Abstract
Hodgkin’s lymphoma is a unique neoplasm of B lymphocytes. Recent data provide new understandings of the pathogenesis and options for staging and therapy of the disease. Three specific topics are addressed in this chapter.
In Section I, Dr. Richard Ambinder reviews implications of the relationship of Epstein-Barr virus (EBV) and Hodgkin’s lymphoma. This relation includes varying geographic epidemiologic associations, including varying associations with the clinical syndrome of infectious mononucleosis. There are plausible mechanisms, including processes initiated by viral proteins, by which EBV might lead to tumorigenesis. These mechanisms include promotion of genetic instability and alteration of normal processes of apoptosis. In addition to an epidemiologic association and potential role in pathogenesis, viral antigens may pose theoretical targets for anti-cancer therapies, including vaccination.
In Section II, Dr. Sigrid Stroobants describes the potential role of positron emission tomographic (PET) scanning. By assessing differences in the metabolic activities of cancer cells, PET scanning may be superior to computerized tomographic scanning, which is limited to showing structural anatomical abnormalities. In patients with Hodgkin’s and non-Hodgkin’s lymphoma, PET scanning has been tested as an initial staging tool, to assess the rate of therapeutic response from a prognostic perspective, and to differentiate residual tumor from fibrotic masses in patients who have completed therapy. Particularly in assessing the nature of a residual mass seen with other post-therapeutic imaging modalities, PET scanning may provide unique information; very high negative predictive values have been reported. However, before this technology can be recommended for incorporation into standard management, properly conducted prospective trials are required to better evaluate the clinical utility of PET with respect to eventual patient outcomes.
In Section III, Dr. Ralph Meyer reviews current data regarding the management of patients with limited-stage Hodgkin’s lymphoma. Over the past decade, standard treatment has evolved to consist of combined-modality therapy that includes an abbreviated course of chemotherapy and involved-field radiation. As this therapy continues to include radiation therapy, patients will remain at risk of long-term toxicities that include the development of second cancers and cardiovascular events. These “late-effects” now account for more deaths than those attributed to progressive Hodgkin’s lymphoma. Comparative data testing the role of chemotherapy alone are now available and demonstrate that omission of radiation therapy results in small but statistically significant reduction in disease control, but no detectable differences in overall survival. Further follow-up will clarify whether chemotherapy alone is the preferred treatment option; at present patients should be informed of the trade-offs involved in choosing between this option and combined modality therapy.
I. Epidemiology of Hodgkin’s Lymphoma and the Role of Epstein-Barr Virus
Richard F. Ambinder, MD, PhD*
Johns Hopkins School of Medicine, 389 Bunting-Blaustein Cancer Research Building, 1650 Orleans Street, Baltimore MD 21231
Hodgkin’s “disease” is a B cell lymphoma.1 The mystery embodied in the term Hodgkin’s disease, which defines neither the affected tissue nor the disease process, is underscored by the name of the cell required for diagnosis: Reed-Sternberg. The malignant character of the disease is now beyond dispute and the cellular lineage is clearly understood to be B cell. The explanation for the difficulty in identifying the tumor may be attributed to two of its defining characteristics: the tumor cells are rare in the mass of the tumor and fail to express many B cell markers—most notably immunoglobulin.
Epidemiology
Whereas the incidence of non-Hodgkin’s lymphoma has been rising since World War II, the incidence of Hodgkin’s lymphoma has been flat. Two age-incidence peaks have long been recognized in North America and Western Europe: young adult and older adult. In developing countries and in parts of Asia, the young adult peak is much less prominent or even absent. Several epidemiologic studies suggested an association with small family size and other factors that might result in delayed exposure to common viral infections.
Epstein-Barr Virus and Hodgkin’s
Serologic studies have suggested modestly higher titers to Epstein-Barr virus (EBV) antigens in patients with Hodgkin’s lymphoma than in controls, but the differences in titers have been modest. EBV is a ubiquitous virus and the vast majority of Hodgkin’s patients (and adults) are EBV seropositive.
Infectious Agents and Lymphomagenesis
There are two very different pathways by which infectious agents drive malignant lymphoproliferative diseases: some infectious agents activate extrinsic pathways through lymphocyte antigen receptors, while others activate intrinsic pathways bypassing antigen receptors2 (Figure 1 ). Helicobacter drives the proliferation of B cells in an antigen-specific manner and treatment with anti-infective agents sometimes results in tumor regression. Hepatitis C may similarly act through immunoglobulin receptors and may similarly respond to treatment with anti-infective agents.3 In contrast, human T cell leukemia virus-1 (HTLV1) drives the proliferation of infected T lymphocytes independent of the specificity of the T cell receptor.4 Similarly, in posttransplant lymphoproliferative disease, EBV drives lymphoproliferation independent of the specificity of the immunoglobulin. Several different EBV genes are required to act in concert to bring about this transformation, including five of the six EBV nuclear antigens.
Detection of EBV in Tumor
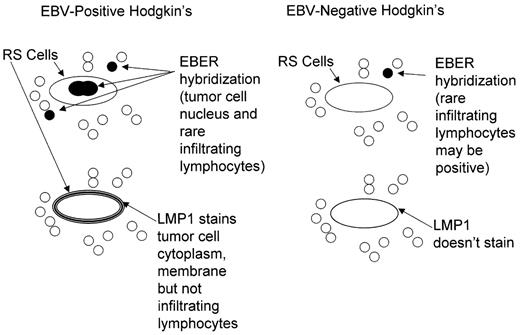
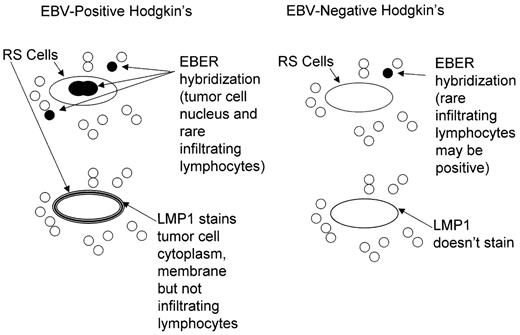
EBV nucleic acids and proteins have been identified in the tumor cells in a subset of patients. Approximately 30% of Hodgkin’s lymphoma in the United States and North America harbor these nucleic acids and proteins, while much higher percentages of tumor cells show evidence of virus in some developing countries (approaching 100% in parts of Latin America, Africa and Asia).5 Formal guidelines for interpretation and comparisons of methods for detecting virus in fixed tissues have recently been published6,7 (Figure 2 ). With some well-documented exceptions EBV is present in each of the tumor cells in a particular patient at all sites of disease, at presentation and at relapse.
Questions
Aspects of the relationship between cases associated with the syndrome of infectious mononucleosis and tumors harboring virus have been problematic. On the one hand, those regions of the world in which Hodgkin’s lymphoma is most consistently associated with EBV are those regions in which the syndrome of infectious mononucleosis is least common. On the other hand, regions in which infectious mononucleosis is most common have been those regions where the presence of EBV in tumor is least common. The questions that emerge are:
Is EBV only associated with Hodgkin’s lymphoma that harbors the genome, or is the virus also associated with lymphoma in which the genome cannot be detected?
