Abstract
Chronic lymphocytic leukemia (CLL) is one of the most commonly diagnosed leukemias managed by practicing hematologists. For many years patients with CLL have been viewed as similar, with a long natural history and only marginally effective therapies that rarely yielded complete responses. Recently, several important observations related to the biologic significance of VH mutational status and associated ZAP-70 overexpression, disrupted p53 function, and chromosomal aberrations have led to the ability to identify patients at high risk for early disease progression and inferior survival. Concurrent with these investigations, several treatments including the nucleoside analogues, monoclonal antibodies rituximab and alemtuzumab have been introduced. Combination of these therapies in clinical trials has led to high complete and overall response rates when applied as initial therapy for symptomatic CLL. Thus, the complexity of initial risk stratification of CLL and treatment has increased significantly. Furthermore, when these initial therapies do not work, approach of the CLL patient with fludarabine-refractory disease can be quite challenging. This session will describe the natural history of a CLL patient with emphasis on important decision junctures at different time points in the disease.
In Section I, Dr. Stephan Stilgenbauer focuses on the discussion that occurs with CLL patients at their initial evaluation. This includes a review of the diagnostic criteria for CLL and prognostic factors utilized to predict the natural history of the disease. The later discussion of risk stratification focuses on molecular and genomic aberrations that predict rapid progression, poor response to therapy, and inferior survival. Ongoing and future efforts examining early intervention strategies in high risk CLL are reviewed.
In Section II, Drs. Ian Flinn and Jesus G. Berdeja focus on the discussion of CLL patients when symptomatic disease has developed. This includes an updated review of monotherapy trials with nucleoside analogs and recent trials that have combined these with monoclonal antibodies and/or alternative chemotherapy agents. Appropriate application of more aggressive therapies such as autologous and allogeneic immunotherapy and less aggressive treatments for appropriate CLL patient candidates are discussed.
In Section III, Dr. John Byrd focuses on the discussion that occurs with CLL patients whose disease is refractory to fludarabine. The application of genetic risk stratification in choosing therapy for this subset of patients is reviewed. Available data with conventional combination based therapies and monoclonal antibodies are discussed. Finally, alternative promising investigational therapies including new antibodies, kinase inhibitors (CDK, PDK1/AKT, PKC) and alternative targeted therapies (DNA methyltransferase inhibitors, histone deacetylase inhibitors, etc.) are reviewed with an emphasis on the most promising agents for this patient population.
I. Genetic Risk Stratification of CLL
Stephan Stilgenbauer, MD*
University of Ulm, Department of Internal Medicine III, Robert Koch Str. 8, Ulm D-89081, Germany Supported by Wilhelm Sander-Stiftung, Deutsche Krebshilfe, Fresenius Stiftung, MedacSchering Onkologie GmbH, Hoffmann-La Roche AG and AMGEN GmbH.
Clinical Course, Prognostic Factors, and Treatment Options
Chronic lymphocytic leukemia (CLL) is the most frequent type of leukemia in the Western world and affects mainly elderly individuals, but about a third of patients are less than 60 years of age at diagnosis.1 CLL follows an extremely variable clinical course with overall survival times ranging from months to decades. Some patients have no or minimal signs and symptoms during their entire disease course and have a survival time similar to age-matched controls. Other patients experience rapidly deteriorating blood counts and organomegaly and suffer from symptoms at diagnosis or soon thereafter necessitating therapy. Because the initiation of therapy for early stage patients has not been shown to prolong survival,2 therapeutic procedures traditionally have been aimed at palliation and were instituted only for advanced stage or symptomatic disease. More recently, however, highly effective and potentially curative approaches such as antibody-chemotherapy and autologous or allogeneic stem cell transplantation have been developed. The therapeutic options vary markedly with regard to efficacy, toxicity and cost, and new risk-stratified algorithms of therapy are becoming increasingly necessary.
