Abstract
Management of hematological malignancies is rapidly evolving from chemotherapy-based regimens toward targeted agents and immunotherapies, including bispecific antibodies (BsAbs). These novel and highly active treatments come with new side effect profiles. The hematological toxicities are common and potentially harmful, and the side effects have hitherto not been reviewed. With many BsAbs recently approved and entering routine clinical use, we have reviewed the rather limited published data and propose recommendations on the management of these toxicities. Our review of the available data confirms that hematological toxicities are among the most common toxicities, with potentially harmful consequences for the patients. Fortunately, hemophagocytic lymphohystiocytosis and disseminated intravascular coagulation are rare. Severe neutropenia and hypogammaglobulinemia are manageable, and their timely treatment and prevention may reduce morbidity and mortality.
Learning Objectives
Review the incidence and severity of hematological toxicities associated with bispecific antibody therapies in hematological malignancies
Discuss the background for such hematological toxicities and their potential complications
Propose management strategies to prevent and/or treat the hematologic toxicities
CLINICAL CASE
A 55-year-old female was referred for experimental treatment of her third relapse of non-germinal center B-cell diffuse large B-cell lymphoma. She was first diagnosed in 2013 and had three times relapsed after initial response to rituximab-containing polychemotherapy, including high-dose chemotherapy with autologous stem cell support. In 2019, a CT scan showed a third relapse in the mediastinum. She was in a good general condition and was enrolled in the phase 1 study of the CD20xCD3 BsAb glofitamab (NP30179) in January 2020. She tolerated the step-up regimen well, with cytokine release syndrome (CRS) grade 1 after the 2.5 mg priming dose and after the 10 mg intermediate dose but with no signs of CRS on subsequent doses, and she achieved a complete response after 2 treatment cycles. After 3 cycles, she encountered recurrent respiratory infections that led to dose delays in cycle 4 and cycle 5 and that were preceded by glofitamab- induced lymphopenia. After cycle 6, the lymphopenia was accompanied by neutropenia, and she was admitted to an intensive care unit with neutropenic sepsis and bilateral pneumonia. She improved on broad-spectrum antibiotics and vasopressor support. The neutropenia resolved after more than one week of granulocyte colony stimulating factor (G-CSF) treatment. After an additional (and again delayed) cycle 7 of glofitamab, she again developed profound neutropenia, this time with a very slow response to G-CSF, and she was taken off protocol per physician's decision. Lymphocyte and neutrophil counts normalized within the following months, and more than 3 years after the end of glofitamab treatment, she remains in complete response. She receives subcutaneous immunoglobulin injections in to reduce the risk of infections.
Introduction
The management of hematologic malignancies is evolving, and immunotherapies are rapidly becoming part of the treatment paradigm. Along with the introduction of these new strategies such as bispecific antibodies, CAR-T cell therapy, and checkpoint inhibiting antibodies,1,2 new toxicities are also emerging. We address adverse events to BsAbs, while the latter modalities will be covered in accompanying articles.
Bispecific antibodies (BsAb) are monoclonal antibodies binding to an effector cell surface antigen (typically CD3 on T-cells) and to a surface antigen on the tumor cell, leading to effector-cell mediated tumor cell killing. These antibodies are so far only recommended for use as single agents, but they are also under study in combination with chemotherapy, other immunotherapies (NCT05849610), checkpoint inhibitors (NCT03533283), other bispecifics (NCT04586426), or costimulatory immune agonists (NCT05219513, NCT04077723).
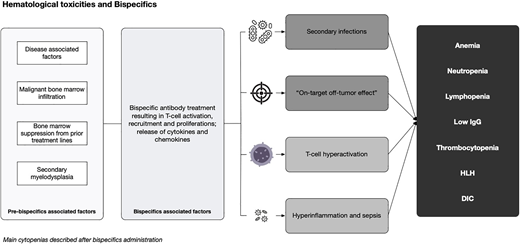
There is a paucity of data on common hematological toxicities observed in the context of BsAbs. While some of these toxicities are well characterized as “on-target, off-tumor” effects of the T-cell bispecifics, particularly the profound lymphopenia and hypogammaglobulinemia seen in most B-cell non-Hodgkin lymphoma (B-NHL) patients, the biological mechanism behind the observed neutropenia, thrombocytopenia, and the rare cases of hemophagocytic lymphohystiocytosis (HLH) are poorly understood.