Is infectious mononucleosis associated with all Hodgkin’s lymphoma or only with those cases in which viral gene expression can be demonstrated in tumor cells? An association with Hodgkin’s lymphoma that does not harbor the genome is not implausible. EBV infection of a lymphocyte might initiate tumorigenesis, perhaps leading to genetic instability or other change that ultimately renders the viral genome superfluous to the maintenance of the malignant state. Evidence has very recently been presented that EBV upregulates expression of activation-induced deaminase, the enzyme responsible for somatic hypermutation in germinal center B cells.8 EBV need not leave any viral genetic sequences behind as a footprint. In contrast with tumor viruses whose DNA integrates into host cell DNA, loss of EBV episomes from malignancies in tissue culture is well documented in EBV tumor cell lines.9
Answers from Scandinavia
Some of these questions were answered in part by a population-based analysis of acute infectious mononucleosis and malignancy in Denmark and Sweden.4 The relative risk of EBV-positive Hodgkin’s lymphoma was increased fourfold in patients with serologically confirmed infectious mononucleosis. There was no increase in the relative risk of EBV-negative Hodgkin’s lymphoma. The median estimated time interval from the diagnosis of mononucleosis to EBV-positive Hodgkin’s lymphoma was approximately 4 years. Thus infectious mononucleosis is clearly linked to EBV-positive Hodgkin’s lymphoma and there is no evidence to suggest that it is linked to EBV-negative Hodgkin’s lymphoma. Nonetheless, it should be noted that most patients with Hodgkin’s lymphoma have no history of documented infectious mononucleosis. Indeed, almost nothing is known of the determinants of symptomatic primary EBV infection except for evidence that infection in young adults is more likely to be symptomatic than in children.
These answers are, however, still incomplete. Why EBV-associated Hodgkin’s lymphoma accounts for highest percentage of Hodgkin’s lymphoma in those regions where infectious mononucleosis is least common is unresolved. Similarly, there are paradoxes in terms of the association with patient age. At the extremes, children under 10 years of age and adults over the age of 70, virtually all cases are EBV-associated. However, infectious mononucleosis is most commonly associated with primary infection in the teenage or early adult years.
Other Infectious Agents and Hodgkin’s Lymphoma
The incidence of Hodgkin’s lymphoma is increased in incidence among human immunodeficiency virus (HIV)-infected patients.10 In contrast to non-Hodgkin’s lymphomas that are “AIDS-defining” malignancies, the increased incidence of Hodgkin’s lymphoma is more subtle. The increased risk of Burkitt’s lymphoma and brain lymphoma in AIDS patients is hundreds- or thousands-fold, whereas the increased incidence of Hodgkin’s lymphoma in AIDS is approximately 3- to 10-fold. A recent population-based study of HIV-Hodgkin’s lymphoma in the San Francisco Bay area showed that 90% of Hodgkin’s tumors in HIV patients were EBV-positive, that nodular sclerosis histology was only half as common among HIV-infected patients as in the general population, and that lymphocyte-depleted disease was much more common.10 HIV patients were also more likely to have advanced-stage disease. Whether the contribution of HIV to Hodgkin’s lymphomagenesis is simply related to generic immunodeficiency or reflects some more specific contribution of particular HIV proteins or HIV-induced immune dysregulation is not clear. However, it is worth bearing in mind that the CD4 T cell count in HIV Hodgkin’s is typically much higher than that in some other EBV-associated malignancies in HIV patients, such as brain lymphoma.
Interesting evidence regarding measles has also been presented.11 Immunohistochemical, reverse transcriptase-polymerase chain reaction and in situ hybridization studies have shown evidence of measles virus in Hodgkin’s tumor tissues in slightly more than half of patients. These include patients with and without EBV in their tumors. Any contribution to pathogenesis remains highly speculative at this point. Despite extensive investigation, no evidence has emerged to support a role for cytomegalovirus, varicella-zoster virus, mumps, pertussis, human herpesvirus 6, 7, or 8, JC virus, adenovirus or HTLV1 or 2.
Viral Proteins in Hodgkin’s Pathogenesis
The EBV latency membrane protein 1 (LMP1) has been recognized as a likely contributor to tumorigenesis. Expression of the protein is transforming in immortalized murine cell lines and leads to lymphoma when expressed in B cells in a transgenic model.12 The protein is a member of the tumor necrosis factor receptor (TNFR) superfamily and most closely resembles CD40. However, in contrast to CD40, LMP1 signaling is constitutively active and requires no ligand. Among the multitude of activities of LMP1, it activates NF-κB by promoting the turnover of IκB alpha. Latency membrane protein 2A (LMP2A) also seems likely to play an important role. As noted above the tumor cells of Hodgkin’s lymphoma do not express immunoglobulin genes. B cells that lack functional Ig would be expected to die by apoptotic mechanisms as has clearly been shown in transgenic mouse models. However, LMP2A has been shown to provide a tonic signal that mimics that associated with immunoglobulin expression so as to prevent apoptosis of such cells—at least in a murine model.13 The role of this viral protein in preventing apoptosis in tumor cells suggests the possibility that related pathways may be targeted by small molecules for therapeutic purposes.14
Might the Presence of EBV Facilitate Diagnosis and Monitoring of EBV-Associated Hodgkin’s Lymphoma?
Posttransplant lymphoma and nasopharyngeal carcinoma are both EBV-associated malignancies in which monitoring of viral genome copy number in peripheral blood mononuclear cells or plasma has proven to be useful diagnostically and prognostically. In particular, plasma levels of viral DNA have been shown to be very important prognostically in nasopharyngeal carcinoma.15,16 Failure to clear viral DNA with therapy is indicative of early progression or relapse. Studies to determine whether EBV DNA in PBMC or plasma is similarly useful in Hodgkin’s lymphoma are underway in the oncology cooperative groups.
Does EBV in Hodgkin’s Lymphoma Provide a Suitable Target for Immunotherapy?
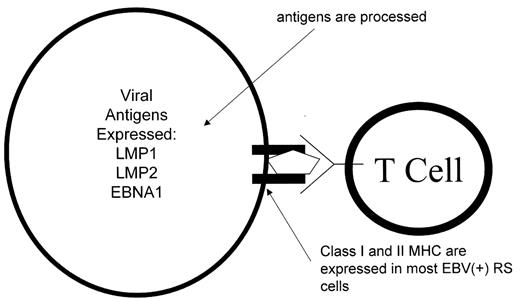
Viral antigens expressed in Hodgkin’s lymphoma are commonly recognized by CD8 T cells (Figure 3 ).17,18 The antigens are generally not mutated in Hodgkin’s lymphoma.19 Most EBV-associated Hodgkin’s tumors express class I MHC antigens and studies of cell lines suggest that the antigen-processing machinery is intact. Studies using adoptive cellular immunotherapy approaches have been initiated with limited success to date.20 However, improved targeting of the T cell lines to antigens such as LMP2A actually expressed in Hodgkin’s lymphoma holds promise as may therapeutic vaccine strategies.
