Abstract
Survivors of childhood hematologic malignancies are at a substantially higher risk of developing subsequent neoplasms (SNs) when compared with the general population. SNs commonly observed in this population include basal cell carcinoma, brain tumors, thyroid cancer, breast cancer, bone tumors, and sarcoma. Radiation is the primary therapeutic exposure associated with the development of these SNs. There is emerging evidence of an association between chemotherapeutic exposures (alkylating agents/anthracyclines) and the development of SNs. Despite a strong dose-dependent association between therapeutic exposures and SN risk, there is significant interindividual variability in the risk for SNs for any given dose of therapeutic exposure. This interindividual variability in risk suggests the role of genetic susceptibility. This article describes the clinical and molecular epidemiology of SNs commonly observed in survivors of childhood hematologic malignancies and also highlights some of the work focusing on the development of risk prediction models to facilitate targeted interventions.
Learning Objectives
Understand the role of genetic susceptibility in the development of subsequent neoplasms in childhood cancer survivors
Explore the role of risk prediction models in identifying survivors at highest risk of subsequent neoplasms
CLINICAL CASE
MH is a 38-year-old woman who was diagnosed with nodular sclerosing Hodgkin lymphoma, stage IIA, at the age of 16 years and treated with splenectomy and radiation to the mantle (42 Gy in 24 fractions) and para-aortic fields (42 Gy in 24 fractions). She did not receive chemotherapy. She has now been off therapy for 22 years. She recently underwent mammography, which revealed a suspicious 0.8-cm lesion in the left breast. Biopsy was positive for infiltrating ductal carcinoma, grade 2, estrogen receptor positive (99%) and progesterone receptor negative and human epidermal growth factor receptor 2 negative by fluorescence in situ hybridization. Staging was T1b, N0, M0. She opted for bilateral mastectomy, has plans to undergo bilateral breast reconstruction surgery, and is currently receiving hormonal therapy (letrozole).
Subsequent neoplasms (SNs) are defined as histologically distinct cancers developing after the occurrence of a first type of cancer. Childhood cancer survivors are at a 3- to 12-fold higher risk of SNs when compared with the general population.1 The 15-year risk of SNs has declined for those diagnosed in the 1990s compared to those diagnosed in the 1970s.2 Nonetheless, the SN risk remains elevated for those diagnosed in the 1990s, and SNs are the leading cause of nonrelapse mortality.3 The following sections describe the clinical and molecular epidemiology of SNs commonly observed in survivors of childhood hematologic malignancies.
Clinical epidemiology of SNs
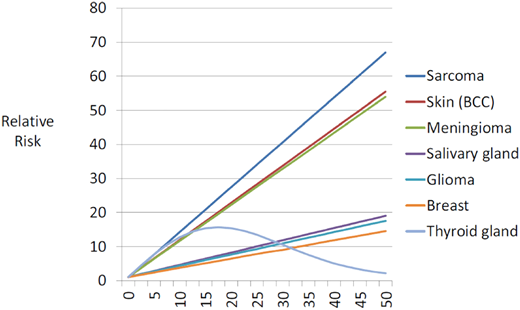
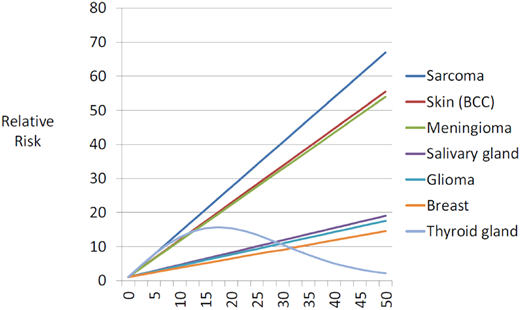
The most common subsequent solid tumors observed in childhood cancer survivors include skin cancer (primarily basal cell carcinoma [BCC]), breast cancer, thyroid cancer, brain tumors, soft tissue sarcoma, bone tumors, gastrointestinal cancers, and salivary gland tumors.1,4 Exposure to radiation is associated with a higher risk of solid tumors; female sex, younger age at exposure to radiation, smoking, infection with oncogenic viruses (Epstein-Barr virus; hepatitis B and C viruses) and germline genetic variants modify the risk.4 There is a dose-dependent association between radiation and solid tumors (Figure 1),5 with the highest risk of tumors developing within or near the treatment field. Alkylating agents and anthracycline chemotherapies also increase the risk of breast cancer, soft tissue sarcoma, thyroid cancer, and melanoma.6,7 The lower risk of solid tumors observed in the 1990s when compared with the 1970s is primarily due to a reduction in therapeutic radiation dose.2
Fitted radiation-dose response by type of second cancer.
