Abstract
Myelofibrosis (MF) is a clonal hematopoietic stem cell neoplasm characterized by constitutional symptoms, splenomegaly, and risks of marrow failure or leukemic transformation and is universally driven by Jak/STAT pathway activation. Despite sharing this pathogenic feature, MF disease behavior can vary widely. MF can generally be categorized into 2 distinct subgroups based on clinical phenotype: proliferative MF and cytopenic (myelodepletive) MF. Compared to proliferative phenotypes, cytopenic MF is characterized by lower blood counts (specifically anemia and thrombocytopenia), more frequent additional somatic mutations outside the Jak/STAT pathway, and a worse prognosis. Cytopenic MF presents unique therapeutic challenges. The first approved Jak inhibitors, ruxolitinib and fedratinib, can both improve constitutional symptoms and splenomegaly but carry on-target risks of worsening anemia and thrombocytopenia, limiting their use in patients with cytopenic MF. Supportive care measures that aim to improve anemia or thrombocytopenia are often ineffective. Fortunately, new treatment strategies for cytopenic MF are on the horizon. Pacritinib, selective Jak2 inhibitor, was approved in 2022 to treat patients with symptomatic MF and a platelet count lower than 50 × 109/L. Several other Jak inhibitors are in development to extend therapeutic benefits to those with either anemia or thrombocytopenia. While many other novel non–Jak inhibitor therapies are in development for MF, most carry a risk of hematologic toxicities and often exclude patients with baseline thrombocytopenia. As a result, significant unmet needs remain for cytopenic MF. Here, we discuss clinical implications of the cytopenic MF phenotype and present existing and future strategies to tackle this challenging disease.
Learning Objectives
Recognize key differences between cytopenic and proliferative myelofibrosis in terms of pathologic features, clinical features, and prognosis
Understand current treatment options for patients with cytopenic myelofibrosis and recognize their limitations
Consider the novel therapies in development and how they relate to cytopenic myelofibrosis phenotypes
Review supportive care options for cytopenias in myelofibrosis
CLINICAL CASE
A 72-year-old man presents with 6 months of fatigue, early satiety with a 20-pound weight loss, and epistaxis. The physical examination reveals splenomegaly, with the spleen edge felt 14 cm below the left costal margin. Laboratory studies show a normal white blood cell count, mild anemia with a hemoglobin level of 10 g/dL, and significant thrombocytopenia with a platelet count of 40 × 109/L. A peripheral blood smear shows frequent teardrop cells and 3% blasts. A bone marrow biopsy reveals extensive reticulin fibrosis (MF-3), and molecular studies identify the JAK2 V617F mutation as well as mutations in ASXL1 and U2AF1. Based on these findings, a diagnosis of high-risk primary myelofibrosis is made.
Approach to myelofibrosis in 2022
Myelofibrosis (MF) is a complex myeloproliferative neoplasm (MPN) with heterogeneous clinical behavior, necessitating a multifaceted, individualized approach to management. Jak/STAT pathway activation is the hallmark pathogenic driver of MF, resulting from mutations in JAK2, CALR, or MPL genes. The resulting clinical behavior is variable, with features including any combination of constitutional symptoms, splenomegaly, cytopenias or cytoses, and a risk of leukemic transformation. Allogeneic hematopoietic cell transplantation (alloHCT) is the only potential cure for MF; however, its utility is often limited by patient-specific factors (ie, age or comorbidities) and disease-related factors (ie, decreased performance status related to symptoms, poor nutrition status, massive splenomegaly, or hepatic dysfunction). Short of transplant, MF treatment is largely aimed at improving symptoms, splenomegaly, and quality of life. To this end, the development of Jak inhibitors has transformed MF therapy. The first 2 approved Jak inhibitors, ruxolitinib and fedratinib, reduce spleen size and symptom burden for the majority of patients with MF; however, both carry the on-target toxicities of anemia and thrombocytopenia, which limit their use in those with baseline cytopenias.
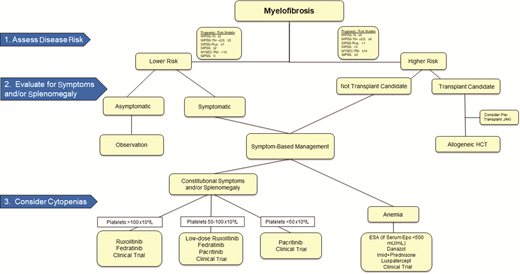
The initial approach to management of MF should be individualized, accounting for clinical, biological, and social/personal factors. How we approach MF step-by-step is detailed in Figure 1. First, we assess disease risk to identify patients in whom alloHCT should be considered. A plethora of prognostic models exist for MF, which incorporate various combinations of clinical, pathologic, and genomic features. A detailed discussion of prognostic models is beyond the scope of this review, though we point out that contemporary models have evolved to incorporate molecular mutations and include the Mutation-Enhanced International Prognostic Scoring Systems for transplantation-age patients (MIPSS70, MIPSS70+/v2.0) and the Genetically Inspired Prognostic Scoring System (GIPSS).1-3 The molecular prognostication of MPNs is discussed in further detail in an associated review in this series. Second, we assess the need for treatment of constitutional symptoms and/or splenomegaly based on a detailed clinical history, including an objective measure of symptoms (ie, MPN-10 score) and physical examination.4 Third, we evaluate the clinical phenotype—proliferative vs cytopenic—to guide the Jak inhibitor agent of choice and dose, as well as supportive therapies. Ruxolitinib and fedratinib both require consideration of baseline platelet count (both require platelet counts >50 × 109/L), and pacritinib is approved for use in those with more severe thrombocytopenia (platelet count <50 × 109/L). While significant progress has been made at each step in this algorithm over the past decade, many unmet needs remain for patients with MF, and clinical trial participation should be considered whenever possible.