The scope of this article is to review hematological complications to bispecific antibody and bispecific-T-cell-engager (BiTE) treatment in hematologic malignancies, based on published trials and abstracts, and to propose some recommendations for their management.
We reviewed the main articles and abstracts published about BsAbs in the treatment of B-NHL, B-cell acute lymphoblastic leukemia (B-ALL), multiple myeloma (MM), with a focus on hematological side effects. Data are summarized and presented in a tabular format, derived from articles with information about anemia, neutropenia, febrile neutropenia, lymphopenia, thrombocytopenia, hypogammaglobulinemia, HLH, and disseminated intravascular coagulation (DIC), by group of disease.
Hematological toxicities are common in the context of BsAbs, regardless of indications and tumor targets. The reasons for these phenomena are multifactorial and associated with the disease (which is often bone marrow based), previous therapies (most patients are heavily pretreated), the patient's immune repertoire, and the effect of the individual bispecific molecule in inflammation, B-cell/plasma cell depletion and T-cells exhaustion.3-5 Production of cytokines by the bone marrow environment may also affect hematopoiesis during BiTE and BsAb therapy.
Acute lymphoblastic leukemia
Although the prognosis of ALL has improved over past decades, a substantial proportion of adults relapse or are refractory (R/R) to first-line treatment. For such patients, the 1- and 5-year overall survival (OS) are 22% and 7%, respectively.6 Blinatumomab, targeting CD19 on B-cells, was the first FDA-approved BiTE used in clinical practice.7
In the phase 2 study of blinatumomab in adult patients with R/R B-ALL, hematological toxicities were the most frequent ≥ grade 3 adverse events (AE). In this trial, 4 of 188 patients (2%) developed DIC, a rare but feared complication of T-cell hyperactivation, while 23/188 (12%) suffered a fatal AE, mainly related to infectious complications.8 In the phase 3 TOWER trial, the hematological adverse events of ≥ grade 3 were more common in the control arm (chemotherapy) than in the blinatumomab arm. The exceptions were hypogammaglobulinemia (6% vs 0.9%) and HLH (1.5% vs 0%), which almost exclusively occurred in the blinatumomab arm.9 The RIALTO study evaluated blinatumomab in a pediatric population with R/R B-ALL. In this study, there was one event of DIC (0.9%) and febrile neutropenia ≥ grade 3 occurred in 9.1% of patients. There was no blinatumomab-related fatal AE, and no reports of HLH.10
Data on the hematological toxicities of the BiTEs in ALL are summarized in Table 1.
Incidence of hematological toxicities on bispecifics for acute lymphoblastic leukemia published trials
| Reference . | Population . | Drug/target . | N* . | Anemia** . | Neutropenia** . | Thrombocytopenia** . | Febrile neutropenia** . | Lymphopenia** . | Low IgG** . | HLH** . | DIC** . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Kantarjian et al. (2017)9 | Adult R/R ALL | Blinatumomab CD19 × CD3 | 267 | 69 (25.8%)/NA | NA/101(37.8%) | 47(17.6%)/NA | NA/57(21.3%) | NA/4(1.5%) | 16(6%)/ 7(2.6%) | 4(1.5%)/ 4(1.5%) | NA/NA |
| Topp et al. (2015)8 | Adult R/R ALL | Blinatumomab CD19 × CD3 | 188 | 38(20%)/ 27(14%) | 33(17%)/ 30(16%) | 21(11%)/16(8%) | 53(28%)/ 48(25%) | NA/NA | NA/NA | NA/NA | 4(2%)/NA |
| Locatelli et al. (2022)10 | Pediatric R/R ALL | Blinatumomab CD19 × CD3 | 110 | 20(18.2%)/ 5(4.5%) | 11(10%)/ 10(9.1%) | 22(20%)/16(14.5%) | NA/ 10(9.1%) | NA/NA | NA/NA | NA/NA | 1(0.9%)/ 1(0.9%) |
| Reference . | Population . | Drug/target . | N* . | Anemia** . | Neutropenia** . | Thrombocytopenia** . | Febrile neutropenia** . | Lymphopenia** . | Low IgG** . | HLH** . | DIC** . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Kantarjian et al. (2017)9 | Adult R/R ALL | Blinatumomab CD19 × CD3 | 267 | 69 (25.8%)/NA | NA/101(37.8%) | 47(17.6%)/NA | NA/57(21.3%) | NA/4(1.5%) | 16(6%)/ 7(2.6%) | 4(1.5%)/ 4(1.5%) | NA/NA |
| Topp et al. (2015)8 | Adult R/R ALL | Blinatumomab CD19 × CD3 | 188 | 38(20%)/ 27(14%) | 33(17%)/ 30(16%) | 21(11%)/16(8%) | 53(28%)/ 48(25%) | NA/NA | NA/NA | NA/NA | 4(2%)/NA |
| Locatelli et al. (2022)10 | Pediatric R/R ALL | Blinatumomab CD19 × CD3 | 110 | 20(18.2%)/ 5(4.5%) | 11(10%)/ 10(9.1%) | 22(20%)/16(14.5%) | NA/ 10(9.1%) | NA/NA | NA/NA | NA/NA | 1(0.9%)/ 1(0.9%) |
ALL, acute lymphoblastic leukemia; DIC, disseminated intravascular coagulation; HLH, hemophagocytic lymphohystiocytosis; NA, not available; R/R, relapsed/refractory.