Reproduced from Inskip et al5 with permission from Elsevier, copyright 2016.
Fitted radiation-dose response by type of second cancer.
Reproduced from Inskip et al5 with permission from Elsevier, copyright 2016.
Clinical epidemiology of subsequent breast cancer
The incidence of breast cancer among young women treated for childhood cancer with chest radiation ranges from 5% to 14% by age 40.8 The latent period after chest radiation ranges from 7 years to 10 years, and the risk of breast cancer increases in a linear fashion with radiation dose (Figure 1).5 Forty percent of identified cases develop contralateral disease. Women treated for childhood cancer with whole-lung irradiation have a greater risk of breast cancer than previously recognized, demonstrating the importance of radiation volume.9 Finally, there is an increased risk of breast cancer in childhood leukemia survivors not exposed to chest radiation; in these patients, alkylators and anthracyclines are associated with an increased risk of breast cancer.7,10
Clinical epidemiology of subsequent thyroid cancer
Thyroid cancer is observed after neck radiation for Hodgkin lymphoma and acute lymphoblastic leukemia. Thyroid cancer has been shown to develop after a median of 19.5 years following exposure to radiation and is associated with an excellent outcome.11 A linear dose-response relationship between thyroid cancer and radiation is observed up to 20 Gy, with a decline in the risk at higher doses, demonstrating evidence for a cell kill effect (Figure 1).5 Female sex and younger age at radiation exposure are significant modifiers of the radiation-thyroid cancer association.12
Clinical epidemiology of subsequent basal cell carcinoma
Therapeutic irradiation is the most significant risk factor for BCC as a subsequent malignancy in childhood cancer survivors, with a well-established dose-response relationship.5 In a large cohort study, the cumulative incidence of BCC 40 years after childhood cancer was 19.1% (95% CI, 16.6%-21.8%) after radiotherapy vs the 0.6% expected based on general population rates.13 After a first BCC, 46.7% had more BCCs later.
Molecular epidemiology of SNs in childhood cancer survivors
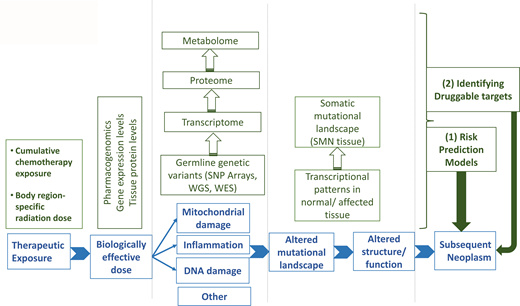
Mutations in high-penetrance genes (eg, RB [retinoblastoma], NF1 [neurofibromatosis], PTCH1 [Gorlin or nevoid BCC syndrome], WT1 [Wilms' tumor], and ATM [ataxia telangiectasia]) could possibly modify the association between therapeutic exposures and SNs. Many genes associated with familial cancer syndromes are responsible for mediating the cellular response to DNA damage (eg, ATM, BRCA) induced by genotoxic insults such as radiation and chemotherapy. Cancer survivors who carry a deleterious high-penetrance mutation are likely to be at increased risk for SNs. However, the low frequency of these mutations in the general population suggests that their attributable risk to the development of SNs is likely very small. The interindividual variability in the risk of SNs is more likely related to common single-nucleotide polymorphisms (SNPs) in low-penetrance genes that regulate drug metabolism/disposition or those responsible for DNA damage response/repair. Between 20% and 95% of the variability in cytotoxic drug disposition can possibly be explained by genetic variation,14 and SNPs in genes involved in drug metabolism/disposition contribute to disease-free survival and drug toxicity.15 Variation in DNA damage response/repair could possibly modify SN risk among cancer patients exposed to DNA-damaging agents, such as radiation and chemotherapy. Finally, it is conceivable that gene-environment (therapeutic exposure) interactions could magnify the functional impact of the polymorphisms.16 The germline genetic contribution to the development of SNs is largely unknown. The limited number of recent studies on this topic is summarized here (Table 1).
