Abstract
Estrogen exposure, in the setting of pregnancy, the postpartum state, combined hormonal contraceptives (CHCs), or hormone therapy use, has been clearly associated with increased rates of venous thromboembolism (VTE). Although recurrence rates are low in these settings, up to 70% of anticoagulated menstruating individuals experience abnormal or heavy menstrual bleeding (HMB), which commonly results in iron deficiency with or without anemia. Patients taking rivaroxaban appear to experience higher rates of HMB compared with those on apixaban, dabigatran, or warfarin. HMB can often be diagnosed in a single visit with a good menstrual history assessing for factors with a known association with increased or heavy bleeding, such as changing pads or tampons more often than every 2 hours, clots larger than a quarter, and iron deficiency (ferritin <50 ng/mL). HMB can be managed with hormonal therapies, including those associated with VTE risk, such as CHCs and depot-medroxyprogesterone acetate (DMPA). In many cases, continuing CHCs or DMPA while a patient is therapeutically anticoagulated is reasonable, so long as the therapy is discontinued before anticoagulation is stopped. Modification of the anticoagulation regimen, such as decreasing to a prophylactic dose in the acute treatment period, is not currently recommended. For patients who are currently pregnant, low-molecular-weight heparin (LMWH) is still standard of care during pregnancy; routine monitoring of anti–factor Xa levels is not currently recommended. Warfarin or LMWH may be considered in the postpartum setting, but direct-acting oral anticoagulants are currently not recommended for lactating patients.
Learning Objectives
Review unique features of hormone-associated thrombosis
Learn diagnosis and management of heavy menstrual bleeding in the anticoagulated menstruating individual
Review management of anticoagulation during pregnancy
CLINICAL CASE
A 26-year-old woman presents to the emergency department with dyspnea. Medical history is significant for heavy menses, for which she was started on combined hormonal contraceptive (CHC) pills. She undergoes computed tomography pulmonary angiogram, which shows a pulmonary embolism. She is subsequently discharged on rivaroxaban, her CHCs are discontinued, and she is referred to hematology.
Hormone-associated thrombosis
Estrogen remains the chief driver of thrombosis risk associated with CHC use. However, different progesterone components likely also play a role, as recent data suggest third- and fourth-generation CHCs (those containing gestodene, desogestrel, norgestimate, or drospirenone) carry a higher risk of venous thromboembolism (VTE) (Table 1) compared with second-generation preparations containing levonorgestrel or norgestrel.1 CHCs carry an odds ratio of 4.03 (95% confidence interval [CI], 1.83-8.89) for VTE, while estrogen alone has an odds ratio of 1.81 (95% CI, 1.06-3.09), although this varies depending on the dose and type of estrogen used. Noncontraceptive estrogen plus progestin carries an odds ratio of 2.53 (95% CI, 1.38-4.63).2 Studies regarding route of contraceptive administration report conflicting results.1 Transdermal estrogen at hormone replacement dosages does not appear to increase the risk of VTE.2 While most estrogen-associated VTE events occur during the first months to year of use, the window of increased risk can persist for 3 months or longer after discontinuation, depending on therapy type.
Thrombosis associated with high-estrogen states such as pregnancy are most often in the deep veins of the legs and pulmonary arteries.1 CHCs use has also been associated with less common sites of thrombosis such as cerebral venous sinus thrombosis, mesenteric venous thrombosis, and retinal vein occlusion, although absolute risk remains low (Figure 1).1
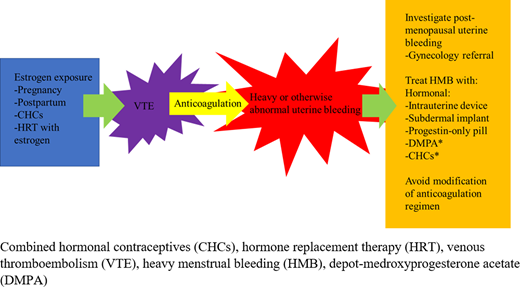
Approach to anticoagulation prescription in the menstruating individual.