N is the population considered for that extracted data, which may be the whole cohort or a subpopulation.
Adverse event, any grade(%)/grade ≥3(%).
B-cell non-Hodgkin lymphoma
B-NHL is a heterogeneous group of diseases, ranging from the aggressive subtypes such as diffuse large B-cell lymphoma (DLBCL) to the more indolent subtypes, including follicular lymphoma.11
DLBCL accounts for approximately 28% of NHL cases.12 DLBCL generally responds well to first-line chemoimmunotherapy, but 40% of patients eventually experience R/R disease and require subsequent treatment.13 For patients who are refractory to second-line chemotherapy and/or unfit for high-dose chemotherapy and autologous stem cell transplant (ASCT), as well as for patients who relapse after ASCT, the prognosis with conventional chemotherapy-based treatment is dismal. CAR-T cell therapy represents a very important improvement for patients, both in first relapse where it replaces ASCT as standard of care as well as in later treatment lines. However, the majority of chemo-refractory patients eventually fail the treatment for their relapsed disease, even with CAR-T cell therapy. The indolent lymphomas are also difficult to control with conventional chemoimmunotherapy in patients with refractory or early relapsing.14 A number of BsAbs have been developed to meet this need that target B-cell surface antigens (CD19, CD20, CD22, CD79) and the T-cell antigen CD3.
Glofitamab is a bivalent CD20-targeting and T-cell-engaging full-length antibody. In the first-in-human phase 1 trial of glofitamab in B-NHL, 2.9% of patients withdrew from treatment because of an AE. At the recommended phase 2 dose (RP2D), the occurrence of hematological toxicity ≥ grade 3 was as follows: neutropenia in 25.7%, thrombocytopenia in 8.6%, and febrile neutropenia in 5.7%, with G-CSF used in 21.6% of patients. Infections were seen in 42.9% at the RP2D, without fatal AEs in this group.15 Also, in the expansion cohort, the most common grade ≥3 AE was neutropenia (27% of patients). Infections ≥ grade 3 occurred in 15% of subjects.16
Epcoritamab also targets CD20 on the malignant B cells and CD3 on the T-cells. The phase 1/2 trial of epcoritamab in R/R B-NHL reported no cases of febrile neutropenia (N = 68).17 In the dose expansion cohort, however, neutropenia occurred in 21.7% of subjects, with febrile neutropenia in 4/157 (2.5%). G-CSF support was used in 16 patients (10.2%).18
Mosunetuzumab is another full-length IgG1-based humanized BsAb targeting CD20 and CD3. In group B of the phase 1 trial, for R/R B-NHL (dose escalation with step-up dosing during cycle 1), the most common ≥ grade 3 AE was neutropenia (25%), with a median duration of neutropenia of 9 days. Febrile neutropenia occurred in only 3.6% of patients, 22.3% received G-CSF, and anemia was reported in 18.8% of group B patients. There were four AE-related deaths in the trial, and one patient developed HLH secondary to Epstein-Barr virus infection.19 In the phase 2 expansion study in R/R follicular lymphoma (grade 1-3a), at baseline the patients had peripheral B-cell counts below normal limits. As expected, mosunetuzumab induced deep and durable B-cell depletion, while NK- and T-cell levels in general remained within normal limits. Most common ≥ grade 3 AE was neutropenia (27% of subjects), with a median duration of 8 days. Of the neutropenic patients, 18 of 26 (69%) received G-CSF support. There were no infection-related deaths.20 In the cohort of R/R DLBCL and transformed follicular lymphoma, data on lymphocytopenia were consistent with those of the previous cohort. The most common hematological AE was neutropenia (27.3%) and anemia (17%). Nineteen patients received G-CSF. There was one case of fatal sepsis.21
Odronextamab is a CD20 × CD3 completely human IgG4-based drug. In the first-in-human study of odronextamab in B-NHL (ELM-1), the distribution of AEs was consistent with other BsAbs. Most common grade ≥3 AEs were anemia (25%), lymphocytopenia (19%), neutropenia (19%) and thrombocytopenia (14%).22
Data on the hematological toxicities of the BsAbs in B-NHL are summarized in Table 2.