Wang et al examined the contribution of pathogenic/likely pathogenic mutations in 60 cancer predisposition genes to their SN risk.17 Pathogenic/likely pathogenic mutations were identified in 5.8% of survivors. Mutations were associated with significantly increased rates of subsequent breast cancer (relative risk [RR], 13.9; 95% CI, 6.0-32.2) and subsequent sarcoma (RR, 10.6; 95% CI, 4.3-26.3) among irradiated survivors and with increased rates of developing any SN (RR, 4.7; 95% CI, 2.4-9.3), breast cancer (RR, 7.7; 95% CI, 2.4-24.4), BCC (RR, 11.0; 95% CI, 2.9-41.4), and 2 or more histologically distinct SNs (RR, 18.6; 95% CI, 3.5-99.3) among nonirradiated survivors. The findings support the referral of all survivors for genetic counseling for potential clinical genetic testing, which should be prioritized for nonirradiated survivors with any SN and for those with breast cancer or sarcoma in the field of prior irradiation.
Qin et al investigated pathogenic germline mutations in 127 DNA repair genes (DRGs) from 6 DNA repair pathways for identification of childhood cancer survivors at increased risk of SNs.18 Overall, pathogenic germline mutations in DRGs (POLG, MUTYH, ERCC2, and BRCA2, among others) were identified in 11.5% of survivors. Mutations in homologous recombination genes were significantly associated with an increased rate of subsequent female breast cancer (RR, 3.7; 95% CI, 1.8-7.7), especially among survivors who received chest radiation of 20 Gy or higher (RR, 4.4; 95% CI, 1.6-12.4) or a cumulative dose of anthracyclines in the second or third tertile (RR, 4.4; 95% CI, 1.7-11.4). Mutations in homologous recombination genes were also associated with an increased rate of subsequent sarcoma among those who received alkylating- agent doses in the third tertile (RR, 14.9; 95% CI, 4.0-38.0). Nucleotide excision repair gene mutations were associated with subsequent thyroid cancer for those treated with neck radiation equal to or higher than 30 Gy (RR, 12.9; 95% CI, 1.6-46.6). These findings have the potential to facilitate the identification of high-risk survivors who may benefit from genetic counseling and/or testing of DRGs, which may further inform personalized cancer surveillance and prevention strategies.
Molecular epidemiology of subsequent radiation-related breast cancer risk
Morton et al conducted a genome-wide association study of breast cancer in female survivors of childhood cancer.19 Among survivors who received breast radiation exposure of 10 Gy or more, a locus on 1q41 was associated with subsequent breast cancer risk (rs4342822, nearest gene PROX1; hazard ratio, 1.92; 95% CI, 1.49-2.44; P = 7.09 × 10−9). Best et al identified 2 variants at chromosome 6q21 associated with SNs (predominantly breast cancer) in survivors of Hodgkin lymphoma treated with radiation therapy as children.20 The variants compose a risk locus associated with the decreased basal expression of PRDM1 and impaired induction of the PRDM1 protein after radiation exposure.
Molecular epidemiology of subsequent thyroid cancer
Gramatges et al showed that shorter telomeres may contribute to subsequent thyroid cancer in childhood cancer survivors. Patients with subsequent thyroid cancer were less likely to have more telomere content (odds ratio, 0.04; 95% CI, 0.00-0.55; P = .01).21 Richard et al annotated the functional impact of variants mapping to 14 telomere maintenance genes in childhood cancer survivors. Functional impact was further assessed by measuring telomere length in leukocyte subsets. The minor allele at protection of telomeres-1 (POT1) rs58722976 was associated with an increased risk of subsequent thyroid cancer (hazard ratio, 6.1; 95% CI, 2.4-15.5).22 These results suggest that intronic variation in POT1 may affect key protein binding interactions that have an impact on telomere maintenance and genomic integrity. Man et al reported that childhood cancer survivors with subsequent thyroid cancer had shorter median lymphocyte telomere length than controls in leukocyte subsets (natural killer cell, naive T cell).23 These findings support immune function studies in survivors to assess the long-term deficits in adaptive and innate immunity that may underlie SN risk.