Management algorithm for patients with chronic-phase myelofibrosis. ESA, erythropoiesis stimulating agent; GIPSS, Genetically Inspired Prognostic Scoring System; MIPSS, Mutation-Enhanced International Prognostic Scoring System; MYSEC-PM, myelofibrosis secondary to PV and ET-prognostic model.
Management algorithm for patients with chronic-phase myelofibrosis. ESA, erythropoiesis stimulating agent; GIPSS, Genetically Inspired Prognostic Scoring System; MIPSS, Mutation-Enhanced International Prognostic Scoring System; MYSEC-PM, myelofibrosis secondary to PV and ET-prognostic model.
Myelofibrosis phenotypes: cytopenic vs proliferative
While clinical heterogeneity exists as a spectrum across MF, 2 distinct phenotypes have been described: (myelo)proliferative and cytopenic (myelodepletive).5 In general, the 2 phenotypes are distinguished by blood counts, with proliferative MF more likely to have leukocytosis and normal to mildly low hemoglobin levels and platelets, and cytopenic MF more likely to have more severe thrombocytopenia, anemia, and at times leukopenia. This subgrouping has important clinical implications, from prognosis (patients with cytopenic MF tend to fare worse) to treatment (thrombocytopenia and/or anemia preclude the use of several therapies).
The development of cytopenias is relatively common throughout a patient's course. Thrombocytopenia (platelet count <100 × 109/L) is present at diagnosis in about 25% of such patients, with approximately 11% having severe thrombocytopenia (platelet count <50 × 109/L). Thrombocytopenia develops at some time during the disease course in about 68% of patients.6-8 Anemia (hemoglobin <10 g/dL) is present in one-third to one-half of patients at the time of MF diagnosis.6 Approximately half of all patients with MF become dependent on red blood cell transfusions within a year of diagnosis.6
Both anemia and thrombocytopenia are well established as adverse prognostic risk factors. Both are incorporated as high-risk features in all of the major prognostic models, and the degree of cytopenias correlates with outcomes as well. A retrospective study of 1100 patients with MF compared survival duration between those with a platelet count lower than 50 × 109/L, 50 to 100 × 109/L, and higher than 100 × 109/L and found overall survival (OS) to be 15 months, 34 months, and 89 months, respectively (P < .001).9 Platelet counts lower than 50 × 109/L have also been associated with an increased risk of leukemic transformation.7 The degree of anemia has shown a similar association, with OS worsening as patients go from having no anemia to mild, moderate, or severe anemia (8 years, 5 years, 3 years, and 2 years, respectively).10
The presence of cytopenias correlates with other clinical and genomic features. Cytopenic MF is more common among those with primary MF, as opposed to secondary (post–essential thrombocytosis or post–polycythemia vera) MF.5 The presence of severe thrombocytopenia (platelet count <50 × 109/L) is also associated with increased circulating blasts and a higher grade of bone marrow fibrosis.11 Among those with JAK2 V617F mutations, the JAK2 variant allele frequency (VAF) tends to be lower among those with cytopenic MF; low JAK2 VAF has been correlated with worse OS and a decreased response to ruxolitinib.12,13 In addition, anemia and thrombocytopenia have been associated with mutations in the spliceosome gene U2AF1, a high-molecular-risk mutation in MF.2,14 These molecular associations support the hypothesis that cytopenic MF may be caused less by Jak/STAT-activating mutations and more by the evolution of an aggressive subclone with different molecular drivers.
Expanding the Jak inhibitor landscape for cytopenic myelofibrosis
Jak inhibitors have revolutionized the treatment of patients with MF, improving spleen size and symptom burden for many. However, the first 2 approved Jak inhibitors, ruxolitinib and fedratinib, both carry significant on-target hematologic toxicities. The thrombocytopenia and anemia related to these agents often limit the dose that can be administered and preclude their use entirely for some patients. Emerging Jak inhibitors with less myelosuppressive potential are likely to expand therapeutic options for those with cytopenias and include pacritinib, approved in 2022 for use in patients with MF and a platelet count lower than 50 × 109/L, and momelotinib, in development for patients with MF and anemia. Table 1 summarizes the current and emerging Jak inhibitor landscape for cytopenic MF.