Questions remain. Is classical Hodgkin’s lymphoma one entity or several entities? Many investigators have argued in favor of two or three “disease” models. However, consideration of familial Hodgkin’s lymphoma illustrates how little we know of the fundamental pathologic processes. Although relatively rare, familial Hodgkin’s lymphoma is well documented. Within a family’s pedigree EBV-positive and EBV-negative Hodgkin’s lymphoma are recognized, as well as nodular sclerosis and mixed cellularity histologies. There must be several paths to Hodgkin’s lymphoma. The relative contributions and temporal relationships of infection, immunodeficiency and other factors have yet to be answered.
II. The Utility of PET in Managing Patients with Hodgkin’s Lymphoma
Sigrid Stroobants, MD, PhD,* Gregor Verhoef, MD, PhD, and Karoline Spaepen, MD, PhD
University Hospital and Catholic University, Department of Nuclear Medicine, Herestraat 49, Leuven B-3000, Belgium
Together with non-Hodgkin’s lymphoma (NHL), Hodgkin’s lymphoma comprises only 8% of all malignancies, but patients are young and potentially curable. In contrast to many solid tumors, lymphomas are highly sensitive to chemotherapy or radiation therapy (RT) and long-term cure rates of greater than 80% for Hodgkin’s lymphoma are achieved. However, the magnitude of late treatment-related morbidity and mortality, especially in early-stage Hodgkin’s lymphoma patients treated with combination chemo-radiotherapy, as well as the fact that many patients with advanced disease cannot be cured with ABVD (Adriamycin®, bleomycin, vinblastine, dacarbazine) chemotherapy, has tempered initial enthusiasm. Accordingly, tailoring the intensity of the treatment to the individual patient has become very topical. Fluorodeoxyglucose (FDG)–positron emission tomography (PET) has a number of potential advantages for the hematologist-oncologist in refining and improving the management of Hodgkin’s lymphoma. In this section, the value of PET for the initial staging, evaluation of residual masses after treatment, and early prediction of response to chemotherapy are discussed.
Staging
Hodgkin’s lymphoma is generally treated according to stage and risk profile; therefore, pretherapeutic staging provides the basis for different treatment strategies such as RT, chemotherapy or a combination of both. Owing to the increasing use of chemotherapy at early stages and the result of the EORTC H6-F study, staging laparotomy has disappeared as a routine staging procedure. Staging of Hodgkin’s lymphoma was, until recently, mainly based on computed tomography (CT). Although structural imaging provides exquisite anatomic detail, it requires perturbation or enlargement of anatomical structure to suggest tumor. Minimally affected lymph nodes of normal size or parenchymal involvement with insufficient contrast to surrounding tissue may therefore be missed, whereas enlarged inflammatory nodes are erroneously seen as tumor deposits. With regard to bone marrow involvement, which is expected in 5%–15% of patients with untreated Hodgkin’s lymphoma, iliac crest biopsy is performed routinely, but due to patchy involvement lesions may be frequently missed.
Recently, metabolic imaging with PET has been increasingly used in the management of lymphoma patients. Since PET relies on the detection of metabolic alterations observed in cancer cells, this examination yields data that are independent of associated structural characteristics. Furthermore, the ability to perform whole body imaging within one examination without increasing the radiation burden makes PET an ideal technique to “screen” patients for cancer deposits. The most frequently used tracer is the glucose analogue FDG. The use of FDG for in vivo cancer imaging is based upon the higher rate of glucose metabolism of cancer cells compared with nonmalignant tissue. With the exception of small lymphocytic and MALT lymphomas, most lymphomas, including Hodgkin’s lymphoma, exhibit moderate to high FDG uptake.1
Several studies have investigated the role of FDG-PET for the initial staging of lymphoma (see also a recent review of Schiepers et al2). The majority of these studies compared the performances of PET with other imaging modalities. Studies were often retrospective and included a mix of NHL and Hodgkin’s lymphoma patients without separately analyzing for these histologic subgroups. Another drawback is the lack of a true gold standard against which new imaging modalities can be compared. Discordant findings between scanning techniques are uncommonly evaluated with biopsy confirmation, and the alternative approaches of follow-up of therapy, or provision of treatment based on the results of one of the tests, have associated limitations as both false positive and negative findings are observed.
Compared with gallium scintigraphy, FDG-PET appears to have advantages that include inherent superior resolution, higher sensitivity (especially in the abdomen and bone), lower radiation burden (10 mSv/PET versus 44 mSv/gallium scintigraphy) and a shorter examination time (2 hours for PET versus 3 days for gallium scintigraphy).3,4 FDG-PET was also found to be more sensitive and specific than bone scintigraphy for the detection of cortical bone involvement5 and complementary to bone marrow biopsy for detection of marrow involvement distant from the biopsy site (Figure 4 ).6
Most reports have focused on comparisons of PET with CT.2 In most cases, PET was found to be more sensitive for detecting of both nodal (e.g., small sized nodes) and extra-nodal (especially spleen and bone) involvement, but PET-negative, CT-positive lesions do occur in a small number of cases. High FDG uptake in brown fat tissue or muscle can hamper the interpretation of the head and neck and mediastinal regions. Moreover, physiological uptake in gut and the renal collecting system can obscure evaluation of lymphoma in adjacent nodal sites. Therefore, optimum use of FDG-PET is likely in combination with CT. Combined use of PET-CT can improve accuracy by increasing the certainty of diagnosis in those difficult regions.7
Incorporating PET in the initial staging in lymphomas results in upstaging or downstaging in 10%–20% of patients, but the impact on treatment management is less obvious. Naumann et al8 analyzed the potential impact of PET staging on therapy decision in 88 patients with Hodgkin’s lymphoma. Concordant findings between PET and CT were found in 70/88 patients (80%). In 11 patients (13%), PET detected additional sites, which would have resulted in treatment intensification in 9 of them, all with early stage disease. Focusing on the patients with stage IA-IIB disease only (n = 44), treatment would have been intensified in 20%. Compared to conventional diagnostics, PET downstaged 7 patients (8%) but this was only correct in 1 patient (inflammatory enlarged cervical node). False negative PET findings would have erroneously led to a minimization of therapy in 6 patients (7%). Therefore, FDG-PET should not be used instead of but in combination with conventional diagnostics.
Evaluation of Residual Masses After Treatment
Approximately two-thirds of patients with Hodgkin’s lymphoma will have a residual mass seen with standard imaging tests at the end of treatment, but only 20% of these patients will eventually relapse. The principal imaging modality for post-treatment follow-up is CT, but these scans are not able to accurately differentiate between a benign fibrotic mass and residual tumor. Early identification or prediction of patients who have not been cured with their primary treatment is important, since better outcomes with further treatment might be expected when this therapy is given at an earlier stage and with a lower tumor burden. It is common practice to consider RT to a residual mass of uncertain significance. However, late morbidities that include cardiovascular toxicity and secondary malignancies are potential risks that challenge this “standard” use of RT.