Incidence of hematological toxicities on bispecifics for non-Hodgkin lymphoma published trials
| Reference . | Population . | Drug/target . | N* . | Anemia& . | Neutropenia& . | Thrombocytopenia& . | Febrile neutropenia& . | Lymphopenia& . | Low IgG& . | HLH& . | DIC& . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hutchings et al. (2021)15 | Adult CD20+ NHL | Glofitamab CD20 × CD3 | 35 | NA/0(0%) | NA/9(25.7%) | NA/3(8.3%) | NA/2(5.7%) | NA/NA | NA/NA | NA/NA | NA/NA |
| Dickinson et al. (2022)16 | Adult CD20+ NHL | Glofitamab CD20 × CD3 | 154 | 47(30.5%)/ 10(6.5%) | 58(37.7%)/ 41(26.6%) | 38(24.7%)/12(7.7%) | NA/4(3%) | NA/5(3.2%) | NA/NA | NA/NA | NA/NA |
| Hutchings et al. (2021)17 | Adult CD20+ NHL | Epcoritamab CD20 × CD3 | 68 | 16(23%)/9(13%) | NA/17(25%) | NA/8(12%) | 0/0 | NA/4(6%) | NA/NA | NA/NA | NA/NA |
| Thieblemont et al. (2023)18 | Adult CD20+ NHL | Epcoritamab CD20 × CD3 | 157 | 28(17.8%)/ 16(10.2%) | 34(21.7%)/ 23(14.6%) | 21(13.4%)/9(5.7%) | 4(2.5%)/NA | NA/NA | NA/NA | NA/NA | NA/NA |
| Budde et al. (2022)20 | Adult CD20+ NHL | Mosunetuzumab CD20 × CD3 | 197 | 37(18.8%)/ 18(9.1%) | 56(28.4%)/ 50(25%) | 5(2.5%)/4(2%) | 7(3.6%)/ 7(3.6%) | 9(4.5%)/8(4%) | NA/NA | NA/NA | NA/NA |
| Budde et al. (2022)19 | Adult CD20+ NHL | Mosunetuzumab CD20 × CD3 | 90 | 12(14%)/7(8%) | 26(28%)/ 24(27%) | 9(10%)/4(4%) | 0/0 | NA/NA | NA/NA | NA/NA | NA/NA |
| Bartlett et al. (2023)21 | Adult CD20+ NHL | Mosunetuzumab CD20 × CD3 | 88 | 15(17%)/8(9.1%) | 24(27.3%)/ 19(21.6%) | NA/3(3.4%) | NA/5(5.7%) | NA/NA | NA/NA | NA/NA | NA/NA |
| Bannerji et al. (2022)22 | Adult CD20+ NHL | Odronextamab CD20 × CD3 | 145 | 55(38%)/36(25%) | 35(25%)/ 27(19%) | 40(28%)/20(14%) | NA/NA | 32(22%)/ 28(19%) | NA/NA | NA/NA | NA/NA |
| Reference . | Population . | Drug/target . | N* . | Anemia& . | Neutropenia& . | Thrombocytopenia& . | Febrile neutropenia& . | Lymphopenia& . | Low IgG& . | HLH& . | DIC& . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hutchings et al. (2021)15 | Adult CD20+ NHL | Glofitamab CD20 × CD3 | 35 | NA/0(0%) | NA/9(25.7%) | NA/3(8.3%) | NA/2(5.7%) | NA/NA | NA/NA | NA/NA | NA/NA |
| Dickinson et al. (2022)16 | Adult CD20+ NHL | Glofitamab CD20 × CD3 | 154 | 47(30.5%)/ 10(6.5%) | 58(37.7%)/ 41(26.6%) | 38(24.7%)/12(7.7%) | NA/4(3%) | NA/5(3.2%) | NA/NA | NA/NA | NA/NA |
| Hutchings et al. (2021)17 | Adult CD20+ NHL | Epcoritamab CD20 × CD3 | 68 | 16(23%)/9(13%) | NA/17(25%) | NA/8(12%) | 0/0 | NA/4(6%) | NA/NA | NA/NA | NA/NA |
| Thieblemont et al. (2023)18 | Adult CD20+ NHL | Epcoritamab CD20 × CD3 | 157 | 28(17.8%)/ 16(10.2%) | 34(21.7%)/ 23(14.6%) | 21(13.4%)/9(5.7%) | 4(2.5%)/NA | NA/NA | NA/NA | NA/NA | NA/NA |
| Budde et al. (2022)20 | Adult CD20+ NHL | Mosunetuzumab CD20 × CD3 | 197 | 37(18.8%)/ 18(9.1%) | 56(28.4%)/ 50(25%) | 5(2.5%)/4(2%) | 7(3.6%)/ 7(3.6%) | 9(4.5%)/8(4%) | NA/NA | NA/NA | NA/NA |
| Budde et al. (2022)19 | Adult CD20+ NHL | Mosunetuzumab CD20 × CD3 | 90 | 12(14%)/7(8%) | 26(28%)/ 24(27%) | 9(10%)/4(4%) | 0/0 | NA/NA | NA/NA | NA/NA | NA/NA |