Current and emerging Jak inhibitor landscape for cytopenic MF
| Jak inhibitor . | Approved agents . | Investigational agents . | ||||
|---|---|---|---|---|---|---|
| Ruxolitinib . | Fedratinib . | Pacritinib . | Momelotinib . | Ilginatinib . | Itacitinib . | |
| Targets | Jak1, Jak2 | Jak2, Jak1, FLT3, BRD4, TYK2, many others | Jak2, IRAK1, FLT3 | Jak1, Jak2, ACVR | Jak2 | Jak1 |
| Indications | Symptomatic MF with platelets ≥50 × 109/L | Symptomatic MF with platelets ≥50 × 109/L | Symptomatic MF with platelets <50 × 109/L | In development for MF with anemia | In development for MF with thrombocytopenia | In development for MF with anemia |
| Key data in cytopenic MF | Phase 2 study of rux in setting of platelets 50-100 × 109/L, starting at 5 mg twice daily and escalating as tolerated. Median SVR 25% in those escalated to 10 mg twice daily vs 13% on lower doses.17 JUMP: phase 3B study of rux in the setting of platelets 50-100 × 109/L, in which 56% of patients had a ≥50% spleen length reduction by palpation, and favorable safety profile.18 EXPAND: phase 1B dose-finding study in setting of platelets 50-100 × 109/L, demonstrated rux 10 mg twice daily as safe starting dose.19 | Subgroup analysis study pooled data from JAKARTA and JAKARTA2 treated with full-dose fedratinib and compared group with platelets 50-100 × 109/L to >100 × 109/L and reported no difference in spleen responses (36% vs 49% in JAKARTA and 36% vs 28% in JAKARTA2). Symptom responses were also similar between groups. No serious bleeding events occurred.25 | PERSIST-1: phase 3 study of up-front treatment with Pac vs BAT, SVR35 in 19% on Pac vs 5% on BAT. Responses similar for those with thrombocytopenia.27 PERSIST-2: in those with platelets <100 × 109/L, SVR10 79% (vs 36% on BAT) and SVR35 18% (vs 3%), in those with platelets <50 × 109/L, SVR35 in 29% on Pac 200 mg twice daily vs 3% on BAT.28 PAC203: dose- finding study with enhanced inclusion/exclusion criteria, establishing Pac dose as 200 mg twice daily.29 PACIFICA: ongoing study of Pac vs physician's choice (including other JAKis) in those with platelets ≤50 × 109/L (NCT03165734). | MOMENTUM: ongoing phase 3 study of momelotinib vs danazol for MF with anemia and prior JAKi treatment, interim data report improved symptom response, spleen response, and anemia parameters among the momelotinib group.34 | Phase 2 study of ilginatinib vs BAT in those with platelets ≤50 × 109/L is ongoing (NCT04854096). | Phase 2 study of itacitinib in those previously treated with JAKi with adequate platelet counts of >50 × 109/L—results awaited (NCT04629508). |
| Clinical practice points | Starting dose based on platelet count and escalate as able. Early anemia is common, with gradual improvement (so dose adjustments not indicated for early anemia). | No starting dose reductions needed for moderate thrombocytopenia, but dose reduce for worsening platelets. Early nausea or diarrhea are common. Monitor thiamine and for signs of encephalopathy. Monitor renal function and liver function. | Less myelosuppression. Early nausea/diarrhea are common and generally improve with supportive care. Caution in those with CV disease or recent hemorrhage. Monitor QTc. Monitor for bleeding. | Rare peripheral neuropathy risk. | TBD | TBD |
| Jak inhibitor . | Approved agents . | Investigational agents . | ||||
|---|---|---|---|---|---|---|
| Ruxolitinib . | Fedratinib . | Pacritinib . | Momelotinib . | Ilginatinib . | Itacitinib . | |
| Targets | Jak1, Jak2 | Jak2, Jak1, FLT3, BRD4, TYK2, many others | Jak2, IRAK1, FLT3 | Jak1, Jak2, ACVR | Jak2 | Jak1 |
| Indications | Symptomatic MF with platelets ≥50 × 109/L | Symptomatic MF with platelets ≥50 × 109/L | Symptomatic MF with platelets <50 × 109/L | In development for MF with anemia | In development for MF with thrombocytopenia | In development for MF with anemia |
| Key data in cytopenic MF | Phase 2 study of rux in setting of platelets 50-100 × 109/L, starting at 5 mg twice daily and escalating as tolerated. Median SVR 25% in those escalated to 10 mg twice daily vs 13% on lower doses.17 JUMP: phase 3B study of rux in the setting of platelets 50-100 × 109/L, in which 56% of patients had a ≥50% spleen length reduction by palpation, and favorable safety profile.18 EXPAND: phase 1B dose-finding study in setting of platelets 50-100 × 109/L, demonstrated rux 10 mg twice daily as safe starting dose.19 | Subgroup analysis study pooled data from JAKARTA and JAKARTA2 treated with full-dose fedratinib and compared group with platelets 50-100 × 109/L to >100 × 109/L and reported no difference in spleen responses (36% vs 49% in JAKARTA and 36% vs 28% in JAKARTA2). Symptom responses were also similar between groups. No serious bleeding events occurred.25 | PERSIST-1: phase 3 study of up-front treatment with Pac vs BAT, SVR35 in 19% on Pac vs 5% on BAT. Responses similar for those with thrombocytopenia.27 PERSIST-2: in those with platelets <100 × 109/L, SVR10 79% (vs 36% on BAT) and SVR35 18% (vs 3%), in those with platelets <50 × 109/L, SVR35 in 29% on Pac 200 mg twice daily vs 3% on BAT.28 PAC203: dose- finding study with enhanced inclusion/exclusion criteria, establishing Pac dose as 200 mg twice daily.29 PACIFICA: ongoing study of Pac vs physician's choice (including other JAKis) in those with platelets ≤50 × 109/L (NCT03165734). | MOMENTUM: ongoing phase 3 study of momelotinib vs danazol for MF with anemia and prior JAKi treatment, interim data report improved symptom response, spleen response, and anemia parameters among the momelotinib group.34 | Phase 2 study of ilginatinib vs BAT in those with platelets ≤50 × 109/L is ongoing (NCT04854096). | Phase 2 study of itacitinib in those previously treated with JAKi with adequate platelet counts of >50 × 109/L—results awaited (NCT04629508). |
| Clinical practice points | Starting dose based on platelet count and escalate as able. Early anemia is common, with gradual improvement (so dose adjustments not indicated for early anemia). | No starting dose reductions needed for moderate thrombocytopenia, but dose reduce for worsening platelets. Early nausea or diarrhea are common. Monitor thiamine and for signs of encephalopathy. Monitor renal function and liver function. | Less myelosuppression. Early nausea/diarrhea are common and generally improve with supportive care. Caution in those with CV disease or recent hemorrhage. Monitor QTc. Monitor for bleeding. | Rare peripheral neuropathy risk. | TBD | TBD |
CV, cardiovascular; Pac, pacritinib; Rux, ruxolitinib; TBD, to be determined.