Several recent reports have shown the effectiveness of FDG-PET in the evaluation of residual disease at the end of treatment, including studies assessing patients with Hodgkin’s lymphoma (Table 1 ).9– 17 A high negative predictive value (NPV) of FDG-PET (81%–100%) has been consistently reported by most studies, clearly showing the ability of PET to identify patients with an excellent prognosis. Relapses are infrequent and occur rarely within the first year after completing treatment, and therefore probably reflect low tumor burdens that are below the limit of PET detection. Since a substantial number of patients received additional RT after a negative PET scan, reported progression-free survivals (PFS) and NPVs may represent overestimations. The question of whether, after first-line chemotherapy, RT can be omitted in patients with a negative PET scan is therefore still unanswered. Prospective randomized trials are needed to compare the PFS, overall survival (OS) and long-term complications observed in patients with a negative PET after chemotherapy who receive no further therapy or standard RT as planned at the initiation of treatment.
In contrast to the NPV, the positive predictive value (PPV) of PET is more variable (25%–100%). We have observed that, for several reasons, false positive rates decrease with increasing experience in evaluating residual masses. First, the timing of PET scanning is crucial. If done immediately after completing RT, inflammatory changes can result in false positive interpretations. Therefore, it is better to perform PET before RT or to wait at least 3 months after completing RT. Second, patients with Hodgkin’s lymphoma are often young and develop thymic hyperplasia at the end of treatment. This produces a typical pattern on FDG-PET with a butterfly-shaped configuration with moderate and homogeneous FDG uptake, whereas residual lymphoma is associated with a more intense and focal pattern (Figure 5 ).18 Thirdly, intense FDG uptake in muscle and brown fat tissue may be seen in lean and young patients and can hamper the detection of residual nodes after treatment19; combined PET-CT images are often necessary to resolve these appearances (Figure 6 ). Finally, inflammatory lesions are known to cause false positive PET findings and occur more frequently after chemotherapy. Correlation of PET findings with pretreatment scans (a new lesion in a previously noninvolved region is rarely lymphoma, Figure 7 ), clinical history or other imaging modalities can help to identify these lesions.
When PET images are interpreted in correlation with clinical history and CT by radiologists/nuclear medicine physicians with specific expertise in PET, the PPV of PET in patients with Hodgkin’s lymphoma is probably equivalent to that observed in NHL (> 80%) and residual positivity is highly suggestive of residual lymphoma for which additional treatment should be considered. In equivocal cases close follow-up or additional diagnostic procedures may be warranted to reduce risks of giving additional treatments that are based on false-positive results.
Early Response Monitoring for Risk Stratification
Since treatments that are more aggressive, but also more toxic, are available, there is a growing interest in the use of risk-directed approaches to utilize prognostic factors that predict for relapse. The International Prognostic Index (IPI) for Hodgkin’s lymphoma20 summarizes different prognostic clinical factors at presentation and has become an established parameter for risk stratification. The clinical features incorporated in the IPI reflect the biologic heterogeneity of the disease. However, the duration of a complete remission (CR) and other long-term outcomes might be more affected by the sensitivity of the tumor to chemotherapy than by the prognostic factors seen at presentation. Reliable predictions of outcome early in the treatment phase could lead to more immediate changes of therapy that might improve outcomes, including survival.
Morphological imaging modalities, as a criterion for early response monitoring, are inefficient at differentiating responders from non-responders, in part because several courses of chemotherapy are necessary before effectiveness can be reliably determined. Using CT findings as a criterion for early response may label an unacceptable number of patients as poor responders and expose them to more aggressive or experimental therapies, even if these patients could achieve durable complete responses with standard chemotherapy. Promising results with FDG-PET can be obtained when evaluating treatment response. Because glucose provides the primary source of carbons for the de novo synthesis of nucleic acids, lipids and amino acids, FDG uptake is closely related to the number and proliferation capacity of viable cells. Treatment-induced changes resulting in tumor cell death or growth arrest reduce FDG uptake, making this a sensitive and early marker of response.21 Romer et al22 described a rapid decrease of FDG-uptake in patients with NHL as early as 7 days after commencing treatment. However, FDG-uptake at 42 days correlated better with long-term outcomes; early FDG reduction probably reflects initial chemosensitivity of the tumor whereas results of later evaluations are more related to the detection of resistant clones. Since that initial description, several studies have correlated PET response during treatment with final outcome, either focusing on the value of PET during initial induction treatment (Table 2 )23–,28 or prior to high-dose therapy and stem cell transplantation (HDT/SCT) (Table 3 ).29– 33
Early response assessment of first-line induction chemotherapy
Persistent PET positivity observed after a few cycles of chemotherapy is associated with a high probability of relapse at the end of treatment (PPV 67%–100%). To date, experience has been almost exclusively gained in patients with aggressive NHL. In the largest prospective study, by Spaepen et al,27 evaluating 70 patients with aggressive NHL, 33 patients showed persistent abnormal FDG uptake after 3–4 cycles of doxorubicin containing first-line therapy and none of these patients achieved a durable CR (median PFS, 45 days). Of the 37 patients with a negative mid-treatment PET, only 6 relapsed with a median PFS of 301 days (P < 0.00001). In a multivariate analysis, FDG-PET at mid-treatment was a stronger predictor for PFS (P < 0.0000001) and OS (P < 0.000009) than the IPI (P < 0.58 and P < 0.03, respectively).
Preliminary data in Hodgkin’s lymphoma by Mikhaeel et al25 show similar results: all 6 patients with persistent disease on PET relapsed compared to only 2 out of 26 patients with a negative PET scan mid-treatment.
Prospective trials are now required to establish the clinical role of PET in this setting by comparing overall survival after randomizing patients with positive mid-treatment FDG-PET findings to either continue standard induction therapy or to switch to a more aggressive approach.
Prognostic value of FDG-PET before HDT/SCT
Improvements in therapy may mean that patients with nonresponding or relapsing disease represent a cohort that has an even more unfavorable prognosis. Currently, many centers consider high-dose therapy with autologous stem cell transplantation as the treatment of choice for these poor prognosis lymphoma patients. The most important prognostic factors to predict favorable outcome after HDT/SCT are the duration of remission prior to progressive disease and the chemosensitivity of the tumor to salvage therapy administered prior to SCT. Since FDG-PET has become a potential tool to differentiate between responders and non-responders early during chemotherapy, several groups have investigated the value of [18F]FDG-PET to identify patients who would benefit from this aggressive treatment. Again, high PPVs with short PFS are recorded in patients with persistent disease on PET. Co-existing inflammatory disease is more frequently seen in this population and can cause false-positive PET results. Correlations of PET, clinical and morphological imaging data are therefore essential. Superior PPVs are obtained if PET is performed just prior to transplantation (> 87%) compared with those performed early during the administration of salvage therapy (Schot et al,32 PPV = 65%). This is not surprising since resistant clones can only become apparent when the sensitive ones are killed. These findings are confirmed by quantitative analyses: only a major decrease in the amount of FDG uptake and not the reduction in metabolic volume is predictive for favorable outcome. Further studies are necessary to define the optimal timing of PET and response criteria before this technique can be used as a standard practice to determine patient eligibility for HDT/SCT.