| Bartlett et al. (2023)21 | Adult CD20+ NHL | Mosunetuzumab CD20 × CD3 | 88 | 15(17%)/8(9.1%) | 24(27.3%)/ 19(21.6%) | NA/3(3.4%) | NA/5(5.7%) | NA/NA | NA/NA | NA/NA | NA/NA |
| Bannerji et al. (2022)22 | Adult CD20+ NHL | Odronextamab CD20 × CD3 | 145 | 55(38%)/36(25%) | 35(25%)/ 27(19%) | 40(28%)/20(14%) | NA/NA | 32(22%)/ 28(19%) | NA/NA | NA/NA | NA/NA |
DIC, disseminated intravascular coagulation; HLH, hemophagocytic lymphohystiocytosis; NA, not available; NHL, non-Hodgkin lymphoma.
N is the population considered for that extracted data, which may be the whole cohort or a subpopulation.
Adverse event, any grade(%)/grade ≥3(/%).
Multiple myeloma
Incorporation of immunomodulatory drugs, proteasome inhibitors, and monoclonal antibodies, in triplet or quadruplet combinations, has dramatically improved the response rates and survival in MM.23,24 However, virtually all patients eventually relapse and ultimately develop disease resistant to conventional therapies. Like in the B-cell lymphomas, T-cell engaging therapies have the potential to improve the landscape of R/R MM.25,26
Teclistamab is a bispecific IgG4 antibody targeting BCMA on plasma cells and CD3 on the T-cells. In the Majestec-1 trial study, among patients treated at the RP2D (N = 40), hematological toxicities were the most frequent grade ≥3 AEs, with neutropenia, anemia, and thrombocytopenia occurring in 40%, 28%, and 20%, respectively. Infectious complications occurred in 45%, including 23% of grade ≥3. In the overall cohort (N = 157), 11% of patients received prophylactic immunoglobulin (Ig) therapy and 6% received Ig therapy for treatment of a hypogammaglobulinemia-related adverse event.27 In the pivotal phase 1-2 study with the RP2D of 1.5 mg/Kg, AEs followed the same pattern, with most common toxicities being hematological, including neutropenia in 70.9%, anemia in 52.1%, thrombocytopenia in 40%, and four cases (2.4%) of febrile neutropenia. Of 117 neutropenic patients, 91 received G-CSF at the discretion of physicians. Hypogammaglobulinemia occurred in 123 patients (74.5%), and immunoglobulin substitution was given in 65 of those 123 patients.28
Elranatamab also targets BCMA and CD3. In the first-in- human trial, for 58 safety-evaluable patients, hematological toxicities followed a similar pattern, with neutropenia ≥ grade 3 in 60% of patients and lymphopenia ≥ grade 3 in 64% of patients.29 In the phase 2 MagnetisMM-3 trial, with 123 patients, neutropenia ≥ grade 3 occurred in 43.1% and lymphopenia ≥ grade 3 in 23.6%.30
ABBV-383 is a fully human monoclonal IgG4 targeting BCMA and CD3. In its phase 1 trial, neutropenia occurred in 37% of patients (≥3 in 34%) and hypogammaglobulinemia in 14%. It is described that 29 of 124 (23%) of patients required Ig substitution.31
Alnuctamab, targeting BCMA × CD3, showed neutropenia ≥ grade 3 in 30% and anemia ≥ grade 3 in 17%.32