The standard clinical procedures to estimate prognosis are the clinical staging systems developed by Rai et al and Binet et al.3,4 These systems define early (Rai 0, Binet A), intermediate (Rai I/II, Binet B) and advanced (Rai III/IV, Binet C) stage disease with median estimated survival times of > 10, 5–7, and 1–3 years, respectively. However, there is heterogeneity in the course of the disease among individual patients within a single stage group. Most importantly, the clinical staging systems do not allow one to predict if and at what rate there will be disease progression in an individual patient diagnosed with early stage disease. Since more than 80% of patients are diagnosed in early disease stages, there is a need to identify markers that may help to refine outcome prediction for these individuals. In addition, in light of the broad therapeutic options available, a risk versus benefit evaluation based on individual disease characteristics would be desirable. There has been intensive work on clinical and biological factors of potential prognostic relevance that may add to the classic assessment provided by the staging systems. Among these are (1) clinical patient characteristics such as age, gender and performance status; (2) laboratory parameters reflecting the tumor burden or disease activity such as lymphocyte count, lactate dehydrogenase (LDH) elevation, bone marrow infiltration pattern or lymphocyte doubling time (LDT)5–,7; (3) serum markers such as soluble CD23, β2-microglobulin (β2-MG) or thymidine kinase (TK),8,9 and (4) genetic markers of tumor cells such as genomic aberrations, gene abnormalities (p53 and ATM), the mutation status of the variable segments of immunoglobulin heavy chain genes (VH), or surrogate markers for these factors (CD38, ZAP-70, LPL, etc.).7,10– 18 Recent research has moved toward a molecular genetic focus that may not only provide insight into the biology and transforming events but may also define mechanisms directly responsible for the clinical behavior of the disease with regard to disease progression, response to treatment and overall survival.
Identification of Prognostic Factors
One of the most important molecular genetic parameters defining pathogenic and prognostic subgroups of CLL is the mutation status of the VH genes.10,11 Since somatically mutated VH genes can be observed in about half of all CLL cases, a separation was made into two different groups: one with unmutated VH genes, assumed to originate from pregerminal center cells, and another with mutated VH genes, thought to originate from postgerminal center cells. However, genome-wide gene expression profiling studies revealed a surprisingly homogeneous pattern of gene expression in both subtypes of CLL with only a limited set of genes being differentially expressed in the subgroups.19,20 Most importantly, it could be demonstrated that the VH mutation status is clinically highly relevant.10,11 While CLL with unmutated VH shows an unfavorable course with rapid progression, CLL with mutated VH often shows slow progression and long survival. Figure 1 shows the survival curves for patients distributed over all stages (n = 300) and separately for patients diagnosed with Binet stage A disease (n = 189) from the largest published cohort.7 Furthermore, and independent of the mutation status, the usage of specific VH genes such as V3-21 may be associated with an inferior outcome.21
To make the estimation of prognosis based on the VH status accessible to the routine hematology laboratory, surrogate markers for the VH status were identified. Originally, a correlation was observed between the VH mutation status and CD38 expression of the CLL cells pointing to CD38 expression as a prognostic marker.10 Based on genome-wide gene expression studies other surrogate markers such as ZAP-70 expression were identified and validated.15,19 ZAP-70 expression appears to strongly correlate with VH mutation status and was therefore a strong prognostic marker in a pivotal study.15 However, for both CD38 and ZAP-70, subsequent studies have yielded controversial results with regard to their validity as a surrogate marker for VH and prognostic indicator. The facts that (1) divergent results have been obtained in different laboratories (CD38 and ZAP-70), (2) the expression level may change over time (CD38), (3) a careful separation of T cells is necessary (ZAP-70), (4) different cut-off values to distinguish “positive” from “negative” cases were defined (CD38 and ZAP-70), and (5) approximately 10%–30% of cases show discordant status for CD38 or ZAP-70 as compared to VH in all series described, indicate that these markers may not be as reliable as initially thought for routine diagnostics.7,15,16,22,23 Furthermore, the relationship of the VH mutation status to other biological disease characteristics of potential pathogenic relevance such as ongoing VH hypermutation as well as VDJ diversification, telomere length, ability for BCR signaling, expression of specific genes such as AID, and genomic aberrations are currently undergoing examination.
Genomic aberrations are the other genetic parameter shown to be of pathogenic and clinical relevance in CLL. Genomic aberrations can be identified in about 80% of CLL cases by fluorescence in-situ hybridization (FISH) of interphase cell nuclei (“Interphase-Cytogenetics”) with a disease-specific comprehensive probe set (Table 1 ).12 Genomic aberrations provide insights into the pathogenesis of the disease since they point to loci of candidate genes (17p13: p53; 11q22-q23: ATM) and identify subgroups of patients with distinct clinical features. Specific genomic aberrations have been associated with disease characteristics such as marked lymphadenopathy (11q deletion) and resistance to treatment (17p deletion, see below). Moreover, such aberrations define specific subgroups that differ in the rate of disease progression as determined by the time from diagnosis to first treatment and the overall survival time of CLL (Figure 2 ).12 VH mutation status and genomic aberrations are two separate genetic parameters of prognostic relevance, but they appear to be correlated. Unfavorable aberrations (11q-, 17p-) occur more frequently in VH unmutated tumors, and favorable aberrations (13q-, 13q- single) occur more frequently in the VH mutated subgroup (Table 2 ).7,13,14 This unbalanced distribution of genomic aberrations emphasizes the different biological background of the CLL subgroups with mutated or unmutated VH and could in part explain their different clinical course. On the other hand, about two-thirds of the VH-unmutated CLL cases show no unfavorable genomic aberrations, indicating a differential influence of these factors (Table 2 ).
