal iron repletion.22 Fetal-neonatal iron deficiency has been linked to neurological impairments in infants23 that may persist into adulthood.22 Animal studies have identified that iron has a crucial role in normal fetal and postnatal brain development, including myelination, dendritic growth, and synapse formation.24-26 Thus, there is a clear association between untreated iron deficiency anemia in pregnancy and long-term adverse neurodevelopmental outcomes.
Oral versus intravenous iron for treating iron deficiency anemia in pregnancy
Iron supplementation is recommended for treating iron deficiency anemia during pregnancy, but the optimal route of delivery remains uncertain. Oral iron, administered in doses higher than found in prenatal vitamins, is the current standard for treating iron deficiency anemia during pregnancy in the United States.3 Ferrous sulfate is the most commonly prescribed oral iron formulation as it is inexpensive, safe, readily available, and, when tolerated, effective. However, a meta-analysis of 43 randomized controlled trials reported that up to 70% of patients prescribed oral iron experienced significant gastrointestinal perturbation, decreasing adherence to therapy.27 Because higher and more frequent doses have not been shown to improve iron uptake but increase adverse medication effects, lower iron doses and alternate-day dosing have been proposed to treat iron deficiency anemia during pregnancy.28,29 Specifically, alternate-date oral iron supplementation has been shown to avoid hepcidin suppression, thereby increasing oral iron supplementation while likely reducing the prevalence of adverse medication effects.30 Though this regimen is commonly used in parts of Europe and the United Kingdom, alternate-day dosing is not yet recommended during pregnancy in the United States.3
Intravenous (IV) iron is another option for treating iron deficiency anemia during pregnancy. IV iron is usually administered in the second or third trimesters, as there are no safety data for first-trimester use. All IV iron products currently on the market have similar safety and efficacy.31 Thus, the choice of formulation is based on cost and administration burden. Formulations such as low-molecular-weight iron dextran, ferric derisomaltose, or ferric carboxymaltose that allow a complete replacement dose in a single visit are preferred to those that require multiple infusions such as ferumoxytol or iron sucrose, namely because they reduce costs associated with infusion and increase likelihood that the pregnant person receives full treatment for iron deficiency anemia.
ACOG currently recommends oral iron repletion as the first-line treatment for iron deficiency anemia during pregnancy, stating that IV iron “may be considered for those who cannot tolerate or do not respond to oral iron or for those with severe iron deficiency later in pregnancy.”3 However, some data suggest that IV iron may be superior to oral iron in rapidly correcting anemia and iron deficiency. Two meta-analyses of randomized trials found that compared with oral iron, IV iron was associated with significantly higher hemoglobin level following therapy among pregnant people with iron deficiency anemia.32,33 One of these meta-analyses also evaluated maternal and neonatal outcomes.33 Among 8 randomized controlled trials with these specific outcomes, IV iron was associated with higher neonatal birth weight (weighted mean difference 58.25 g [95% CI 5.57 g, 110.94 g]), higher neonatal ferritin levels (weighted mean difference 21.38 ng/mL [95% CI 5.50 ng/mL, 37.25 ng/mL]), and less frequent adverse effects (relative risk 0.34 [95% CI 0.20, 0.57]) and therapy discontinuation (0.02% with IV and 2% with oral iron).33 However, the primary trials did not assess clinically meaningful maternal or neonatal outcomes and included small sample sizes (50-252).33
After these meta-analyses were published, two large randomized controlled trials—one in India34 and another in Malawi35 — were published. In the study from India, pregnant people at 20 to 28 weeks with a hemoglobin of 5-8 g/dL or at 29 to 32 weeks with a hemoglobin of 5-9 g/dL were randomly assigned to receive up to 5 divided doses of IV iron sucrose infusions or oral iron (100 mg elemental iron twice daily).34 The primary outcome was a maternal composite outcome, defined as one of the following conditions: postpartum hemorrhage, blood transfusion during and after delivery, sepsis, shock, prolonged hospital stay and intensive care unit admission, or referral to higher centers.34 The results showed no difference in the primary outcome, serious maternal adverse events, or serious fetal and neonatal adverse events.34 However, this study is limited by using iron sucrose, which required multiple infusions, resulting in a wide range of IV iron doses infused (200-1600 mg), and the mean iron sucrose dose of 400 mg is subtherapeutic for treatment during pregnancy. In addition, this study is limited by the fact that the primary composite outcome includes multiple conditions not directly associated with anemia, such as sepsis.34