Talquetamab is a bispecific directed to GPRC5D on plasma cells and CD3 on T-cells. MonumenTAL-1 was the first-in- human, phase 1 study with 232 R/R MM subjects. As other BiTEs, most common grade ≥3 AEs were hematological. For RP2D SC cohorts (N = 130), grade ≥3 hematological events were neutropenia (45.4%), anemia (27.7%), lymphopenia (32.3%), and thrombocytopenia (20%). Low IgG serum levels (<500 mg/dL) were observed in 71% to 87% of patients, depending on the cohort.33
In the ongoing phase 1 study of the GPRC5D × CD3 targeting BsAb forimtamig (N = 105), hematological toxicities are infrequent, with anemia ≥ grade 3 ranging from 5.2% to 13.7% and neutropenia ≥ grade 3 from 11.8% to 16.7% for different routes of administration.34
Cevostamab targets FcRH5 and CD3. FcRH5 is expressed on B-cells and is highly expressed on MM plasma cells. In the phase I data (NCT 03275103) of cevostamab in R/R MM anemia, ≥ grade 3 was seen in 21.9% of patients, neutropenia ≥3 in 30.1% of patients, with one reported case of fatal HLH.35
Data on the hematological toxicities of the BsAbs in MM are summarized in Table 3. The higher rates of cytopenias compared to data from the B-NHL studies perhaps is no surprise, as MM is a disease of the bone marrow, more so than B-NHL. Nevertheless, given that MM patients are generally more prone to infections than B-NHL patients, the frequent cytopenias are clinically important.
Incidence of hematological toxicities on bispecifics for multiple myeloma published trials
| Reference . | Population . | Drug/target . | N* . | Anemia& . | Neutropenia& . | Thrombocytopenia& . | Febrile Neutropenia& . | Lymphopenia& . | Low IgG& . | HLH& . | DIC& . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Usmani et al. (2021)27 | Adult R/R MM | Teclistamab BCMA × CD3 | 40 | 20(50%)/ 11(28%) | 26(65%)/ 16(40%) | 18(45%)/8(20%) | NA/NA | 7(9.6%)/ 7(9.6%) | 14(9%)/NA | NA/NA | NA/NA |
| Moreau et al. (2022)28 ¥ | Adult R/R MM | Teclistamab BCMA × CD3 | 165 | 86(52.1%)/ 61(37%) | 117(70.9%)/ 106(64%) | 66(40%)/35(21%) | 4(2.4%)/NA | 57(34.5%)/ 54(32.7%) | 123(74.5%)/NA | NA/NA | NA/NA |
| D'Souza et al. (2022)31 | Adult R/R MM | ABBV-383 BCMA × CD3 | 124 | 36(29%)/ 20(16%) | 46(37%)/ 42(34%) | 29(23%)/15(12%) | NA/NA | 19(15%)/16(13%) | 17(14%)/NA | NA/NA | NA/NA |
| Chari et al. (2022)33 # | Adult R/R MM | Talquetamab GPRC5D × CD3 | 130 | 63(48.5%)/ 36(27.7%) | 67(51.5%)/ 59(45.4%) | 39(30%)/26(20%) | NA/3(2.3%) | 42(32.3%)/ 42(32.3%) | NA/NA | NA/NA | NA/NA |
| Reference . | Population . | Drug/target . | N* . | Anemia& . | Neutropenia& . | Thrombocytopenia& . | Febrile Neutropenia& . | Lymphopenia& . | Low IgG& . | HLH& . | DIC& . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Usmani et al. (2021)27 | Adult R/R MM | Teclistamab BCMA × CD3 | 40 | 20(50%)/ 11(28%) | 26(65%)/ 16(40%) | 18(45%)/8(20%) | NA/NA | 7(9.6%)/ 7(9.6%) | 14(9%)/NA | NA/NA | NA/NA |
| Moreau et al. (2022)28 ¥ | Adult R/R MM | Teclistamab BCMA × CD3 | 165 | 86(52.1%)/ 61(37%) | 117(70.9%)/ 106(64%) | 66(40%)/35(21%) | 4(2.4%)/NA | 57(34.5%)/ 54(32.7%) | 123(74.5%)/NA | NA/NA | NA/NA |
| D'Souza et al. (2022)31 | Adult R/R MM | ABBV-383 BCMA × CD3 | 124 | 36(29%)/ 20(16%) | 46(37%)/ 42(34%) | 29(23%)/15(12%) | NA/NA | 19(15%)/16(13%) | 17(14%)/NA | NA/NA | NA/NA |