To examine the individual prognostic value of genomic aberrations, the VH mutation status and other clinical and laboratory features, we performed a multivariate analysis of the survival time.7 The VH mutation status, 17p deletion, 11q deletion, age, leukocyte count and LDH were identified as independent prognostic factors. When the VH mutation status and 11q and 17p aberrations were included in the model, the clinical stage of disease according to the systems of Rai or Binet was not identified as an independent prognostic factor for survival, indicating that in the context of these genetic parameters, the clinical stage of the disease may lose its independent prognostic value.7 Two other independent series have confirmed the strong prognostic and independent impact of VH mutation status and genomic aberrations on clinical course.13,14 Therefore, four subgroups of CLL with markedly differing survival probabilities can be defined by the VH mutation status, 11q deletion and 17p deletion (Figure 3 ). These molecular features also provide insight into the biological bases of the clinical heterogeneity of CLL and may lead to future risk-adapted treatment strategies for individual patients. As a guide for the following sections on the role of genetic prognostic markers in different clinical situations, Table 1 gives a summary of the incidences of genomic aberrations and the VH mutation status observed in a single center cohort of patients distributed over all stage groups and from several multicenter trials of the GCLLSG (see also www.dcllsg.de). Of note is the fact that there are significant differences with regard to the occurrence of high-risk (17p-, 11q-, unmutated VH) and low-risk (13q- single, mutated VH) markers in the different studies, indicating the different biological background of the diseases/patients enrolled in these studies designed for different clinical situations.
In order to further improve our understanding of molecular pathogenesis and clinical outcome prediction in CLL, microarray platforms have been developed as tools to evaluate genome wide parameters and defects. On the genomic level matrix CGH (comparative genomic hybridization against a matrix of defined DNA fragments) is a sensitive test allowing the detection of novel recurrent aberrations of potential pathogenic and prognostic importance (Figure 4; see Color Figures, page 509).24 On the level of gene expression, comprehensive profiling studies of CLL based on DNA chip technology have indicated that the global gene expression “signature” of VH mutated and unmutated CLL is very similar and that only the expression of a small number of genes discriminates between the two groups.19,21 In addition to the characterization of expression signatures associated with the VH mutation subgroups of CLL a study of 100 CLL samples characterized for VH status and genomic aberrations described a significant number of differentially expressed genes clustering in chromosomal regions affected by the respective genomic losses or gains.25 Deletions affecting chromosome bands 11q22-q23 and 17p13 led to a reduced expression of the genes in the corresponding genomic region, such as ATM and p53, while trisomy 12 resulted in the upregulation of genes mapping to chromosome arm 12q (Figure 5; see Color Figures, page 509). The finding that the most significantly differentially expressed genes were located in the corresponding aberrant chromosomal regions suggests that a gene dosage effect may exert a pathogenic role in CLL.
Validation of Prognostic Factors in Prospective Trials
In retrospective series of heterogeneously treated patients with CLL, the relationship between stage of disease and genetic parameters was assessed. Distinct subgroups of CLL patients defined by specific genomic aberrations showed significantly different rates of disease progression as defined by the time from diagnosis to first treatment (Figure 2a ). In addition, the prognostic impact of VH mutation status and genomic aberrations with regard to overall survival was observed for patients both with early stage (Binet A) and advanced stage disease (Figure 3 ). However, this data was derived from heterogeneous single center cohorts of patients. Data from patients prospectively enrolled in multicenter trials are just emerging.
Risk for Progression in Early Stage CLL
In the CLL1 trial of the German CLL Study Group (GCLLSG) CLL patients with Binet A disease are stratified into a high risk arm if they have a LDT < 12 months and/or a diffuse bone marrow infiltration pattern and a TK level > 7 U/L and/or β2-MG level > 3.5 mg/L (see also www.dcllsg.de).26 Based on this stratification the high risk group is randomized between immediate treatment with fludarabine versus watch and wait while the low-risk group is followed up. In addition at enrollment genomic aberrations and VH mutation status are analyzed. Table 1 gives a summary of the genetic results obtained so far. These results show that high-risk aberrations (11q- or 17p-: 14%) and unmutated VH (41%) occur in a significant number of asymptomatic early stage patients. When comparing the results from CLL1 with our single center study containing patients diagnosed in all stages and the CLL4 study (fludarabine vs fludarabine/cyclophosphamide for untreated Binet B/C patients, see also www.dcllsg.de),27 it is interesting to note that the incidence of low-risk markers (mutated VH, 13q- single) is higher and the percentage of high-risk markers (unmutated VH, 11q-, 17p-) is lower. The low incidence of 11q- in the CLL1 trial as compared to the other studies is likely due to the strong association of this abnormality with marked lymphadenopathy and rapid disease progression leading to recruitment of such patients in a trial for advanced stage symptomatic disease.