In the study from Malawi, pregnant people at 13 to 26 weeks with a hemoglobin of less than 10.0 g/dL and negative malaria rapid diagnostic test were randomized to a single dose of up to 1000 mg of ferric carboxymaltose or oral iron (60 mg elemental iron twice daily for 90 days).35 The primary outcome was anemia at 36 weeks' gestation (defined as hemoglobin <11.0 g/dL), and the primary neonatal outcome was birth weight. There was no difference in the primary outcome—or in rates of anemia or moderate-to-severe anemia 4 weeks posttreatment, at delivery, or 4 weeks postpartum—or primary neonatal outcome and no significant difference in adverse events.35 However, those randomized to ferric carboxymaltose had lower rates of iron deficiency and iron deficiency anemia (defined as anemia with a ferritin <15 mg/L or <30 mg/L if C-reactive protein >5 mg/L) at 36 weeks' pregnancy, birth, and 4 weeks' postpartum compared with those randomized to oral iron. Importantly, only approximately 40% of the study sample had iron deficiency anemia, with a median ferritin at randomization slightly less than 30 mg/L, suggesting that approximately half of those randomized did not have iron deficiency. Of note, subgroup analyses limiting the analytic population to those with iron deficiency or iron deficiency anemia at randomized demonstrated no difference in maternal anemia at 36 weeks' gestation or neonatal birth weight. In addition, those randomized to oral iron were prescribed treatment for only 90 days after randomization,35 which does not align with ACOG recommendations to continue oral iron supplementation until delivery.3
There is, therefore, an urgent need for a study to test the clinical effectiveness, safety, and cost-effectiveness of IV iron on clinically relevant maternal and neonatal outcomes among pregnant people with iron deficiency anemia. Our group and others are conducting such trials.36-40 In our trial, the only multicenter, placebo-controlled, double-blinded randomized controlled trial, 746 pregnant people with moderate-to-severe iron deficiency anemia (hemoglobin <10 g/dL and ferritin <30 ng/mL) at 24 to 28 weeks' gestation are randomized 1:1 to either a single 1000 mg dose of intravenous ferric derisomaltose and oral placebo (1 to 3 times daily) or a single placebo infusion with 1 to 3 times daily 325 mg ferrous sulfate tablets containing 65 mg of elemental iron.36 The primary outcome is peripartum blood transfusion, defined as blood transfusion from delivery to 7 days postpartum. This primary outcome is clinically meaningful and plausible. In our pilot randomized trial, there was a 100% reduction in blood transfusion among pregnant people with iron deficiency anemia treated with IV iron compared with oral iron (0% vs 15%).41 Secondary outcomes include adverse medication reactions, maternal and neonatal hematologic indices, and offspring neurodevelopment as measured via nonsedated MRI and neurodevelopmental assessment at 6 months of life. Results from this and the other ongoing trials will help define the optimal route of iron repletion among pregnant people with iron deficiency anemia.
Summary
Anemia is common during pregnancy, and while most causes are physiologic, the most common pathologic cause is iron deficiency. ACOG recommends confirmation of iron deficiency anemia with iron studies when anemia is diagnosed during pregnancy but acknowledges that presumptive treatment for suspected iron deficiency anemia is common in practice. Currently ACOG does not recommend treating iron deficiency without anemia during pregnancy. Though the benefits of treating iron deficiency anemia during pregnancy are clear, the optimal route of iron repletion remains uncertain. Results of ongoing large randomized trials will help define the optimal route of iron treatment for pregnant patients diagnosed with iron deficiency anemia.
Conflict-of-interest disclosure
Adam K. Lewkowitz served on medical advisory boards for Shields Pharmaceutics in 2021 and Pharmacosmos Therapeutics Inc. in 2022.
Methodius G. Tuuli: no competing financial interests to declare.
Off-label drug use
Adam K. Lewkowitz: nothing to disclose.
Methodius G. Tuuli: nothing to disclose.