| Chari et al. (2022)33 # | Adult R/R MM | Talquetamab GPRC5D × CD3 | 130 | 63(48.5%)/ 36(27.7%) | 67(51.5%)/ 59(45.4%) | 39(30%)/26(20%) | NA/3(2.3%) | 42(32.3%)/ 42(32.3%) | NA/NA | NA/NA | NA/NA |
DIC, disseminated intravascular coagulation; HLH, hemophagocytic lymphohystiocytosis; MM, multiple myeloma; NA, not available; R/R, relapsed/refractory; RP2P, recommended phase 2 dose.
N is the population considered for that extracted data, which may be the whole cohort or a subpopulation.
Adverse event, any grade(%)/grade ≥3(/%).
Data from cohort of phase 1 and cohort of phase 2 that used the RP2D; N = 165.
Data from all subcutaneous cohorts (supplement material); N = 130.
How to manage hematological toxicities to biscpecific antibodies
Hematological toxicities are among the most common adverse events and the most common grade ≥3 AEs related to bispecific antibody therapy. Although we do not have prospective trials guiding the management of these toxicities, data from the early-phase trials provide some guidance. Different authors have proposed guidelines on these toxicities3,4 that are mainly extrapolated from similar situations in the context of CAR-T cell therapy and hematopoietic stem cell transplant. The following points represent a proposal that should take into account institutional standards:
- A)
Anemia and thrombocytopenia: No clear recommendations can be made regarding eryhtropoietin and thrombopoitin agonists because we only have anecdotal reports of beneficial effects in this context.36 Transfusions should be administered according to clinical indication.
- B)
Lymphopenia and hypogammaglobulinemia: They may be present before the initiation of BsAbs. We propose monthly monitoring and replenishing IgG to keep levels at ≥400 mg/dL. In the case of repetitive infections, replenishing to ≥600 mg/dL should be considered, in keeping with most recommendations for patients with hypogammaglobulinemia after CART.37 Both lymphopenia and hypogammaglobulinemia can last many months after discontinuation of BsAb therapy (as discussed in the clinical case).
- C)
Neutropenia: We advise using G-CSF in case of neutropenia <1000/mm3 while avoiding dosing delays. The pathogenesis behind neutropenia remains elusive, if it is not due to infection or dysplasia. Cytokine-mediated damage to hemopoietic precursors or an effect similar to what is seen in delayed-onset neutropenia post-rituximab could be speculated.37,38
- D)
Broad-spectrum antibiotics for primary antibacterial prophylaxis: They may be considered in individual cases but cannot be generally recommended. Antifungal prophylaxis should be considered in cases of neutropenia <500/mm3 for more than 7-14 days in patients with recent invasive fungal infections or long-term high-dose corticosteroid use.
- E)
We recommend prophylaxis for herpes simplex virus and/or varicella-zoster virus and prophylaxis against Pneumocystis jirovecii during treatment and until total lymphocyte and CD4 counts approach normal levels.