In the CLL1 study preliminary correlations of genetic parameters with progression-free survival (PFS) among untreated patients showed that unmutated VH as well as +12q, 11q- and 17p- are associated with more rapid disease progression (Figure 6 ).28 Moreover, the genetic parameters and the other parameters used for risk stratification appear to be correlated. Unmutated VH and high-risk aberrations (17p-, 11q-, +12q) were significantly associated with the trial-defined high-risk group and with the individual parameters defining this group. However, there was discordance in 20%–40% of cases between individual parameters, i.e. among the trial-defined “high-risk” patients 37% had mutated VH, while in the “low-risk” group 28% had unmutated VH. In univariate analysis the following prognostic indicators were significant for a shorter PFS: TK (P < .001), LDT (P = .001), lymphadenopathy (P = .002), β2-MG (P = .006), absolute lymphocytes (P = .004), unfavorable genomic aberrations (11q-, 17p-, +12q) (P < .001) as well as unmutated VH status (P = .003). In multivariate analysis TK, LDT, unfavorable genomic aberrations (11q-, 17p-, +12q) as well as unmutated VH status were identified as independent variables. Therefore, it appears that a combination of several different factors may allow the best prediction of an individual patient’s risk for disease progression. The future CLL7 trial will therefore include the parameters TK, LDT, genomic aberrations and VH mutation status for initial risk stratification among Binet A CLL patients. Furthermore, young early stage patients in the “high risk” group as defined in the CLL1 trial or by genetic risk factors (unmutated VH, 11q-, 17p-) and active disease as defined by National Cancer Institute (NCI) criteria are eligible for the autologous and allogeneic transplantation protocols of the GCLLSG (see below).
Predictors for Response to Treatment and Survival in Advanced Stage CLL
The observation that the rate of disease progression is associated with genomic aberrations and VH mutation status indicates that these factors may determine the behavior of the disease. However, overall survival reflects additional parameters such as response to treatment. The fact that overall survival was inferior for the subgroups with unmutated VH, 11q-, or 17p-, despite the fact that comparable treatment modalities were used for patients with or without these markers, suggests that response to therapy may be different in genetic subgroups.
In particular, the deletion 17p- and/or abnormalities of the p53 gene involved in this aberration have been associated with failure after treatment with alkylating agents, purine analogs and rituximab.29–,32 In a chromosome banding study of patients treated in a prospective trial based on alkylating agents, 17p aberrations were the only chromosomal aberration of prognostic relevance.30 An interphase-FISH study also showed that patients whose leukemia cells showed a 17p-/p53 deletion had significantly shorter survival times than patients without this aberration, and a relationship was found between the deletion and the response to treatment.31 While 56% of patients without p53 deletion went into remission after treatment with purine-analogs, none of the patients with p53 deletion showed a response. Similarly, the monoclonal anti-CD20 antibody rituximab did not show efficacy in CLL with p53 deletion.32 In contrast, there is anecdotal evidence that durable therapeutic success can be achieved in CLL with 17p-/p53 mutation using the monoclonal anti-CD52 antibody alemtuzumab.33 This observation has been expanded in a retrospectively evaluated series of CLL cases mostly refractory to fludarabine therapy.34 Treatment with intravenous alemtuzumab resulted in a CR or PR in 11 of 36 (31%) and in 6 of 15 (40%) patients with p53 mutations or deletions. In the CLL2H study of the GCLLSG (alemtuzumab for fludarabine refractory CLL, see also www.dcllsg.de) a high incidence (27%) of 17p- aberrations was observed, confirming the association of this abnormality with fludarabine-resistant disease (Table 1 ). An interim analysis of this ongoing prospective trial has shown a response (CR or PR) in 10 of 21 VH unmutated, 5 of 10 11q-, and 6 of 10 17p- cases, providing evidence from a controlled trial that alemtuzumab may be effective in CLL with 17p-/p53 mutation.