Molecular epidemiology of basal cell carcinoma
Sapkota et al performed a genome-wide association study of subsequent BCC among irradiated survivors.24 They identified a risk locus in HTR2A that was specific to survivors with reduced nongenetic risk. HTR2A encodes 1 of the receptors for serotonin, which is a multifunctional neurotransmitter with an important role in many physiological processes such as sleep, hormone secretion, and appetite. Recently, the HTR2A SNP rs6311 was shown to be associated with ultraviolet-induced BCC in women.25
Predicting the risk of SNs in childhood cancer survivors
Significant interindividual variability in the risk of SNs provides an opportunity to identify childhood cancer survivors at increased risk for SNs. As summarized below, there is increasing evidence that inclusion of genetic predictors with clinical and demographic factors can refine the prediction models, allowing for identification of survivors at increased risk of SNs. This information can be used to inform surveillance for early detection of SNs.
Opstal-van Winden et al examined 211 155 germline SNPs for gene-radiation interaction on subsequent breast cancer risk in Hodgkin lymphoma patients after chest radiation.26 Nine SNPs showed statistically significant interaction with radiation on breast cancer risk, of which 1 SNP in the PVT1 oncogene attained the Bonferroni threshold for statistical significance. A polygenic risk score (PRS) composed of these SNPs (RT*PRS) and a previously published breast cancer PRS derived from the general population were evaluated in a case-control analysis comprising chest-irradiated Hodgkin lymphoma patients with breast cancer and chest-irradiated Hodgkin lymphoma patients without breast cancer. Patients in the highest tertile of the RT*PRS had a 1.6-fold higher breast cancer risk than those in the lowest tertile. On a continuous scale, radiation-related breast cancer risk increased 1.4-fold per standard deviation of the breast cancer PRS, similar to the effect size found in the general population.
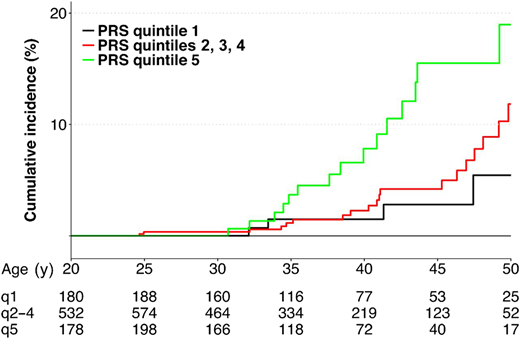
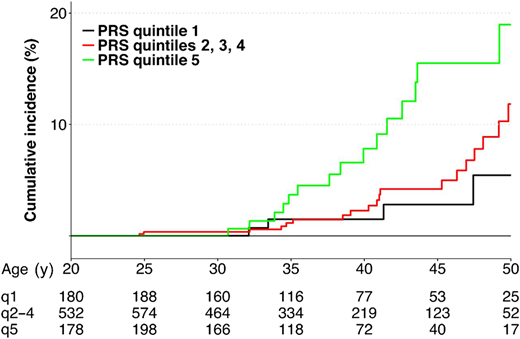
Wang et al aimed to determine genetic contributions to the risk of radiation-related breast cancer, focusing on the polygenic determinants implicated in breast cancer susceptibility in the general population.27 A PRS was constructed for each survivor using 170 established common risk variants. By the age of 45 years, the cumulative incidence was 15.5%, 4.2%, and 2.8%, respectively, for survivors with a PRS in the highest quintile, in 3 intermediate quintiles, and in the lowest quintile (Figure 2). RRs for survivors with a PRS in the highest vs lowest quintiles were 2.7 (95% CI, 1.0-7.3), 3.0 (95% CI, 1.1-8.1), and 2.4 (95% CI, 0.1-81.1) for all survivors and survivors with and without chest irradiation, respectively.
Cumulative incidence of subsequent breast cancer by PRS.
Reproduced from Wang et al27 with permission from the American Association for Cancer Research, copyright 2018.
Cumulative incidence of subsequent breast cancer by PRS.