Future Directions and Clinical Implementation
For post-treatment evaluation, especially if a residual mass is present, PET has become a standard procedure in routine clinical practice and has replaced gallium scintigraphy as an adjunct to CT. In cases where, after completing first-line treatment, abnormal FDG-uptake is seen in initially involved sites and there is a low suspicion of co-existing inflammation, strong consideration for providing alternative therapy should be given. In Hodgkin’s lymphoma patients with stage III or IV disease, negative PET results do not exclude minimal residual disease and/or the risk of late relapse. These patients might benefit from repeated follow-up scans. Patients with early-stage Hodgkin’s lymphoma and a negative FDG-PET scan after therapy are considered as CR and follow-up scanning is indicated only if recurrent disease is suspected. The increasing use of integrated PET-CT systems requires systematic evaluation, including assessment of the radiation burden associated with repetitive combined imaging studies.
For prognostic determination in the early phases of therapy, there are promising data for the predictive role of FDG-PET, but larger patient populations and longer follow-ups are necessary for confirmation. As current studies are extended, data regarding the timing of the interim scan, definition of PET response criteria, and utility in tailoring RT, stem cell transplantation and radio-immunotherapy will become available. These assessments of utility, potentially supported with the results of economic evaluations, will inform the process to determine new standards of practice.
III. Balancing Treatment Efficacy and Long-term Toxicities: The Model of Limited Stage Hodgkin’s Lymphoma
Ralph M. Meyer, MD*
Juravinski Cancer Centre and McMaster University, 699 Concession Street, Hamilton ONT L8V 5C2, Canada
The objectives of this review are to place into context what might now constitute standard therapy for patients with limited-stage Hodgkin’s lymphoma and to suggest a potential next generation of hypotheses to be tested to further improve the outcomes of these patients. These objectives will be addressed by considering a broad group of issues that frame this topic, reviewing results of some recently completed randomized trials that have tested new treatment strategies in patients with limited-stage disease, and summarizing some recent reports that have evaluated long-term complications that are considered to be treatment-related.
Statement of the Problem
Hodgkin’s lymphoma is an uncommon form of cancer. In Canada, a country of approximately 32 million people, the most recent available annual statistics (from 20001) show that 134,413 people were diagnosed with cancer and 62,672 deaths were attributed to this disease. The most common hematologic neoplasm was non-Hodgkin’s lymphoma, with an incidence of 5444 new cases and 2537 deaths. In contrast, a new diagnosis of Hodgkin’s lymphoma was made in 811 people and 128 deaths observed. These data exemplify the success of current therapies that have resulted in high expectations of cure for patients with Hodgkin’s lymphoma, but also demonstrate the nature of future challenges; strategies are needed to identify and provide successful therapy to the 15%–20% of patients who are not cured, and for the more than 80% of patients who have had their cancer successfully eradicated, long-term threats to quality of life and survival need to be reduced. Given the uncommon nature of Hodgkin’s lymphoma, evaluation of interventions to improve long-term outcomes will require international collaborations and, by definition, long periods of follow-up.
For patients with limited-stage Hodgkin’s lymphoma, the development of successful therapies that control the malignancy makes evaluation of the competing objectives to eradicate the cancer while reducing the risks of long-term treatment-related toxicities particularly important. This balance has evolved even over the past 15 years. In 1989, an international collaboration of single institutions and cooperative groups led to the Paris International Workshop and Symposium. At this workshop, the outcomes of 14,702 patients diagnosed with Hodgkin’s disease between the early 1960s and 1987 were pooled, allowing for an analysis of results that extended over approximately 20 years.2 This analysis included 9041 patients assessed as having “early-stage” Hodgkin’s disease; 1957 (22%) of these patients had died. An actuarial analysis of cumulative deaths by cause demonstrated that over the first decade of follow-up, most deaths were directly attributed to Hodgkin’s disease. However, by 13 years of follow-up, deaths attributed to other causes surpassed those due to progressive Hodgkin’s disease. Furthermore, the observed number of deaths attributed to other causes exceeded those expected in comparison with an age- and gender-matched control group. Specific “other” causes of death included an increased occurrence of cardiovascular events and second cancers. As new treatment strategies improve control of Hodgkin’s lymphoma and reduce the incidence of death from this cause, recent randomized controlled trials evaluating therapy in patients with limited-stage disease already demonstrate, with much shorter durations of follow-up, that causes other than progressive Hodgkin’s lymphoma or acute treatment-related toxicity will account for the majority of deaths. In a randomized trial comparing extended-field radiation therapy with combined-modality therapy, Sieber et al observed that with a median follow-up of 22 months, 17 deaths had been observed in 572 patients; 10 deaths (59%) were attributed to causes other than progressive Hodgkin’s lymphoma or acute treatment-related toxicity.3 In a randomized trial comparing a strategy that includes radiation therapy with chemotherapy alone, Meyer et al observed that with a median follow-up of 4.2 years, 15 deaths had been observed in 399 patients; 9 deaths (60%) were attributed to causes other than progressive Hodgkin’s lymphoma or acute treatment-related toxicity.4 Thus, to further improve long-term survival, reduction in deaths from other causes will become the major focus of new interventions.
Deriving Optimum Treatment: Methodologic Issues
Two basic principles of clinical trial design require attention in order to reduce the risk of bias that might confound resulting conclusions.5 The first principle requires that when a study aim is to determine the clinical course of a disease or prognosis of specific patient groups, it is necessary to evaluate an inception cohort of patients with the specific disease who have been identified at a relatively uniform time point in the disease process. The second principle requires that when a study aim is to evaluate whether new interventions should replace an existing form of therapy, these interventions should be tested in randomized controlled trials. Particularly in evaluating new modalities of therapy, systematic reviews that include meta-analyses of randomized trials testing similar interventions can enhance an understanding of the consistency, precision and generalizability of trial results.6
Randomized controlled trials and meta-analyses need to include evaluations of relevant outcome measures that would properly inform determinations of standard treatment policies. For most clinical problems, a hierarchy of relevant outcomes can be constructed.7 This hierarchy includes specific domains such as survival, quality of life and health care economics. Often other outcomes that serve as proxy measures for these specific domains will be reported; these surrogate measures can include parameters of disease control or treatment-related toxicity that might predict for overall survival or quality of life. The use of surrogate outcomes to formulate standard treatment practices requires caution as the predictive properties of the surrogate marker for a specific domain may vary. The management of patients with limited-stage Hodgkin’s lymphoma exemplifies this dilemma. Historically, as new treatment strategies were developed to deal with an otherwise fatal malignancy, it is understandable that evaluations of disease control would be a central outcome measure. As it is now apparent that, in these patients, long-term survival and likely quality of life have complex determinants, the need to directly evaluate these specific outcomes is recognized, with a reduced reliance upon use of proxy measures, such as the more narrow evaluation of disease control.