The observation that in a multivariate analysis 17p-, 11q-, and unmutated VH were independent adverse prognostic markers with regard to overall survival indicated that these factors may be associated with different outcomes after treatment. Support for this hypothesis requires prospective evaluation of the best currently available prognostic markers in controlled clinical trials. VH mutation status, genomic aberrations, ZAP-70, etc. are currently being evaluated in different treatment trials from which so far only preliminary data have been available.16,27,35
Risk Versus Benefit Evaluation of Autologous and Allogeneic Stem Cell Transplantation in CLL
Autologous and allogeneic stem cell transplantation (SCT) are increasingly considered in the management of medically fit patients with active CLL. These procedures may confer therapeutic benefit but are also associated with considerable toxicity and cost. The efficacy of autologous SCT relies solely on the cytotoxic therapy administered. Allogeneic SCT offers the potential additional benefit of the immune-mediated graft-versus-leukemia effect but also harbors the danger of graft-versus-host disease. Hence, there is a need to identify prognostic factors that may help to determine whether a patient is a candidate for SCT and if an allogeneic SCT or autologous SCT should be considered.
Emerging data from prospective autologous SCT trials are demonstrating safety, improved remission after transplant and long survival times. In the Medical Research Council (MRC) series the early transplant-related mortality (TRM) was 1.5% and the 5-year overall and disease-free survival following transplantation were 77.5% and 51.5%, respectively.36 In the multicenter prospective autologous SCT study of the GCLLSG (CLL3, see also www.dcllsg.de) the TRM was 5% with a 2-year overall survival rate of 88% among 105 patients.37 This result appears promising considering the high-risk features present in the majority of patients (see also Table 1 for the CLL3 trial: 68% unmutated VH, 25% 11q- or 17p-). However, the continuing clinical and molecular relapses observed in all series of autologous SCT in CLL are evidence against the curative potential of the procedure in the majority of patients.
Furthermore, genetic risk factors appear to retain their adverse impact after autologous SCT. In the CLL3 trial the median time to recurrence of a clonal CDR3 PCR analysis as molecular evidence of disease was 17 months in the group with 11q- versus 37 months in the group without 11p- (P = .0019). Similarly, the time to clinical relapse and the time to disease recurrence as assessed by consensus primer CDR3 PCR was significantly shorter among patients with unmutated VH genes.38 Nevertheless, the median treatment-free interval of 49 months in the VH unmutated cohort suggested a beneficial effect of autologous SCT for this high-risk population. This was confirmed in a matched pair analysis of autologous SCT versus conventional treatment including the VH mutation status as matching variable.39 In this study auto-SCT resulted in significantly longer overall survival as compared to conventional chemotherapy (autologous SCT: last death at 139 months, corresponding survival rate of 0.57, conventional: median survival 119 months) and this effect was primarily based on the benefit of the subgroup of cases with unmutated VH (Figure 7 ).
As compared to autologous SCT the primary therapeutic advantage of allogeneic SCT after dose-reduced conditioning is the graft-versus-leukemia effect, which may offer long-term disease control and eventual cure. Indeed, a recent comparative study of minimal residual disease (MRD) as detected by consensus primer CDR3 PCR provided evidence that the graft-versus-leukemia effect is operational in CLL with unmutated VH.40 In this study only a modest decrease in MRD levels was observed immediately after allogeneic SCT, but MRD became undetectable in 7 of 9 (78%) CLL patients with unmutated VH after tapering immunosuppression, chronic graft-versus-host disease or donor lymphocyte infusions. After a median of 25 (14–37) months, these 7 patients remain in clinical and molecular remission. In contrast, PCR negativity was achieved in only 6 of 26 (23%) control CLL cases with unmutated VH after autologous SCT after dose-reduced conditioning and was not durable (Figure 8 ). Therefore, allogeneic SCT appears to combine the favorable features of low TRM with the activity of the graft-versus-leukemia effect, making this procedure a valid option when aiming at cure for high risk CLL.