Ruxolitinib
Ruxolitinib, a potent and selective inhibitor of Jak1 and Jak2, was the first Jak inhibitor approved for patients with MF. Ruxolitinib's approval was based on 2 randomized phase 3 studies: COMFORT-I and COMFORT-II. In both COMFORT studies, the majority of patients experienced reduction in their spleen volume and symptoms, with 28% to 42% reaching the end points of a 35% or greater reduction in spleen volume (SVR35) and 46% reaching a reduction in symptoms of 50% or higher (TSS50, in COMFORT-I).15,16 However, both COMFORT studies limited inclusion to patients with platelet count higher than 100 × 109/L, and even among this select population, drug-induced cytopenias were limiting. In COMFORT-I, thrombocytopenia of any grade was seen in 70% of patients on ruxolitinib (vs 30% on placebo), and grade 3 or higher thrombocytopenia occurred in 13% (vs 1% on placebo). Most cases of ruxolitinib-induced thrombocytopenia were able to be managed with dose reduction rather than discontinuation. In practice, close attention should be paid to the patient's platelet count to guide both the starting dose and subsequent dose adjustments of the agent, and thrombocytopenia may limit eligibility or the ability to reach an effective dose for some.
Several studies have investigated the safety and utility of ruxolitinib in the setting of lower platelet counts. A phase 2 study examined ruxolitinib dosing, safety, and efficacy among patients with MF and moderate thrombocytopenia (platelet count 50-100 × 109/L).17 Ruxolitinib was started at a dose of 5 mg twice daily and escalated as high as 15 mg twice daily, provided that the platelet count remained equal to or higher than 40 × 109/L. Of the 66 patients enrolled, 52 were treated for at least 24 weeks, no one discontinued due to thrombocytopenia, and 25 were able to have their ruxolitinib doses escalated to 10 mg twice daily or more. Responses were best among those on ruxolitinib at a dosage of 10 mg twice daily compared to those on 5 mg once or twice daily, with a median spleen volume reduction of 25% vs 13% and a median total symptom score improvement of 57% vs 21%, respectively. JUMP, a phase 3b expanded access study, included a small number of patients with platelet counts in the same range who were started on ruxolitinib at 5 mg twice daily.18 Meaningful reductions in spleen length and symptoms were still observed in the low-platelet cohort, although to a lesser degree than those with a platelet count higher than 100 × 109/L; a lack of protocol-mandated dose escalations may have contributed to this. Not surprisingly, worsening thrombocytopenia was more common in the low-platelet cohort, as was drug discontinuation due to thrombocytopenia (12%). Similarly, a phase 1b dose-finding study (EXPAND) demonstrated that ruxolitinib at 10 mg twice daily is a safe starting dose among patients with MF and a baseline platelet count between 50 and 100 × 109/L.19 These studies demonstrated that ruxolitinib is a reasonable option for patients with moderate thrombocytopenia, provided that the dose is started low and escalated as needed. Among those with more severe thrombocytopenia (platelet count <50 × 109/L), ruxolitinib has not been formally studied.
Fedratinib
Fedratinib was the second Jak inhibitor approved for MF. Similar to ruxolitinib, fedratinib is a potent inhibitor of Jak2; however, fedratinib also inhibits several other kinases such as FLT3 and the BET family protein BRD4, which may contribute to differences in efficacy and toxicity between the agents.20 Fedratinib was studied in the up-front setting in the phase 3 placebo-controlled JAKARTA study, in which SVR35 was reached in 38% of the fedratinib-treated group vs 1% of the placebo group and TSS50 in 35% and 7%, respectively.21 In the second-line setting, after ruxolitinib failure or intolerance (JAKARTA2), SVR35 was still reached in 55% of all patients. After selecting a subgroup that met strict criteria for prior Jak inhibitor failure, 30% still achieved SVR35.22,23 Both JAKARTA studies limited inclusion to those with a platelet count higher than 50 × 109/L but did not adjust the starting dose for baseline platelet count. Treatment-emergent thrombocytopenia was relatively common with fedratinib in JAKARTA2, with any grade of thrombocytopenia developing in 27% and grade 3 to 4 thrombocytopenia in 22%, which resulted in fedratinib discontinuation in 2 patients. Treatment-emergent anemia was also common, in 49% of patients (grade 3-4 anemia in 38%). While these hematologic toxicities resemble those of ruxolitinib, several distinct toxicities may be seen with fedratinib, including diarrhea, nausea, increased creatinine, increased transaminases, and, very rarely, Wernicke's encephalopathy, which prompted a black box warning and a recommendation for thiamine monitoring.24
The efficacy of fedratinib in those with moderate thrombocytopenia has been examined in a pooled analysis of patients enrolled in JAKARTA and JAKARTA2 and treated with full-dose fedratinib (400 mg/d).25 Those with baseline thrombocytopenia of 50 to 100 × 109/L were compared to those with a platelet count higher than 100 × 109/L and were found to have no difference in spleen volume response or symptom control. Not surprisingly, worsening or new thrombocytopenia was more common in patients with low baseline platelet values (44%) compared to the high-platelet cohort (9%) but was generally manageable with dose reductions, with only 3 of 48 patients discontinuing therapy due to thrombocytopenia. Similarly to ruxolitinib, fedratinib has not been systemically studied in patients with platelet counts lower than 50 × 109/L.
Pacritinib
A third Jak inhibitor, pacritinib, was approved in 2022 to address the need for spleen volume and symptom control in patients with MF and severe thrombocytopenia (platelet count <50 × 109/L). Pacritinib is a potent and specific inhibitor of Jak2 and interleukin 1 receptor–associated kinase 1 (IRAK1), a mediator of nuclear factor kappa B (NF-kB) and inflammatory signaling.26 The IRAK1/NF-kB inhibition and sparing of Jak1 likely contribute to its lower myelosuppressive potential; pacritinib can be administered at full doses in patients who are severely thrombocytopenic.