Further complicating evaluation of new therapies for patients with limited-stage Hodgkin’s lymphoma is the discrepancy of time lines for evaluating different outcomes. Assessments of disease control, such as progression-free survival, can be reported with reliability after relatively short follow-up periods of 3–5 years, whereas evaluations of long-term overall survival will require more than a decade of follow-up. This discrepancy results from the time required to observe the long-term toxicities of therapy. Recognition of this requirement is particularly important given the young age of most patients with limited-stage Hodgkin’s lymphoma; these patients are unlikely to have confounding comorbidities that would otherwise influence long-term survival, meaning that detrimental late effects of therapy will have a greater proportionate effect on survival. As a result of this time line discrepancy, completion of accrual to randomized trials evaluating new forms of therapy, and reporting of surrogate outcomes such as progression-free survival, will be possible well before the long-term outcomes of previously tested therapies are completely understood.
Synopsis of Recent Randomized Controlled Trials
The historical complexity of managing patients with limited-stage Hodgkin’s lymphoma is exemplified by the number of different interventions that have been tested in randomized controlled trials. These include assessments of invasive staging by laparotomy, different fields of radiation therapy, radiation therapy as a single modality versus combined-modality therapy, different chemotherapeutic regimens, and chemotherapy as a single modality. One landmark report was the H6 trial of the European Organisation for Research and Treatment of Cancer (EORTC), which demonstrated that no benefits were detected through use of surgical in comparison with clinical staging.8 Another landmark report on two individual patient-data meta-analyses demonstrated that more extended fields of radiation therapy reduced the risk of disease recurrence at 10 years but did not alter overall survival, and that use of combined-modality therapy in comparison with radiation therapy alone also reduced the risk of recurrent disease but did not significantly alter 10-year overall survival.9 These reports contributed to evolution of treatment from previous practices of evaluating disease stage and risk by laparotomy and complex prognostic factor schema with treatment including extended-field radiation, to more recent practices in which evaluation of stage emphasizes use of imaging modalities and treatment is with an abbreviated course of chemotherapy combined with involved-field radiation therapy. The choice of chemotherapy to be combined with radiation therapy has also been clarified. Randomized trials have confirmed that in comparison with mechlorethamine, vincristine (Oncovin®), prednisone and procarbazine (MOPP), treatment with doxorubicin (Adriamycin®), bleomycin, vinblastine and dacarbazine (ABVD) or MOPP-ABV(D) (with or without dacarbazine) provides superior disease control.10 Randomized trials comparing ABVD with MOPP-ABV(D) have failed to detect differences in disease control or overall survival10; treatment with ABVD is associated with less treatment-related toxicity and is considered to carry a lower long-term risk of infertility and development of acute leukemia or myelodysplasia. These results have led to ABVD becoming a standard regimen in treating patients with limited-stage disease.
Based on the above results, a recent generation of randomized trials conducted by multiple cooperative groups has been reported. These trials have tested combined-modality therapy, in which the extent of a therapeutic modality is reduced, or use of chemotherapy alone (Table 4 ). In three studies of an abbreviated course of chemotherapy (ABVD,3 AV,11 or MOPP-ABV12) combined with radiation therapy (extended3,11 or involved-field12) compared with extended-field radiation therapy, disease control was superior in each trial in the groups receiving combined-modality therapy. In one trial, overall survival was also superior.12 These results have led to a reasonable choice of standard therapy including 2–3 cycles of ABVD and involved-field radiation therapy. The National Cancer Institute of Canada Clinical Trials Group (NCIC-CTG) led a trial involving 399 patients with non-bulky stages I–IIA disease that compared 4–6 cycles of ABVD with standard therapy that included radiation therapy (extended-field radiation alone in favorable risk patients and combined-modality therapy in unfavorable risk patients).4 With a median follow-up of 4.2 years, 5-year freedom from disease progression was superior in patients allocated to radiation therapy (93% vs. 87%; P = 0.006), but no difference in overall survival was detected (94% with radiation therapy and 96% with ABVD; P = 0.4). The potential role of this trial in determining current standard practices will be discussed below.
Through cooperative group mechanisms, the above trials have included larger sample sizes with homogeneous groups of patients with limited-stage Hodgkin’s lymphoma. Two randomized trials conducted at single institutions also provide supporting data to the findings described above, although conclusions from these trials are limited by inclusion of smaller numbers and more heterogeneous groups of patients. To evaluate the role of radiation field size, Bonadonna et al have reported the outcomes of 140 patients with stage I-II A/B disease including 20% who had bulky mediastinal disease.13 Patients received six cycles of ABVD followed by involved-field or subtotal nodal radiation; no differences in 12-year freedom from progression (94% vs 93%) or overall survival (94% vs 96%) were detected in the two respective groups. To test the role of chemotherapy alone, Straus et al have reported the results of a randomized trial comparing ABVD plus involved or more extended fields of radiation to ABVD alone in 152 patients with stage I-II A/B and IIIA disease.14 No differences in 5-year freedom from progression (86% vs 81%) or overall survival (97% vs 92%) were detected in the groups who were or were not randomized to receive radiation therapy.
Late Effects: Second Cancers and Cardiovascular Events
Assessments of the risks of second cancers and cardiovascular events in patients treated for Hodgkin’s lymphoma have been facilitated by the availability of large inception cohorts evaluated by co-operative groups and single institutions. Particularly for assessment of second cancers, large geographically based registries allow comparison of the observed risk of developing a second neoplasm with the expected risk of developing cancer in a control population. Pooling of data by multiple groups further strengthens these evaluations. Dating back to the 1989 Paris Workshop, analyses have clearly identified that patients treated for Hodgkin’s disease were at increased risk of developing second cancers, including solid tumors, acute leukemia and non-Hodgkin’s lymphoma.15 For each of these malignancies, older age at the time of treatment predicted for an increased risk of developing the malignancy. The risk of developing a solid tumor was also associated with wide-field radiation therapy and combining any radiation therapy with MOPP-like chemotherapy.
A summary of recent studies evaluating the occurrence of second malignancies is shown in Table 5 . A review of these reports requires an appraisal of variables that differ between studies including patient and disease-related factors (e.g., age at diagnosis, stage or other risk features that might influence clinical course or treatment received, need for second or subsequent-line therapy), treatment factors (e.g., radiation therapy, chemotherapy, combined modality therapy, stem cell transplantation), and methodologic features of the study (e.g., details of constructing the inception cohort, duration of follow-up, loss to follow-up, calculation of relative risk and/or absolute excess risk). Studies that report both the relative risk of developing a second malignancy and the absolute excess risk observed are particularly informative. Relative risk refers to the ratio of observed cancers to that expected; high relative risks might be associated with the frequent development of a specific cancer in patients previously treated for Hodgkin’s lymphoma, or conversely the observation of malignancies that would be uncommonly expected in control populations. Absolute excess risk refers to the absolute number of excess cancers observed in comparison with those expected with conversion of this value to account for the number of patient-years of follow-up; this value more appropriately reflects the additional burden of the second malignancy.
The studies described in Table 5 demonstrate several consistent features:
Solid tumors comprise the most common type of second malignancy, accounting for 59%–80% of cases and 58%–75% of absolute excess risk.
Leukemia and non-Hodgkin’s lymphoma make up a relatively small number of second cancers observed, but are associated with very high relative risks because of the uncommon expectation of these malignancies in the control populations.