II. The Initial Management of Patients with Chronic Lymphocytic Leukemia
Ian W. Flinn, MD, PhD,* and Jesus G. Berdeja, MD
Johns Hopkins University, 1650 Orleans St., Room 388, Baltimore MD 21231-1000
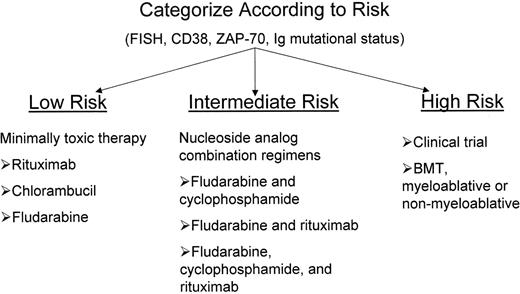
The definition of more accurate prognostic factors has made possible a risk-adapted approach to therapy for patients with CLL akin to what has been commonplace for patients with acute leukemia for years. Clearly, one can define patient populations with wide divergent survival expectations as one can with acute leukemia. However, in patients with acute myeloid leukemia (AML) the outcome is quite black and white. Patients either are cured of their disease or they are not. For patients with CLL, the outcome is closer to shades of gray. With the possible exception of blood or marrow transplantation (BMT), no one will be cured of their disease. Furthermore, the anticipated survival for even the worst group of patients with CLL is several years. Thus, the decision of when to initiate treatment and what type of therapy to initiate with a CLL patient is not straightforward. In fact, treatment of low-risk patients may actually have deleterious effects on survival as has been shown by the French Cooperative Group.1 Currently the decision to treat patients is based on multiple factors including advanced clinical staging, symptomatic disease, burden of disease, age, co-morbid illnesses, adverse prognostic factors, and availability of treatments that alter survival. Patients in early clinical stages with enough poor prognostic criteria could be considered candidates for enrollment in clinical trials or therapies such as stem cell transplantation that offer the potential for cure.
Once the decision to treat a patient has been made, there are multiple treatments known to be effective for CLL. Chlorambucil is the oldest and best-known treatment for CLL. It can induce partial remissions (PR) in 60%–70% of previously untreated patients, but no significant complete remissions (CR). Alkylator/anthracycline combinations such as CVP (cyclophosphamide, vincristine, prednisone) and ChOP (cyclophosphamide, doxorubicin, vincristine, prednisone) are frequently used with similar and perhaps better responses to those seen with chlorambucil, but no change in survival.2
Purine Analogues
The purine analogues (i.e., fludarabine and cladribine) are nucleoside analogues that inhibit DNA polymerase and ribonucleotide reductase, promoting apoptosis.3 Of these, fludarabine has been the most widely tested and used nucleoside analogue in CLL.
The efficacy of fludarabine was studied in a large North American intergroup trial in which more than 500 patients were randomized to receive fludarabine, chlorambucil, and fludarabine and chlorambucil. Overall response (OR) favored the fludarabine group at 63% (20% CR + 43% PR) versus 37% (4% CR + 33% PR).4 The median duration of remission and the median progression-free survival (PFS) in the fludarabine group were 25 months and 20 months, respectively, whereas both values were 14 months in the chlorambucil group (P < 0.001 for both comparisons). Leporrier et al also reported their results in patients with previously untreated CLL treated with either fludarabine or an anthracycline-containing regimen (CAP [cyclophosphamide, doxorubicin, prednisone] or ChOP). Nine hundred and thirty-eight patients (651 stage B and 287 stage C) were randomized in 73 centers. Compared to ChOP and fludarabine, CAP induced lower overall remission rates (58.2%; ChOP, 71.5%; fludarabine; 71.1%; P < .0001 for each), including lower clinical remission rates (CAP, 15.2%; ChOP, 29.6%; fludarabine, 40.1%; P = .003). By contrast, median survival time did not differ significantly according to randomization (67, 70, and 69 months in the ChOP, CAP, and fludarabine groups, respectively).5 An important observation in all these trials is the higher percentage of CRs seen in the fludarabine treated groups. Fludarabine is well tolerated with major side effects being hematologic and immunologic toxicities. Also seen is autoimmune hemolytic anemia (AIHA) and, at very high doses, significant neurotoxicity. The issue of treating patients with fludarabine who have a history of AIHA or ITP, either primary or secondary to previous purine analogue therapy, is a topic of continued controversy.6,7 The decision to treat these patients should be done with extreme caution and close monitoring.
Likewise, there is evidence that cladribine (2-CdA) produces similar responses as fludarabine in both previously treated and untreated populations. Juliusson and Liliemark reported on the response to 2-CdA of 52 patients with previously treated CLL. They reported a 31% CR and 27% PR, with a median PFS of 23 months for CR patients and 16 months for PR patients. Toxicities were similar to those observed with fludarabine.8 Recently, Robak et al reported their experience with 2-CdA on 378 patients with CLL. One hundred ninety-four patients were previously untreated and 184 had relapsed or refractory disease. Results showed a CR of 45.4% and a PR of 82.5% in the previously untreated group with a median survival of 19.4 months, and a CR of 12.5% and PR of 48.4% in the previously treated group with a median survival of 16.3 months.9 The accumulating 2-CdA data is provocative but not much different from the fludarabine data. Unfortunately, crossresistance is seen among all the nucleoside analogues, so a sequential treatment algorithm is unlikely.