Pacritinib has now been examined in multiple phase 3 clinical trials, which all included patients with baseline thrombocytopenia. PERSIST-1 was an up-front study in which patients with higher-risk MF and no prior Jak inhibitor exposure were randomized to receive either pacritinib at 400 mg/d or the best available therapy (BAT), excluding other Jak inhibitors.27 The primary end point of SVR35 at week 24 was met in 19% of patients in the pacritinib group vs 5% in patients receiving BAT. Response rates were similar between subgroups with and without baseline thrombocytopenia. In light of this observation, the PERSIST-2 trial focused specifically on patients with platelet counts lower than 100 × 109/L.28 PERSIST-2 included both Jak inhibitor–naive and previously treated patients (48% had received prior ruxolitinib) and randomized patients to 2 doses of pacritinib (400 mg/d or 200 mg twice daily) or BAT; ruxolitinib was the most commonly chosen BAT, in 45%. Even in this historically difficult to treat setting, most patients experienced some degree of spleen volume reduction: a decrease of 10% or more was seen in 72% and 79% in the pacritinib arms, compared to 36% on BAT. The primary end point of SVR35 was reached in 18% in the pooled pacritinib arms, vs 3% on BAT (P = .001). A subgroup analysis showed that those with severe thrombocytopenia (platelet count <50 × 109/L) fared similarly to those with moderate thrombocytopenia (<100 × 109/L), with SVR35 reported in 18% on pacritinib at a dose of 400 mg/d, 29% on pacritinib at 200 mg twice daily, and 3% on BAT. Of note, there was no difference in the incidence of hematologic adverse events (AEs) between those with moderate and those with severe thrombocytopenia when treated with pacritinib.
The development of pacritinib was marred by a clinical hold imposed after a pooled analysis of PERSIST-1 and -2 showed concern for excess deaths related to cardiovascular and bleeding events. These concerns prompted a subsequent dose-finding study, PAC203, in which 3 doses of pacritinib were studied for safety and efficacy in a more select patient population (ie, exclusion for recent serious bleeding or anticoagulation, QTc >450 ms, and left ventricular ejection fraction <45%).29 PAC203 established pacritinib at 200 mg twice daily as an efficacious dose with an acceptable safety profile, with no excess of grade 3 or greater hemorrhagic or cardiac events. The 200-mg dose twice daily has been carried forward into the ongoing PACIFICA study, which focuses exclusively on patients with platelet counts lower than or equal to 50 × 109/L and no (or limited) prior Jak inhibitor exposure randomized to receive either pacritinib or physician's choice therapy, including low doses of other Jak inhibitors (NCT03165734).
Pacritinib has emerged as the new standard of care for patients with symptomatic MF and severe thrombocytopenia (platelet count ≤50 × 109/L). In those with moderate thrombocytopenia (platelet count 50-100 × 109/L), pacritinib is also a reasonable Jak inhibitor option, as are fedratinib or low-dose ruxolitinib. Patient selection for pacritinib eligibility is key to minimize the risks of cardiovascular, hemorrhagic, or gastrointestinal AEs. Looking forward, pacritinib represents an intriguing partner for combination strategies with the many non–Jak inhibitor investigational MF treatments in development due to the potential for less combined myelosuppression.
Investigational Jak inhibitors
Momelotinib
Momelotinib is in development specifically for the subset of patients with MF and anemia. Momelotinib inhibits Jak1, Jak2, and activin A receptor type 1, which is thought to suppress hepcidin production, thus mobilizing sequestered iron to support erythropoiesis.30,31
Momelotinib has been examined in 2 phase 3 studies, SIMPLIFY-1 in the up-front setting and SIMPLIFY-2 in the later-line setting. SIMPLIFY-1 was a noninferiority study in which patients were randomized to receive either momelotinib or ruxolitinib as their first Jak inhibitor.32 In this setting momelotinib was noninferior to ruxolitinib in terms of spleen responses but not symptom responses. SIMPLIFY-2 compared momelotinib to BAT for patients previously treated with ruxolitinib and found that momelotinib was not superior to BAT for inducing spleen responses in that setting.33 Notably, patients receiving momelotinib in both SIMPLIFY 1 and 2 were found to have improvements in anemia, prompting a shift in therapeutic focus of this agent toward the subgroup of patients with MF and anemia. An ongoing phase 3 study, MOMENTUM (NCT04173494), compares momelotinib to danazol in prior Jak inhibitor–exposed patients with anemia. Patients with platelet counts higher than or equal to 25 × 109/L were included. Reported results from MOMENTUM demonstrate the superiority of momelotinib compared to danazol in terms of symptom response (TSS50 in 25% vs 9%, respectively; P = .0095), spleen response, and transfusion requirements.34 Overall momelotinib is relatively well tolerated, though low-grade peripheral neuropathy should be noted (4% in MOMENTUM, all grade 2 or less, with no discontinuations as a result).
Ilginatinib
Ilginatinib (formerly NS-018) is a selective Jak2 inhibitor that spares Jak1.35 Ilginatinib was evaluated in a phase 1/2 dose-escalation study in patients with prior Jak inhibitor exposure and demonstrated improvements in symptoms and spleen size with minimal myelosuppression.36,37 A phase 2 randomized study comparing ilginatinib to BAT in patients with MF and severe thrombocytopenia is ongoing (NCT04854096).