Reproduced from Wang et al27 with permission from the American Association for Cancer Research, copyright 2018.
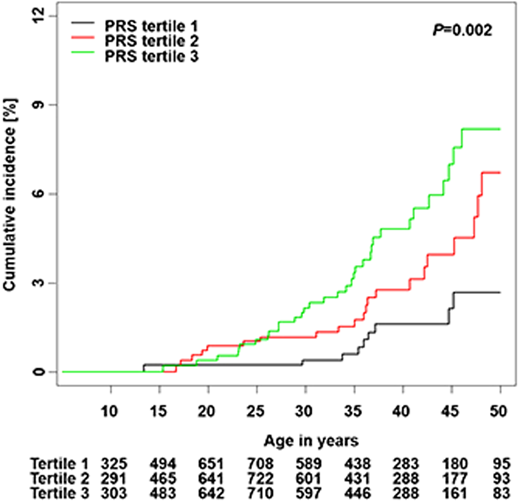
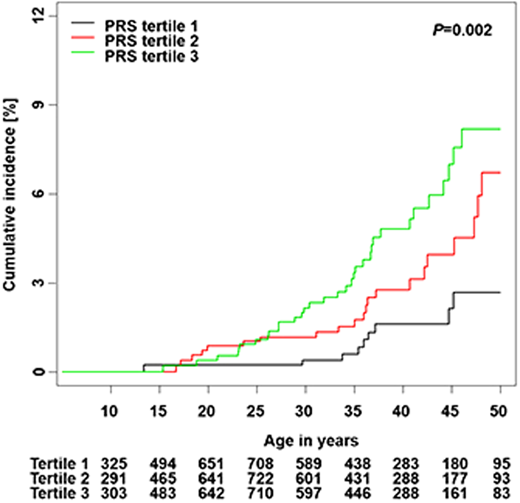
Song et al calculated a PRS from 12 independent SNPs associated with thyroid cancer risk in the general population and developed a risk prediction model integrating the PRS and clinical factors.28 The cumulative incidence of subsequent thyroid cancer was significantly different by PRS tertiles among SJLIFE survivors (P = .002) (Figure 3). The standardized PRS was associated with an increased rate of subsequent thyroid cancer in a discovery cohort (RR, 1.57; 95% CI, 1.24-1.98) and was successfully replicated (RR, 1.52; 95% CI, 1.25-1.83; P < .001). A risk prediction model integrating the PRS with clinical factors showed better performance than the model considering only clinical factors in discovery (area under the curve [AUC], 83.2% vs 82.1% at age 40), and replication (AUC, 72.9% vs 70.6%).
Cumulative incidence of subsequent thyroid cancer by PRS tertiles.
Adapted from Song et al28 with permission from the American Association for Cancer Research, copyright 2021.
Cumulative incidence of subsequent thyroid cancer by PRS tertiles.
Adapted from Song et al28 with permission from the American Association for Cancer Research, copyright 2021.
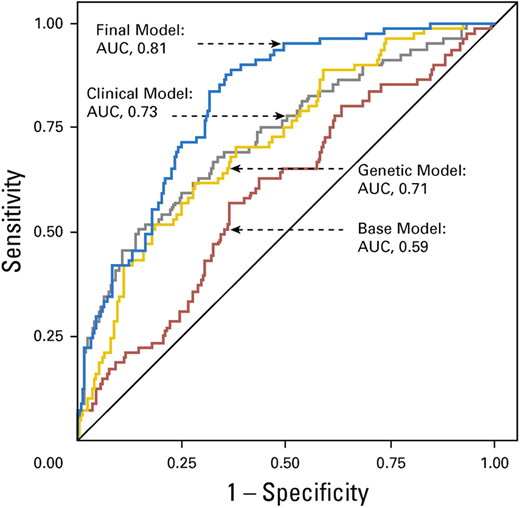
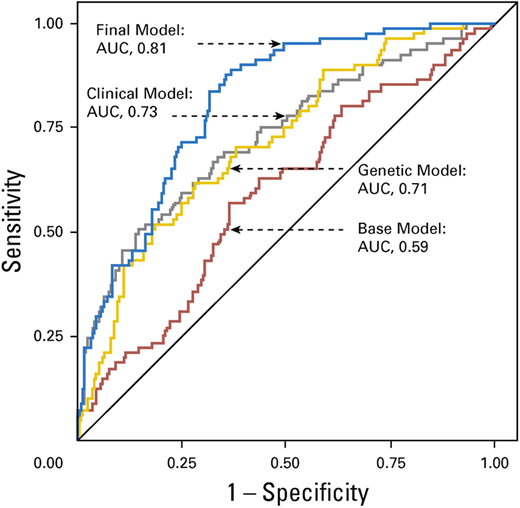
Wang et al curated candidate genetic variants from previously published studies in adult-onset primary central nervous system tumors and replicated these in survivors of childhood cancer with and without subsequent brain tumors.29 They identified an association between 6 previously published SNPs (rs15869 [BRCA2], rs1805389 [LIG4], rs8079544 [TP53], rs25489 [XRCC1], rs1673041 [POLD1], and rs11615 [ERCC1]) and subsequent brain tumors in survivors of childhood cancer. Including genetic variants in a final model containing age at primary cancer, sex, and cranial radiation therapy dose yielded an AUC of 0.81 (95% CI, 0.76-0.86), which was superior (P = .002) to the clinical model (AUC, 0.73; 95% CI, 0.66-0.80) (Figure 4). The prediction model was successfully validated.
Receiver operating characteristic curves from the risk prediction models for subsequent brain tumors.
Reproduced from Wang et al29 with permission from the American Society of Clinical Oncology.
Receiver operating characteristic curves from the risk prediction models for subsequent brain tumors.
Reproduced from Wang et al29 with permission from the American Society of Clinical Oncology.
CLINICAL CASE (Continued)