Importantly, VTE occurring during pregnancy or the postpartum state, or in association with CHCs or hormone replacement therapy, is associated with a reduced risk of recurrence after anticoagulant discontinuation compared with unprovoked VTE (6% vs 17% at 5 years for estrogen users vs nonusers at time of first VTE).2,3 Women also experience a lower risk of recurrent VTE compared with men in general.4
Data on arterial thrombosis suggest that estrogen is associated with an increased risk of myocardial infarction and stroke, although absolute risk is low (21.4 strokes and 10.1 myocardial infarctions per 100 000 women aged 15-49 years taking CHCs).1
Diagnosis of heavy menstrual bleeding
Heavy menstrual bleeding (HMB) has historically been defined as menstrual blood loss of >80 mL per cycle.5 The pictorial blood loss assessment chart (PBAC) can be used to gauge the number and degree of saturation of menstrual pads and tampons used during menses, with a sensitivity and specificity >80% for HMB for scores above 100.6 The PBAC does not account for differences in absorbency between products or the potential use of other menstrual products, such as menstrual cups and discs, and can be quite time-consuming.
The Menorrhagia 1 study identified 3 predictors of menstrual blood loss >80 mL/cycle: patient-reported passage of clots >1 in. in diameter, low ferritin, and the need to change menstrual products more frequently than every hour during the heaviest day of menses.7
A more recent and easily applied, although subjective, definition of HMB recommended by the International Federation of Gynecology and Obstetrics and the National Institute for Health and Care Excellence Guidelines of the National Institute of Health and Clinical Excellence of the United Kingdom is “excessive menstrual blood loss which interferes with the woman's physical, emotional, social and material quality of life, and which can occur alone or in combination with other symptoms.”8
HMB on anticoagulation
Overall, women and men on anticoagulation have similar rates of bleeding if uterine/vaginal bleeding is excluded (2.9% patient-years for women vs 2.1% for men).9 If included, then anticoagulated women have higher rates of bleeding than men (3.5% vs 2.1%). Around 70% of menstruating individuals on anticoagulation have HMB or other abnormal uterine bleeding.10 Lack of reporting and underdiagnosis have made direct comparisons between drugs challenging, although rivaroxaban and edoxaban have both demonstrated a higher risk of uterine bleeding compared with warfarin.11,12 Apixaban appears to have similar risk to warfarin and low-molecular-weight heparin (LMWH),11 and dabigatran may have a lower risk compared with warfarin.13 A 2021 retrospective study of women taking apixaban, rivaroxaban, or warfarin found that, compared with apixaban and warfarin, women taking rivaroxaban were more likely to have HMB.14 Patients taking rivaroxaban also experience longer menses and are more likely to require medical or surgical intervention.10 People who experience HMB while taking rivaroxaban are at up to 5-fold increased risk of recurrent VTE compared with those who did not experience HMB, possibly due to increased rates of anticoagulation modification, including shortened courses and missed doses.15
Evaluating for HMB on anticoagulation
HMB should be assessed for at the time anticoagulation is initiated and should include current and past symptoms, which are of particular importance for individuals who develop VTE while taking CHCs, which may have initially been prescribed for HMB. Patients reporting symptoms consistent with HMB should undergo a complete blood count (CBC) and ferritin check. While many factors contribute to choosing an anticoagulant agent, in patients with a history of HMB, consideration should be given to options other than rivaroxaban, and shared decision-making should include consideration for the risk of new or worsened HMB.
Patients who do not report symptoms of HMB should be educated and advised to notify the prescriber if these symptoms develop. Patients should be asked about HMB symptoms again on follow-up evaluations and at least annually for individuals on long-term anticoagulation. HMB can develop at any point, particularly during adolescence and the perimenopausal years when ovulation and bleeding can be most irregular. Periodic laboratory monitoring with CBC and ferritin is also appropriate in patients having menses, with frequency to be determined based on clinical context and past or current history of iron deficiency/anemia.