Itacitinib
Jak1 inhibition is another potential avenue for MF treatment, in hopes of modulating cytokine production and potentially improving symptoms and anemia. Itacitinib, a selective Jak1 inhibitor, was investigated in a phase 2 study at 3 dose levels in patients with higher-risk MF and a platelet count higher than 50 × 109/L.38 Symptom and spleen responses were reported, but the most notable outcome was that 21 of 39 (54%) patients who required red blood cell transfusions prior to study entry experienced a 50% or greater reduction in transfusion requirements. Thrombocytopenia may be limiting with this approach, as new or worsening grade 3 to 4 thrombocytopenia was reported in 29% in this study, though only 1 patient discontinued treatment for this reason. Results are awaited from a phase 2 study of itacitinib that enrolled patients with MF previously treated with Jak inhibitors with adequate platelet counts higher than 50 × 109/L (NCT04629508).
CLINICAL CASE (Continued)
For this patient with higher-risk MF characterized by bothersome constitutional symptoms, splenomegaly, and thrombocytopenia, the initial treatment chosen is pacritinib, at a dose of 200 mg twice daily. A workup for alloHCT is initiated, but the patient decides to defer transplant given the excellent spleen and symptom response with pacritinib. One year later, his spleen is enlarging, and his constitutional symptoms have worsened. Investigational options are considered.
Novel agents for cytopenic myelofibrosis
Despite the expanding Jak inhibitor landscape for MF, many unmet needs remain. Not all experience a sufficient response to Jak inhibitors, and even among responders, eventual resistance is common. Novel strategies aimed at epigenetic regulators, apoptosis mechanisms, non–Jak/STAT signaling pathways, and alternate mechanisms driving fibrosis are all under investigation in MF. In cytopenic MF, investigational options are often limited by the potential for hematologic toxicities. However, several of these agents have demonstrated an ability to improve anemia in some patients. Other strategies such as transforming growth factor β (TGF-β) inhibitors and antifibrosing agents may also provide some clinical benefit in this subset, with less myelosuppressive potential. However, very few current trials focus specifically on the cytopenic patient population; such investigations are desperately needed.
Pelabresib
Bromodomain and extraterminal (BET) inhibitors disrupt binding to acetylated histone lysine residues, resulting in the modified expression NF-κB target genes, and may subsequently decrease the production of pro-inflammatory cytokines and disrupt fibrogenesis.39 The BET inhibitor pelabresib has been useful in controlling spleen enlargement and symptoms as well as improving anemia, but at the cost of lowering platelets. In the MANIFEST trial (NCT02158858), pelabresib was studied as a single agent in Jak inhibitor refractory settings, as well as in combination with ruxolitinib in both up-front and Jak inhibitor refractory MF. The single- agent, prior Jak-inhibitor-treated arm enrolled patients with platelet counts higher than or equal to 75 × 109/L and reported promising spleen and symptom responses (SVR35 in 30%, TSS50 in 48%) and improvements in anemia parameters, with 3 of 14 (21%) transfusion-dependent patients converting to transfusion independence.40 The most common treatment-emergent AEs was thrombocytopenia, in 30%. The combination arms limit enrollment to those with platelet counts higher than or equal to 100 × 109/L due to the myelosuppressive potential of both agents, but it is worth mentioning that encouraging improvements in spleen size, symptoms, anemia parameters, and marrow fibrosis have all been described in these arms, though at the expense of frequent treatment-emergent thrombocytopenia in 52% of patients.41 In the ongoing MANIFEST-2 study (NCT04603495), up-front treatment with ruxolitinib plus pelabresib is compared to ruxolitinib plus placebo and limits enrollment to those with platelet counts of 100 × 109/L or higher.
Navitoclax
The prosurvival genes B-cell lymphoma 2 (BCL2) and BCL-xL are upregulated in MF and provide a potential target for therapy.42 Navitoclax, a BCL2/BCL-xL inhibitor, has also garnered excitement based on clinical responses in combination with ruxolitinib; however, this agent is also associated with significant thrombocytopenia. In data reported thus far from the phase 2 REFINE study (NCT03222609), patients with progression or a suboptimal response on ruxolitinib and platelet counts higher than 100 × 109/L received navitoclax in escalating doses as an add-on strategy.43 Clinical responses were demonstrated, including SVR35 in 26.5%, TSS50 in 30%, and impressively, a sustained improvement in hemoglobin levels of 2 g/dL or higher in 7 of 11 evaluable patients (64%). Thrombocytopenia was a common AE even among this patient population with reasonable baseline platelet counts, occurring in 88% of patients. A post hoc biomarker analysis has also described improvements in driver mutation VAF and marrow fibrosis in a subset of patients, which warrants optimism about the disease- modifying potential of this combination, although further studies are needed to clarify what, if any, clinical impact these findings connote.44 Randomized phase 3 settings are ongoing with navitoclax plus ruxolitinib vs ruxolitinib alone in the up-front setting (TRANSFORM-1, NCT04472598) and navitoclax plus ruxolitinib vs physician's choice therapy in the second-line setting (TRANSFORM-2, NCT04468984), both with exclusion criteria for platelet counts (<100 × 109/L).
TGF-β inhibitors
Excess TGF-β signaling plays a role in MF pathophysiology through multiple mechanisms, including promoting the differentiation of neoplastic monocytes to fibrocytes, thus contributing to marrow fibrosis and enhancing the dormancy of normal hematopoietic stem cells, contributing to cytopenias.45,46 Multiple TGF-β inhibitors have been studied in an attempt to reverse fibrosis and improve cytopenias in patients with MF. TGF-β1/3 “trap” AVID200 is one example. A phase 1b study tested AVID200 in the post–Jak inhibitor setting and allowed for the inclusion of patients with baseline thrombocytopenia down to a platelet count of 25 × 109/L (platelets could have been transfused to that level).47 AVID200 treatment had variable impact on spleen size and symptoms, but the results were notable for an increase in platelet counts in the majority of patients treated, suggesting a possible role for this agent in cytopenic MF or in combination settings with more myelosuppressive agents. The other TGF-β inhibitors luspatercept and sotatercept have elicited anemia improvements in patients with MF (Table 2).48,49 An ongoing randomized phase 3 study is assessing luspatercept compared to placebo in patients with MF on Jak inhibitor therapy who require transfusions (NCT04717414).