Studies evaluating age of treatment for Hodgkin’s lymphoma demonstrate that younger age is associated with higher relative risks for developing a solid tumor16,17; this in part reflects the relatively lower incidence of malignancies expected in matched control populations. Within adult populations, absolute excess risks appears to increase with increasing age—this finding may be particularly weighted by risks associated with developing lung cancer.16,17,19
The cumulative risk of developing a solid tumor continuously increases, until at least 25 years of follow-up. In contrast, the risk of developing acute leukemia is predominantly confined to the first 10 years of follow-up.16
Treatment received is associated with different risks of developing specific second cancers. The risk of developing a solid tumor is increased in patients receiving radiation therapy in comparison with those receiving chemotherapy alone.16,19,21 This risk may be further increased by use of larger radiation fields18 and, at least for some solid tumors, by use of combined-modality therapy.16–,19,21 Risk of a second malignancy progressively increases over time in patients who have received radiation therapy, whereas a plateau in cumulative incidence is suggested in patients who receive chemotherapy alone.16
Data regarding the risk of long-term cardiovascular events in patients treated for Hodgkin’s lymphoma are less comprehensive as geographic registries for cardiovascular disease are less sophisticated than those seen for cancer. In addition, different types of cardiovascular events exist (e.g., coronary and non-coronary artery disease, valvular and pericardial disease) and these can be defined by different outcome measures (e.g., symptomatic or asymptomatic detection, association with hospitalization and/or surgical intervention, death). Recent reports of cardiovascular outcomes in inception cohorts of patients with Hodgkin’s lymphoma treated with radiation therapy are shown in Table 6 . Observations from these studies include the following:
The actuarial risk of developing symptomatic coronary artery disease appears to be in the range of 6% at 10 years and 10%–20% at 20 years.22,23 These rates represent relative risks for surgical intervention or hospitalization of 1.5–2.0. The actuarial risks of death from cardiac ischemia appear to be 2%–6% at 10 years and 10%–12% at 15 to 25 years. These rates represent relative risks of approximately 5 in comparison with control populations.23,24
The actuarial risk of non-cardiac (e.g., carotid or subclavian vessel) vascular events is approximately 3% at 10 years and 7% at 20 years.22
The actuarial risk of valvular heart disease is 4% at 15 years and 6% at 20 years. These represent relative risks of 8 to 9 with respect to the need for valve replacement.22
Increased risks of cardiac events are particularly observed in patients who have known cardiac risk factors such as hypertension and hyperlipidemia. Use of chemotherapy, in addition to radiation treatment, does not appear to confer a higher risk.22,23
In summary, patients treated for Hodgkin’s lymphoma are at increased risk for second cancers and cardiovascular complications; these events now comprise the major causes of death in patients with limited-stage Hodgkin’s lymphoma. These risks are principally due to the use of radiation therapy. While it is reasonable to propose that reductions in radiation field size and dose might reduce these risks, long-term follow-up of randomized trials testing these strategies, and associated meta-analyses, will be required to confirm these hypotheses.
Current Treatment Options and Future Strategies
Based on current data, there are two reasonable options of therapy for patients with limited-stage Hodgkin’s lymphoma. The choice between these options requires that patients be informed of the trade-offs of potential superior disease control when radiation therapy is included versus the risks of late effects associated with this therapy. One standard option is treatment with combined-modality therapy that includes 2–3 cycles of ABVD and involved-field radiation therapy.26 Details within this option may be clarified by the results of new trials, including the role of 2 versus 4 cycles of ABVD, based on data from the German Hodgkin’s Study Group (GHSG) HD-10 trial, and the role of different doses of involved-field radiation therapy, such as 20 versus 30 Gy (GHSG HD-10 trial) and 20 versus 36 Gy (EORTC H9 trial). Further minimization of chemotherapy, such as use of AV as reported by Press et al in a trial conducted by the Southwest Oncology Group,11 is now being tested by the GHSG in their H-13 trial that compares ABVD with AV, ABV and AVD. However, all of these options will continue to include some long-term risk associated with use of radiation therapy, and while reporting of disease control may be possible within the next 3–5 years, evaluations of long-term toxicities are unlikely to become apparent for another decade.
An alternative current option is to consider treatment with ABVD alone. Based on the data of the NCIC-CTG trial, long-term disease control can be expected in 87% of patients. While this degree of disease control is less than that observed in patients receiving combined modality therapy, the risks of inferior disease control may be counterbalanced by a reduced risk of long-term toxicities. Further analysis of this trial may permit identification of specific patient groups that are particularly likely to experience long-term disease control, and evaluations of quality of life may facilitate determination of patient preferences in considering risk-benefit trade-offs during the decision-making process.
Future advances may result from developments in diagnostic testing. Application of new imaging modalities, such as PET scanning, may facilitate abilities to more confidently identify at earlier points in therapy those patients who have received sufficient treatment to provide long-term disease control, thus potentially permitting an ability to avoid exposure to additional therapies. Enhanced understandings of the basic pathogenic mechanisms of Hodgkin’s lymphoma, including molecular profiling, may also assist in identifying specific prognostic groups that might receive different therapies. However, advances in these, and other related fields, will require the completion of randomized controlled trials that have sufficient follow-up to properly assess overall survival.
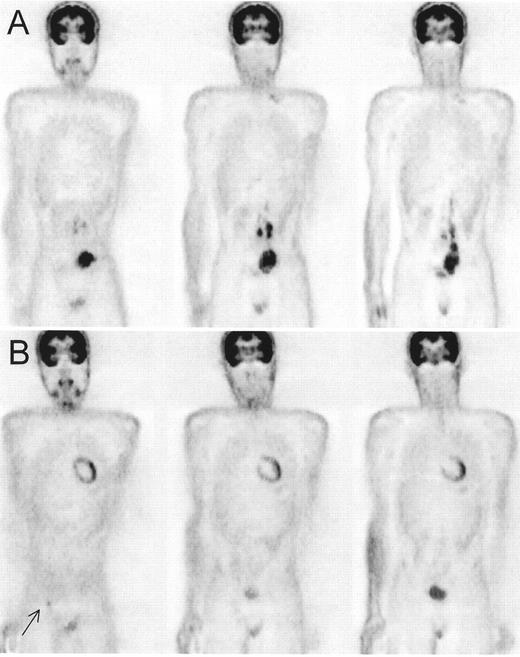
Patient with Hodgkin’s lympoma, nodular sclerosis, stage IIB based on conventional imaging modalities (computed tomography [CT] and iliac crest biopsy).
Positron emission tomography (PET) also showed increased fluorodeoxyglucose (FDG) uptake in the lumbar spine suspected for bone involvement (A) upgrading this patient to stage IVB. PET after 4 cycles of ABVD (Adriamycin®, bleomycin, vinblastine, dacarbazine), (B) only showed increased FDG uptake in bone marrow secondary to marrow regeneration. The hot spot in the spine has become cold representing replacement of the bone marrow by nonviable tumor tissue.
Patient with Hodgkin’s lympoma, nodular sclerosis, stage IIB based on conventional imaging modalities (computed tomography [CT] and iliac crest biopsy).