Managing HMB on anticoagulation
Hormonal therapies
Hormonal therapy (or continuation of hormonal therapy) can be safe and effective in managing HMB (Table 2). Hormonal therapies also provide contraception, which is important given the increased risk of VTE during pregnancy, as well as for those taking warfarin, a teratogen, and direct-acting oral anticoagulants (DOACs), which cross the placenta. The levonorgestrel intrauterine device (LNG-IUD) can reduce median menstrual blood loss by 80% after 4 months of use,16 with a 44% amenorrhea rate at 6 months and 50% at 1 year.17 The etonogestrel subdermal implant can result in amenorrhea in around 20% of cases, although irregular spotting is common and can be bothersome to patients.18 Both are >99% effective for pregnancy prevention. Neither the etonogestrel implant nor the LNG-IUD have been linked with a significant increase in VTE risk.19–21 One study suggested a trend toward increased VTE with the etonogestrel implant, but this was not statistically significant (relative risk, 1.4; CI, 0.6-3.4) and represents a favorable risk profile compared to pregnancy, with the added benefit of potentially decreasing menstrual blood loss and enabling continued toleration of anticoagulation.19
Depot-medroxyprogesterone acetate (DMPA) induces amenorrhea in 55% of users at 1 year and 68% at 2 years.22 It too is >99% effective in pregnancy prevention when used perfectly. However, it is associated with a 2.2- to 3-fold increased risk of VTE and is therefore not preferred in the absence of anticoagulation for women at risk of recurrent VTE.2,20
Progestin-only pills result in amenorrhea in 5% to 10% of women but often cause other menstrual irregularities and require strict adherence to maintain contraceptive efficacy.23
CHC pills, including both an estrogen and a progesterone component, can be prescribed with or without scheduled interruptions in hormone exposure, allowing for the possibility of inducing amenorrhea. Breakthrough bleeding, which commonly occurs with this method, can be reduced by “scheduling” bleeding periodically (eg, every 3 months). No difference in rates of recurrent VTE were found in post hoc analysis of the EINSTEIN randomized controlled trial data of rivaroxaban vs enoxaparin/vitamin K antagonists in a comparison of women who were and were not prescribed combined contraceptives while on anticoagulation, although high-quality data are still lacking.24
As discontinuation of CHCs can precipitate bleeding, menstruating individuals started on anticoagulation for VTE in the setting of stopping CHCs are particularly vulnerable to HMB. Consideration should be given to continuing the current hormonal therapy while anticoagulation continues, transitioning to an alternative therapy such as an LNG-IUD or even starting hormonal therapy if the patient develops HMB while anticoagulated. While a patient can stay on CHCs or DMPA while anticoagulated, transition to an alternative contraceptive method should be arranged prior to anticoagulation discontinuation. Likewise, if CHCs or DMPA are initiated in the setting of recent or remote VTE, therapeutic anticoagulation should be in place first.
Antifibrinolytics
While useful in the nonanticoagulated menstruating patient for control of HMB, studies of the combination of anticoagulants and antifibrinolytics are lacking—although one, the MEDEA trial, is currently ongoing.25 This combination may be a consideration if use enables continuation of anticoagulation without interruptions. If used, antifibrinolytics would ideally be avoided during the acute phase of VTE, in order to permit fibrinolysis of existing thrombus.
Modification of anticoagulation
Temporary or early discontinuation of anticoagulation results in increased risk of recurrent VTE and is therefore not recommended, particularly in patients with VTE in the past 3 months.15 A study of women treated with 20 mg or 10 mg rivaroxaban or aspirin after completing 6 to 12 months of therapeutic anticoagulation demonstrated no statistical difference among the groups in menstrual flow duration or intensity, raising questions about theoretical benefits of dose adjustments.26 More prospective studies of this strategy are needed, ideally with a variety of DOACs. Use of an alternative anticoagulant may be beneficial, particularly for menstruating patients who experience HMB while taking rivaroxaban, in which case apixaban, dabigatran, or warfarin may be considered.
Gynecology referral
Abnormal uterine bleeding while on anticoagulation does not necessarily mean the anticoagulation is the chief or only culprit. Uterine bleeding in a postmenopausal patient requires gynecologic evaluation for etiologies such as endometrial cancer. Painful and heavy menses can be associated with fibroids or endometriosis, among other causes, which can benefit from gynecologic interventions. Additionally, gynecologists may offer surgical therapies, such as endometrial ablation, which may be of great benefit in reducing HMB.