Supportive care for cytopenic MF
| Drug class . | Indication . | Agent . | Key data . | Toxicities of note . | References . |
|---|---|---|---|---|---|
| ESAs | Anemia, if endogenous Epo level <500 mU/mL | Darbopoetin alpha Epoetin alpha | Retrospective, multicenter study of patients with MF and anemia: - Anemia response in 53%. - Median duration of response 19 mo. Retrospective study in the setting of concurrent Rux treatment showed anemia response in 54%. | - Very few toxicities but hypertension and thromboses are possible. | 59,60 |
| TGF-β inhibitors | Anemia | Luspatercept | Phase 2 study of patients with MF and anemia: - On luspatercept monotherapy, 10% converted from transfusion dependent to independent. - On luspatercept plus Rux, 27% converted to transfusion independence. - Similar response rates among transfusion-independent groups. Ongoing phase 3 study of luspatercept vs placebo in patients with MF on Jak inhibitor therapy who require transfusions (NCT04717414). | - Very few toxicities but hypertension and bone pain are possible. | 48 |
| Sotatercept | Phase 2 study showed anemia responses in 30% with sotatercept monotherapy and 32% with combination therapy with Rux. | 49 | |||
| IMiDs | Anemia, thrombocytopenia | Thalidomide | Phase 2 study in 63 patients with MF, increase in platelet count by >50 × 109/L in 22% with baseline thrombocytopenia. Pooled analysis of MF studies using thalidomide at doses ≥100 mg/d; 38% had an increase in platelet counts, and 29% had an increase in Hb. | - Constipation - Fatigue - Peripheral neuropathy - Neutropenia - Sedation - Edema | 61,62 |
| Lenalidomide | Pooled data from MF studies showed that 50% of patients had a platelet response (>50% improvement from baseline to absolute count of >50 × 109/L). | 63 | |||
| Pomalidomide | Phase 3 study showed platelet response in 22% on Pom vs 0% on placebo. | 64 | |||
| Androgen | Anemia, thrombocytopenia | Danazol | In 50 patients with MF and anemia: - WHO IWG-defined anemia response achieved in 15 (30%), including 5/27 (18%) of those with transfusion dependency. - Median time to response 5 mo - Median duration of response 14 mo - Among 13 patients with thrombocytopenia, a platelet response was seen in 3 (23%). | - Hepatic toxicity - Prostate cancer risk | 65 |
| TPO receptor agonists | Thrombocytopenia (not routinely recommended) | Eltrombopag | Among 6 patients with MF on Rux with thrombocytopenia, 0 had a sustained platelet response. | - Has not been effective - Theoretic risk of worsening fibrosis - Not routinely recommended | 66 |
| Drug class . | Indication . | Agent . | Key data . | Toxicities of note . | References . |
|---|---|---|---|---|---|
| ESAs | Anemia, if endogenous Epo level <500 mU/mL | Darbopoetin alpha Epoetin alpha | Retrospective, multicenter study of patients with MF and anemia: - Anemia response in 53%. - Median duration of response 19 mo. Retrospective study in the setting of concurrent Rux treatment showed anemia response in 54%. | - Very few toxicities but hypertension and thromboses are possible. | 59,60 |
| TGF-β inhibitors | Anemia | Luspatercept | Phase 2 study of patients with MF and anemia: - On luspatercept monotherapy, 10% converted from transfusion dependent to independent. - On luspatercept plus Rux, 27% converted to transfusion independence. - Similar response rates among transfusion-independent groups. Ongoing phase 3 study of luspatercept vs placebo in patients with MF on Jak inhibitor therapy who require transfusions (NCT04717414). | - Very few toxicities but hypertension and bone pain are possible. | 48 |
| Sotatercept | Phase 2 study showed anemia responses in 30% with sotatercept monotherapy and 32% with combination therapy with Rux. | 49 | |||
| IMiDs | Anemia, thrombocytopenia | Thalidomide | Phase 2 study in 63 patients with MF, increase in platelet count by >50 × 109/L in 22% with baseline thrombocytopenia. Pooled analysis of MF studies using thalidomide at doses ≥100 mg/d; 38% had an increase in platelet counts, and 29% had an increase in Hb. | - Constipation - Fatigue - Peripheral neuropathy - Neutropenia - Sedation - Edema | 61,62 |
| Lenalidomide | Pooled data from MF studies showed that 50% of patients had a platelet response (>50% improvement from baseline to absolute count of >50 × 109/L). | 63 | |||
| Pomalidomide | Phase 3 study showed platelet response in 22% on Pom vs 0% on placebo. | 64 | |||
| Androgen | Anemia, thrombocytopenia | Danazol | In 50 patients with MF and anemia: - WHO IWG-defined anemia response achieved in 15 (30%), including 5/27 (18%) of those with transfusion dependency. - Median time to response 5 mo - Median duration of response 14 mo - Among 13 patients with thrombocytopenia, a platelet response was seen in 3 (23%). | - Hepatic toxicity - Prostate cancer risk | 65 |
| TPO receptor agonists | Thrombocytopenia (not routinely recommended) | Eltrombopag | Among 6 patients with MF on Rux with thrombocytopenia, 0 had a sustained platelet response. | - Has not been effective - Theoretic risk of worsening fibrosis - Not routinely recommended | 66 |
Epo, erythropoietin; ESA, erythropoiesis stimulating agent; Hb, hemoglobin; Pom, pomalidomide; Rux, ruxolitinib; TPO, thrombopoietin; WHO IWG, World Health Organization International Working Group.