Reports of synergism between alkylators and nucleoside analogues by alkylators inducing DNA damage and nucleoside analogues inhibiting DNA repair prompted trials combining these two modalities.10,11 Early reports of the combination of fludarabine and cyclophosphamide showed higher OR rates than fludarabine alone in previously treated patients with alkylators or fludarabine.10,12–,14 In a multicenter Phase II study conducted by ECOG, 36 patients received cyclophosphamide 600 mg/m2 intravenous (iv) day 1 and fludarabine 20 mg/m2 iv days 1 through 5, followed by filgrastim 5 mg/kg subcutaneous starting approximately day 8. Fifteen of 36 (41.7%) evaluable patients achieved complete response and 8 (22.2%) achieved a partial response, giving an OR rate of 63.9% (90% CI: 48.4%, 77.2%).15 Preliminary results from a North American Intergroup trial comparing this combination regimen to single-agent fludarabine revealed the combination produced a superior CR rate compared to fludarabine alone, 23% versus 6%, respectively (personal communication, Ian Flinn). Alternative regimens using 3-day schedules of fludarabine and cyclophosphamide have produced encouraging results. The German CLL Study Group noted a higher CR rate with fludarabine and cyclophosphamide than with fludarabine alone (20.2% vs 8.6%).16 While these CR rates were disappointingly low in both arms, the difference in response translated into a longer PFS in the combination arm. In a single center Phase II arm study, CR rates were not found to be substantially increased. However, the quality of these CRs was improved, since only 8% of CR patients demonstrated residual CD5+ cells in the marrow compared with 33% after fludarabine and prednisone.17 Taken together these studies suggest that the combination of fludarabine and cyclophosphamide improves CR in patients with previously untreated CLL. However, it is not clear as to what regimen might serve as the best backbone by which to add new agents such as rituximab. Numerous other fludarabine combination regimens have been tested with promising results in Phase II trials.18– 20
Single-Agent Antibodies
Rituximab is a chimeric anti-CD20 monoclonal antibody, which has afforded excellent responses in patients with B-cell lymphomas. Early disappointing results in small lymphocytic leukemia (SLL)/CLL from the pivotal trial led to skepticism about its ultimate utility in CLL. However, subsequent studies examining alternative schedules, doses, and patient populations have clearly demonstrated rituximab’s efficacy in CLL. One explanation for the meager response rates in patients with SLL is the low serum trough levels seen in these patients, which are known to be inversely related to response rates. To overcome the apparent altered pharmacokinetics, the antibody has been given thrice weekly for 4 weeks at standard doses (375 mg/m2) as well as very large single doses weekly for 4 doses. The thrice weekly regimen has resulted in 45% OR rate in patients with previously treated CLL.21 Five of 6 previously untreated patients responded. A dose escalation study has also been completed in which cohorts of patients with CLL and other mature B cell malignancies received increasing doses of rituximab weekly for 4 doses. Response correlated with dose and 75% of patients at the highest dose (2250 mg/m2) responded.22 Forty-four previously untreated patients with CLL/SLL received rituximab 375 mg/m2 weekly for 4 consecutive weeks. All patients were required to have one or more indications for treatment.23 Patients with objective response or stable disease continued to receive identical 4-week rituximab courses at 6-month intervals, for a total of 4 courses. The response rate after the first course of rituximab was 51% (4% CR). While many patients treated with rituximab will respond, these are predominantly partial responses, with the bone marrow being the most difficult compartment to treat adequately.
Alemtuzumab is a CDR-grafted, human IgG, monoclonal antibody against the CD52 antigen expressed on normal and leukemic B and T lymphocytes, macrophages and monocytes. In patients with heavily pretreated B-CLL, a response rate of 30%–40% has been reported with a median response duration of 12 months. The toxicity related to the infusion of the antibody has hampered its early use in patients. When administered subcutaneously, the incidence of rigors and hypotension is significantly reduced. In a Phase II study of previously untreated patients given alemtuzumab for 18 weeks, an OR of 87% (95% CI, 76%–98%; CR, 19%; PR, 68%) was achieved in 38 evaluable patients (81% of intent-to-treat population). CLL cells were cleared from blood in 95% patients in a median time of 21 days. CR or nodular PR in the bone marrow was achieved in 66% of the patients and most patients achieved this after 18 weeks of treatment. An 87% OR (29% CR) was achieved in the lymph nodes. Transient injection site skin reactions were seen in 90% of patients. Rigor, rash, nausea, dyspnea, and hypotension were rare or absent. Alemtuzumab induces a profound lymphopenia, which may have contributed to the high incidence of infectious complications seen in the early studies. This risk has been substantially reduced by the use of prophylactic trimethoprim/sulfamethoxazole, acyclovir, and fluconazole. In this study of previously untreated patients infections were rare, but 10% of patients developed cytomegalovirus (CMV) reactivation. In contrast to rituximab, alemtuzumab has its most pronounced effects in blood and bone marrow, with minimal effect on bulky disease.