CLINICAL CASE (Continued)
The patient presents for follow-up. She has been taking her rivaroxaban as prescribed but has been experiencing increased menstrual bleeding, saturating 2 tampons every hour during her heaviest day and passing several clots of at least an inch in size. Concerned for HMB, you order a CBC, which shows a hemoglobin of 12.0 g/dL and a ferritin that returns at 15 ng/mL. Although she is not currently anemic, she is iron deficient. You discuss options for iron replacement and treatment of her HMB. As she has had nausea with oral iron in the past, you decide to prescribe intravenous iron. She also decides to have an LNG-IUD placed and to change from rivaroxaban to apixaban.
Pregnancy
Risk of VTE increases 4-fold during pregnancy and 5-fold or more during the postpartum period compared with nonpregnant women of childbearing age.2,27 Preconception counseling is key for patients on anticoagulation who may become pregnant. LMWH remains the mainstay of anticoagulation in pregnancy due to warfarin's teratogenicity, although warfarin remains an option during breastfeeding.28,29 While warfarin is known to be a teratogen, limited data on DOACs prevent a more definitive statement on their risk.
Given the known teratogenicity and relatively long half-life, we favor transitioning patients on long-term warfarin for VTE to LMWH when contraception is discontinued in the hopes of a pregnancy, while recognizing the controversy and lack of data guiding these decisions. Some providers may opt to continue warfarin until conception, understanding that there is significant risk of teratogenicity if pregnancy is not detected early and a switch to LMWH made before 6 weeks' gestation. In the absence of clear evidence of an increased risk of teratogenicity with DOACs in very early pregnancy, we offer continuation of a DOAC until the first positive pregnancy test, at which time the transition should be made to LMWH.28 However, given the paucity of data overall, some patients and/or providers may favor switching to LMWH while attempting conception. DOACs do appear to be able to cross the placenta, and prescribing information identifies some reported fetal complications in animals: dabigatran (fetal growth restrictions, birth defects), edoxaban (gallbladder anomalies), rivaroxaban (slowed or progressed ossification, increase of common malformations and placental changes), and apixaban (none).30 The significance of this in humans when exposure is limited only to the time between conception and a positive pregnancy test is unclear. A retrospective cohort study found that of 336 pregnancies complicated by DOAC exposure, 74 ended in miscarriages, and 6 to 12 had major birth defects for which potential association with the DOAC could not be excluded.30 The birth defects that occurred were also variable, not following a pattern such as would be expected if induced by drug exposure. In this study, DOAC-exposed pregnancies had a 2% to 4% rate of birth defects compared with 6% reported for warfarin and ~3% for unexposed pregnancies. Likewise, the 22% rate of miscarriages in DOAC-exposed pregnancies is similar to the general population's risk and lower than the 30% miscarriage rate in vitamin K antagonists-exposed pregnancies.
Informed, shared decision-making is critical in this situation. Some individuals may prioritize absolute minimization of risk to a fetus over the inconvenience of injections while others may prioritize avoiding additional months of LMWH (in addition to those required during pregnancy) over an unclear but likely very small risk of brief early pregnancy exposure to a DOAC.
Patients who develop new VTE during pregnancy should be therapeutically anticoagulated throughout pregnancy and for 6 weeks after, or for a total of 3 months, whichever is longer. There are no data to support routine monitoring and dose adjustments based on anti–factor Xa levels in pregnancy.29 However, certain patients, such as those with a history of VTE on anticoagulation and/or variable renal function, may benefit from this approach.
In women with prior VTE in the setting of high-estrogen states, thromboprophylaxis is recommended in both the antepartum and postpartum periods.1 Antepartum prophylaxis is not recommended in those who had VTE in the setting of a nonhormonal, temporary, now-resolved risk factor with no other risk factors, although postpartum prophylaxis is still recommended.29 Recommendations regarding thromboprophylaxis to prevent first-time VTE in women with thrombophilias, based on American Society of Hematology 2018 guidelines, can be found in Table 3.29 Recent data from the Highlow study, presented at the 2022 International Society on Thrombosis and Haemostasis meeting, which looked at prophylactic and intermediate-dose anticoagulation antepartum and postpartum, suggest a benefit of intermediate-dose LMWH (once daily, roughly half therapeutic dose) for preventing superficial but not deep vein thrombosis without an increased risk of bleeding.31 The authors concluded that fixed, prophylactic dose LMWH is safe and efficacious for prevention of VTE and may benefit from increased access to neuraxial anesthesia. Discussion regarding indications for thrombophilia testing is beyond the scope of this article. Those with antiphospholipid syndrome on anticoagulation should use therapeutic LMWH or unfractionated heparin (UFH) during pregnancy, typically in combination with low-dose aspirin.1
Anticoagulation in the setting of mechanical heart valves and pregnancy is beyond the scope of this article. However, risks to the fetus (greatest with warfarin) and risks to the mother (greatest with LMWH) must be carefully weighed and discussed.