Antifibrosing agents
Mechanisms of fibrogenesis in MF are complex and incompletely understood. Agents that target the profibrotic factors in the marrow microenvironment rather than hematopoietic cells may have less potential for myelosuppression and may be useful in cytopenic MF. PRM-151 is a recombinant form of the molecule pentraxin-2 that may prevent and even reverse fibrosis at sites of tissue damage and was initially developed for pulmonary fibrosis but subsequently repurposed for MF.47,50 Clinical studies with PRM-151 in patients with MF showed modest improvements in symptoms, spleen size, and marrow fibrosis.51,52 While studies with PRM-151 are ongoing in pulmonary fibrosis, its future in MF is unclear. Another potential antifibrosing target is signaling lymphocytic activation molecule F7 (SLAMF7), which is aberrantly expressed on monocytes and monocyte-derived fibrocytes in patients with MF.53 A phase 2 study of elotuzumab, an anti-SLAMF7 monoclonal antibody, is ongoing (NCT04517851).
Other novel agents
Many other novel targets are being explored in MF, and several may have utility in the context of cytopenic subsets. The telomerase inhibitor imetelstat demonstrated modest clinical activity in MF in a phase 2 study in terms of spleen size (SVR35 in 10%) and symptom burden (TSS50 in 32%), and the observed median OS was longer than expected from historical controls.54 As a result, a phase 3 study is underway in which patients with MF and prior Jak inhibitor exposure are randomized to imetelstat vs BAT (excluding Jak inhibitors) with OS as the primary end point (NCT04576156). In the setting of myelodysplastic syndromes, imetelstat has demonstrated improvements in anemia parameters and rates of transfusion independence, lending hope that similar improvements may be possible in cytopenic MF.55 Other agents in development for MF-associated anemia aim to suppress hepcidin production and therefore increase iron availability. These include INCB000928, an ALK2 inhibitor that is being studied as monotherapy and in combination with ruxolitinib (NCT04455841), and DISC-0974, a monoclonal antibody against hemojuvelin that is being studied as monotherapy in this setting (NCT05320198). A phase 2 study of the interleukin 1β monoclonal antibody canakinumab is starting as well to assess for anemia improvements. It is allowing participants with platelet counts as low as 25 × 109/L (NCT05467800).
CLINICAL CASE (Continued)
Our patient with cytopenic MF, who has begun to experience disease progression after an initial response to pacritinib, returns to the office. AlloHCT has been deemed too risky due to his age, comorbidities, and decline in performance status. He now requires regular red blood cell transfusions, on the average of 2 units received every 2 to 3 weeks. After considering his options, including investigational trials, he opts for a strategy of supportive care.
Supportive care for cytopenic myelofibrosis
Anemia is common and relentless in patients with MF, and treatment options are often insufficient. Anemia may result from the disease or its treatment with Jak inhibitors and is one of the most common reasons for ruxolitinib discontinuation.56 Supportive care measures including red blood cell transfusions, erythropoiesis stimulating agents, danazol, and immunomodulatory agents (IMiDs) are frequently attempted, though all have drawbacks and limited efficacy (Table 2). Fortunately, newer Jak inhibitors (ie, momelotinib) and non–Jak inhibitor treatments (ie, pelabresib and navitoclax) may help address this problem for some.
Supportive management of thrombocytopenia is also limited and rarely effective. Many of the same agents can be attempted, including danazol and IMiDs, though response rates are low, and any improvements are often short-lived (Table 2). DNA hypomethylating agents are more commonly deployed in the setting of accelerated or blast phase disease. They have also demonstrated clinical activity in higher-risk chronic-phase MF, including in patients with baseline thrombocytopenia, but at the expense of initial myelosuppression.57 The role of spleen-directed therapy (ie, splenectomy or splenic irradiation) in MF is controversial, but the lack of disease-modifying potential and the high risk for morbidity and mortality have generally relegated this approach to the palliative setting in those with refractory splenomegaly-related symptoms that do not respond to any other medical therapy.58 Hypersplenism likely contributes to thrombocytopenia in some patients, and transient improvements in platelet counts in thrombocytopenic MF have been reported after spleen-directed therapy, though thrombocytopenia itself should not be an indication for this approach.
Conclusions
Cytopenic MF is a distinct subset that tends to harbor high-risk clinical and pathologic features. The management of cytopenic myelofibrosis continues to present a significant therapeutic challenge and represents a major area of unmet need. However, the therapeutic landscape for cytopenic MF is expanding. Recent data show that the Jak inhibitors ruxolitinib and fedratinib can be administered safely in those with moderate thrombocytopenia (platelet count of 50-100 × 109/L), with close monitoring. For those with more severe thrombocytopenia, pacritinib is now approved and available. Other Jak inhibitor and non–Jak inhibitor therapies are in development, in the hopes of filling treatment gaps for patients with anemia and those with refractory disease, relapse, or intolerance to the current approved agents. Further treatment options are desperately needed for patients with cytopenic MF, but the current pace of research in the field provides reason for optimism.
Conflict-of-interest disclosure
Samuel B. Reynolds: no competing financial interests to declare.
Kristen Pettit: advisory board member: AbbVie, CTI Biopharma; research funding: AbbVie, Blueprint Medicines, CTI Biopharma, Imago Biosciences, Kura Oncology, Macrogenics, PharmaEssentia, Protagonist Therapeutics.
Off-label drug use
Samuel B. Reynolds: nothing to disclose.
Kristen Pettit: nothing to disclose.