- F)
Hemophagocytic lymphohystiocytosis (HLH): This hyperinflammatory state is most often driven by infection, malignancy, autoimmune diseases, or drugs. It is the extreme end of a hyperinflammatory continuum and should be regarded as a potential complication of BsAb, along with macrophage activation syndrome. HLH can occur immediately post-treatment in the direct context of CRS, but more classic HLH is typically delayed from treatment onset. Diagnosis can be supported by HLH-2004 criteria38,39 or the calculation of the HScore.40 In a scenario of BsAbs-induced HLH, the use of tocilizumab and corticosteroids is recommended (extrapolated from the experience in CAR T cell-treatment). HLH also occurs in rare instances of checkpoint inhibiting therapies and often responds to glucocorticoid monotherapy.4,41 Other anti-inflammatory agents (e.g., anti-IL1ra, JAK-inhibition, anti-IFNγ) should be considered in refractory cases. Etoposide and cytostatic agents probably play a limited role in this context.4,42 It is mandatory to search for causal pathogens (i.e., Epstein-Barr virus, cytomegalovirus, COVID-19, Aspergillus fumigatus), including local triggers like leishmaniasis and rickettsial disease or histoplasmosis in the US. We use microbiome evaluation of bone marrow examinations in HLH patients because some infections may be latent for many years,43 and some infections (e.g., the newly diagnosed HLH-trigger Neoehrlichia mikurensis) are undetectable by serology and blood cultures).4,44
- G)
Disseminated intravascular coagulation: This is a very rare but potentially fatal complication, especially in the context of CRS or sepsis. There is no data to suggest that management of BsAb-associated DIC should differ from that of conventional DIC.
- H)
In all cases of severe hematological toxicities, discontinuation of BsAb therapy should be carefully considered.
BsAbs have significant potential for adverse events. It is important to acknowledge that the agents they replace may have worse infectious complications and less efficacy (e.g., blinatumomab vs conventional chemotherapy).9 Prevention of infectious complications remains important. This includes immunizations, immunoglobulin substitution, and G-CSF support. Physicians should be aware of the risk of hyperinflammation leading to HLH in the context of CRS.
Conflict-of-interest disclosure
Luiz Henrique de Assis: no competing financial interests to declare.
Daniel El Fassi: no competing financial interests to declare.
Martin Hutchings: honoraria: AbbVie, AstraZeneca, Celgene, Genmab, Janssen, Merck, Roche, and Takeda; research support (paid to the institution): AbbVie, AstraZeneca, Bristol Myers- Squibb, Celgene, Genentech, Genmab, Incyte, Janssen, Merck, Novartis, Roche, and Takeda.
Off-label drug use
Luiz Henrique de Assis: This paper discusses use of drugs which are still under early-phase investigation. Blinatumumab is approved for the treatment of r/r B-ALL and for the treatment of B-ALL n first or second complete remission with minimal residual disease. Glofitamab and Epcoritamab are approved for the treatment of r/r DLBCL with at least two prior lines of treatment. Mosunetuzumab is approved for the treatment of r/r FL with at least two prior lines of treatment. Teclistamab, Talquetamab, and Elranatamab are approved for the treatment of r/r MM with at least four prior lines of therapy, including a proteasome inhibitor, an immunomodulatory agent and an anti-CD38 monoclonal antibody. All other drugs discussed are purely investigational and their use regarded as investigational or off-label, including the use of tocilizumab in the treatment of HLH.
Daniel El Fassi: This paper discusses use of drugs which are still under early-phase investigation. Blinatumumab is approved for the treatment of r/r B-ALL and for the treatment of B-ALL n first or second complete remission with minimal residual disease. Glofitamab and Epcoritamab are approved for the treatment of r/r DLBCL with at least two prior lines of treatment. Mosunetuzumab is approved for the treatment of r/r FL with at least two prior lines of treatment. Teclistamab, Talquetamab, and Elranatamab are approved for the treatment of r/r MM with at least four prior lines of therapy, including a proteasome inhibitor, an immunomodulatory agent and an anti-CD38 monoclonal antibody. All other drugs discussed are purely investigational and their use regarded as investigational or off-label, including the use of tocilizumab in the treatment of HLH.
Martin Hutchings: This paper discusses use of drugs which are still under early-phase investigation. Blinatumumab is approved for the treatment of r/r B-ALL and for the treatment of B-ALL n first or second complete remission with minimal residual disease. Glofitamab and Epcoritamab are approved for the treatment of r/r DLBCL with at least two prior lines of treatment. Mosunetuzumab is approved for the treatment of r/r FL with at least two prior lines of treatment. Teclistamab, Talquetamab, and Elranatamab are approved for the treatment of r/r MM with at least four prior lines of therapy, including a proteasome inhibitor, an immunomodulatory agent and an anti-CD38 monoclonal antibody. All other drugs discussed are purely investigational and their use regarded as investigational or off-label, including the use of tocilizumab in the treatment of HLH.