Positron emission tomography (PET) also showed increased fluorodeoxyglucose (FDG) uptake in the lumbar spine suspected for bone involvement (A) upgrading this patient to stage IVB. PET after 4 cycles of ABVD (Adriamycin®, bleomycin, vinblastine, dacarbazine), (B) only showed increased FDG uptake in bone marrow secondary to marrow regeneration. The hot spot in the spine has become cold representing replacement of the bone marrow by nonviable tumor tissue.
Residual increased fluorodeoxyglucose (FDG) uptake after ABVD therapy in two patients with Hodgkin’s lymphoma.
The typical butterfly-shaped configuration is suggestive for thymic hyperplasia (A), and this patient remained in complete remission after a follow-up of 23 months. The more focal uptake seen in patient (B) is more suspicious for residual tumor, progression in the residual mass was seen 6 months later on CT.
Residual increased fluorodeoxyglucose (FDG) uptake after ABVD therapy in two patients with Hodgkin’s lymphoma.
The typical butterfly-shaped configuration is suggestive for thymic hyperplasia (A), and this patient remained in complete remission after a follow-up of 23 months. The more focal uptake seen in patient (B) is more suspicious for residual tumor, progression in the residual mass was seen 6 months later on CT.
Persistent fluorodeoxyglucose (FDG) uptake in the cervical and mediastinal regions, 3 months after completion of chemo-radiotherapy.
Based on positron emission tomography (PET) alone, no discrimination is possible between residual lymphoma and physiological uptake in muscle and/or brown fat tissue (A). Fusion images located all hot spots to be in brown fat tissue. Based on PET–computed tomography (CT), it was concluded that the patient achieved a complete remission.
Persistent fluorodeoxyglucose (FDG) uptake in the cervical and mediastinal regions, 3 months after completion of chemo-radiotherapy.
Based on positron emission tomography (PET) alone, no discrimination is possible between residual lymphoma and physiological uptake in muscle and/or brown fat tissue (A). Fusion images located all hot spots to be in brown fat tissue. Based on PET–computed tomography (CT), it was concluded that the patient achieved a complete remission.
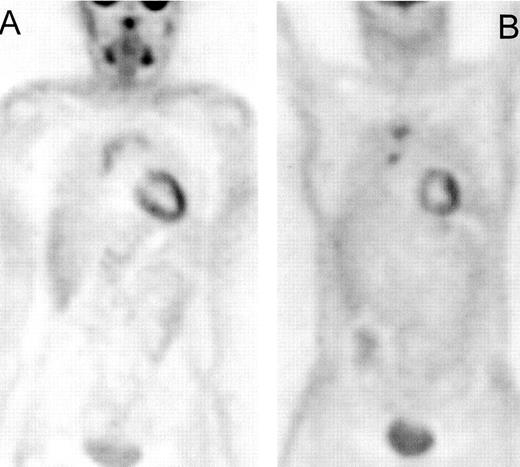
Positron emission tomography (PET) scan of a patient with Hodgkin’s lymphoma, nodular sclerosis, stage IIIB, prior to the start of chemotherapy (A).
PET after therapy (B) showed a complete normalization of the initially involved sites, but a new hot spot developed in the right groin (→). Correlation with clinical history revealed erysipelas of the right leg with probably inflammatory nodes in the groin. Repeat PET scan after antibiotics was completely normal. The patient remains in complete remission after a follow-up of 4 years.
Positron emission tomography (PET) scan of a patient with Hodgkin’s lymphoma, nodular sclerosis, stage IIIB, prior to the start of chemotherapy (A).
PET after therapy (B) showed a complete normalization of the initially involved sites, but a new hot spot developed in the right groin (→). Correlation with clinical history revealed erysipelas of the right leg with probably inflammatory nodes in the groin. Repeat PET scan after antibiotics was completely normal. The patient remains in complete remission after a follow-up of 4 years.


![Figure 4. Patient with Hodgkin’s lympoma, nodular sclerosis, stage IIB based on conventional imaging modalities (computed tomography [CT] and iliac crest biopsy). / Positron emission tomography (PET) also showed increased fluorodeoxyglucose (FDG) uptake in the lumbar spine suspected for bone involvement (A) upgrading this patient to stage IVB. PET after 4 cycles of ABVD (Adriamycin®, bleomycin, vinblastine, dacarbazine), (B) only showed increased FDG uptake in bone marrow secondary to marrow regeneration. The hot spot in the spine has become cold representing replacement of the bone marrow by nonviable tumor tissue.](https://ash.silverchair-cdn.com/ash/content_public/journal/hematology/2004/1/10.1182_asheducation-2004.1.184/4/m_meyer_fig4.jpeg?Expires=1765928077&Signature=Lcj5t9WzRXO0Hw46q~eEMEz~1z-uIS5Kg33WRXGg0GY0dqWDY9W3EGeCJGoLmDhMuqKKEZKYPf9zRFx~whUXG8aIgecVwH5PvSkxPaQhnapBQgdIDRAJulBLwgHaZvqAMQ7KFyxICDKqxWhux9TDAz6OfJaIPlsSZYxDUNrbZPY6In2QWh8Ac~v9KoSZx8NX2MXN0GU20oF~m2Ubz4rvECF9Ffxcy32lCk0h3osWJDlWGUqu9zl~dObUPseAXnu~Z3qF3lNUEgLWfmX0p2lTFm8UORhzrrDCFAmPzL3Gx1gB6O3CmBXQHiLqD9LzzoiuoH6q6tx0I3XWFNJyJONRyQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)




![Figure 4. Patient with Hodgkin’s lympoma, nodular sclerosis, stage IIB based on conventional imaging modalities (computed tomography [CT] and iliac crest biopsy). / Positron emission tomography (PET) also showed increased fluorodeoxyglucose (FDG) uptake in the lumbar spine suspected for bone involvement (A) upgrading this patient to stage IVB. PET after 4 cycles of ABVD (Adriamycin®, bleomycin, vinblastine, dacarbazine), (B) only showed increased FDG uptake in bone marrow secondary to marrow regeneration. The hot spot in the spine has become cold representing replacement of the bone marrow by nonviable tumor tissue.](https://ash.silverchair-cdn.com/ash/content_public/journal/hematology/2004/1/10.1182_asheducation-2004.1.184/4/m_meyer_fig4.jpeg?Expires=1766007440&Signature=LXNMNVcVfXP~GTFG2L31vXwAnTuxLDlc6uLdFIC-5P4TXVfEDqi8n1V4aPgyMOfiQVZoOlm8wVYVL72Ng74RDOz4T4XmVi7ax47VE1k3tGkfraSHx2uB24BzVzlA8zhJVwGesrwSLuT8HhWL5GO9-zVYeS1egFYMDzU52Mlc0NXJvXweEs~SD3P9s4tYpELJq3dHqlOgwKaQk3KMqO~mFkqrmKOhQX6Jcb3c7a0kL2KK5aFuA~lZYHRCNOsJsbPszIkvAzYXXlP2OiOBgG37KVgs5V1T~iA3BjMLWXeoNXqXYvSqNprjj3rSlOdveqiKer0xz0UbIj-yuO2OCHKC~Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)