MH developed radiation-related breast cancer 22 years after exposure to chest radiation. She carried significant risk factors (a high dose of radiation to the mantle field). Therapeutic strategies have changed significantly over the past 2 decades, with significant reductions in both the dose (<25 Gy) and the field (the involved field). While these have resulted in a reduction in the risk of breast cancer, the risk remains significantly higher than the risk in the general population. Nonetheless, the considerable interindividual variability in risk suggests that we need to identify those at highest risk in order to institute targeted screening/intervention strategies. We also need to understand the pathogenesis of radiation-related neoplasms so we can disrupt the process of carcinogenesis and mitigate this risk.
Future directions
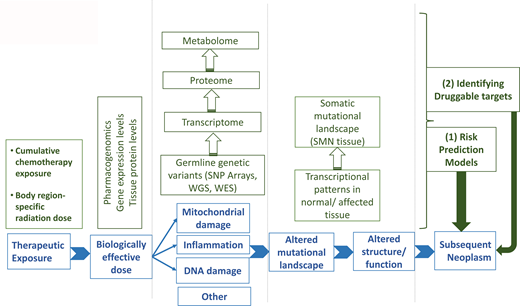
SNs are a devastating complication following the successful treatment of childhood cancer. Ample evidence exists for a causal relationship between key therapeutic exposures and the development of specific types of SNs. There is also significant interindividual variability in the risk of an SN for any given dose of therapeutic exposure, suggesting the role of genetic susceptibility in the development of SNs. Technological and analytical advances have allowed evidence to emerge regarding the role of genetics in the development of treatment-related SNs. This has been possible primarily because of the availability of germline DNA in large comprehensively followed cohorts with detailed therapeutic exposure data. There are also attempts to harness this information to identify survivors at highest risk for the development of SNs, allowing for a targeted approach to prevention/intervention. However, much remains to be done in this field. The relative rarity of the outcome often precludes the identification of genetic associations, yielding false- negative findings or unstable/imprecise associations. Almost all studies use survivors as the study population, possibly resulting in survival bias and an inability to apply these findings to cancer patients at the time of diagnosis. Finally, there is a critical need to understand the functional relevance of the genetic associations identified; this would allow a deeper understanding of the mechanism of carcinogenesis and an opportunity to identify druggable targets.
Acknowledgment
Funding for this research came from the National Institutes of Health (R35CA220502; R01CA139633).
Conflict-of-interest disclosure
Smita Bhatia: no competing financial interests to declare.
Off-label drug use
Smita Bhatia: nothing to disclose.