Delivery
Anticoagulation management around delivery is a controversial topic and one with little data. Pregnant individuals taking LMWH at the time of delivery have been shown to be at increased risk of postpartum hemorrhage (relative risk, 1.45; 95% CI, 1.02-2.05) compared with those not taking LMWH but with a similar mean blood loss.32 However, the presence of therapeutic or even prophylactic levels of anticoagulation may preclude placement of epidural analgesia and, in the case of unplanned cesarean section, necessitate general anesthesia for pain control. For this reason, many practitioners favor scheduled inductions rather than waiting for spontaneous labor, to ensure that therapeutic anticoagulation may be held at least 24 hours in advance (12 hours for prophylactic dosing) to ensure neutralization of an anticoagulant effect prior to labor or epidural placement. Other practitioners favor switching to UFH, with its shorter half-life, as the due date approaches, in the hopes of improving odds of epidural access and delivery off anticoagulation. However, it should be noted that subcutaneous doses >7500 to 10 000 units twice daily still necessitate a 12-hour separation from last dose and a normal partial thromboplastin time (PTT) prior to epidural placement. Doses >10 000 units twice daily or 20 000 units total daily require a separation of 24 hours from last dose and a normal PTT.33 Additionally, prolonged use of subcutaneous UFH can result in a “depot effect,” resulting in prolongation of PTT for >24 hours after the last dose.34 Our practice is to continue LMWH up until the time of delivery. The decision-making process around induction is shared among the patient, obstetrician, and hematologist. We favor induction for patients on therapeutic anticoagulation in order to avoid the need for general anesthesia at the time of delivery if unplanned cesarean section is required, and we counsel all patients on this potential risk as well as the option of planned induction prior to delivery.
CLINICAL CASE (Continued)
Unfortunately, your patient experiences a second VTE event, this one unprovoked, and requires indefinite anticoagulation. Several months later, she has her LNG-IUD removed in the hopes of achieving pregnancy. After a discussion of the risks and benefits, she opts to continue on apixaban until her first positive pregnancy test. A few months later, after a positive pregnancy test, she transitions to LMWH, which she continues for the duration of her pregnancy. Following discussion with her obstetrician, she elects to pursue a planned induction and receives her last dose of LMWH 24 hours prior. She wishes to breastfeed and ultimately opts to transition to warfarin for the duration of breastfeeding before transitioning back to apixaban.
In summary, estrogen-associated thrombosis tends to occur in the pulmonary arteries and deep veins of the legs, and thrombosis can be associated not only with estrogen but also with DMPA. Individuals who menstruate may benefit from avoiding rivaroxaban due to its association with HMB, and patients who experience HMB while on anticoagulation may benefit from trialing an alternative anticoagulant. CHCs and DMPA can be continued while on anticoagulation, and indeed some form of hormonal birth control can be helpful in reducing the risk of HMB. However, if transitioning off anticoagulation, CHCs or DMPA should be discontinued and an alternative form of contraception started prior to stopping anticoagulation. LMWH is still the mainstay of therapy during pregnancy.
Conflict-of-interest disclosure
Emma DeLoughery: no competing financial interests to declare.
Bethany Samuelson Bannow: no competing financial interests to declare.
Off-label drug use
The only off-label drug use discussed in this article was the use of the subdermal implant for treatment of heavy menstrual bleeding, although we recommend it both for its contraceptive use (which it is approved for) and the benefit of reducing menstrual blood loss, which has been demonstrated in studies.