The increased specificity of monoclonal antibodies for CLL compared to chemotherapy with relative sparing of normal tissues has raised the question as to whether MABs could be used as adjuvants to chemotherapy to target minimal residual disease or in a maintenance approach to maintain remissions. Rituximab has been used in other low-grade lymphoid malignancies for maintenance and it has prevented relapse but the duration of rituximab benefit was not superior to using the antibody when patients relapsed.24 In patients with CLL, PFS (18.6 months) was relatively poor when rituximab was used as front-line therapy and then administered at 6 month intervals as maintenance.24 Further investigation is needed to learn whether rituximab might be useful in this setting if used at different doses or schedules.
The efficacy and safety of alemtuzumab in patients with CLL who had residual disease after chemotherapy have also been investigated. Alemtuzumab was administered to 41 patients 3 times weekly for 4 weeks in a Phase I/II study following maximum response to induction chemotherapy. The OR was 46%. The major reason for failure to respond was the presence of adenopathy. Residual bone marrow disease cleared in most patients, and 11 of 29 patients (38%) achieved a molecular disease remission. Infections were reported to occur in 15 patients (37%), and 9 of these infections were reactivation of CMV. Three patients developed Epstein-Barr virus–positive, large cell lymphoma.25 Given the significant infectious toxicity, larger studies are needed before this approach can be used on a routine basis.
Combination Chemo-immunotherapy
The encouraging high rate of durable responses seen with fludarabine-based regimens may be further improved by the addition of other novel agents. Fludarabine, cyclophosphamide and rituximab (FCR) is currently under investigation as a first-line therapy for patients with CLL. A single center study has shown good preliminary results including a 71% CR rate,26 with 57% of complete responders tested (35/61 patients) showing molecular remission.27 Fludarabine and rituximab (FR) in combination have also been reported to result in very high response rates in previously untreated patients with CLL, with CRs seen in 33% of patients when FR was administered concurrently.28 The CR rate improved to 47% when responding patients were consolidated with rituximab. This group retrospectively compared the treatment outcome of the patients on this trial (n = 104) to patients with similar clinical characteristics enrolled on a US Intergroup who received fludarabine alone (n = 178). In multivariate analyses controlling for pretreatment characteristics, the patients receiving fludarabine and rituximab had a significantly better PFS (P < 0.0001) and overall survival (OS) (P = 0.0006) than patients receiving fludarabine therapy. Two-year PFS probabilities were 0.67 versus 0.45, and 2-year OS probabilities were 0.93 versus 0.81. Infectious toxicity was similar between the two treatment approaches.29
The combination of fludarabine and alemtuzumab has also been investigated in patients with previously untreated CLL. Treatment included fludarabine 25 mg/m2/day for 5 days IV, monthly × 4, followed by observation for 2 months when an evaluation of initial response was performed. Patients were evaluated 2 months after completing fludarabine and those who achieved stable disease (SD) or better were treated with a 6-week course of alemtuzumab. Alemtuzumab was given at 30 mg IV 3 times a week after a stepped up dosing during the first week (3 mg as the first dose and increasing, as tolerated, to 10 mg, and to 30 mg). Prophylaxis with trimethoprim-sulfamethoxazole and acyclovir was required during and for 6 months after alemtuzumab therapy. Among 36 patients who entered the alemtuzumab phase of therapy, there were 15 CRs (42%) and 18 PRs (50%) with an OR rate of 92%.30 Out of 11 patients with SD after fludarabine, 3 achieved CR and 5 had PR following alemtuzumab. Among all 56 patients (intention-to-treat population) the CR and PR incidence was 27% and 43%, respectively. Grade 3 or worse major infections were recorded in 12 patients during or following alemtuzumab therapy. Infections with CMV occurred in 8 patients during or within 4 months following alemtuzumab. The outcome of CMV was fatal in 1 case, complete or partial resolution occurred in 6, and persistent CMV in 1.31
Blood and Marrow Transplantation for CLL
The use of BMT in CLL was slow to develop given the perceived “good prognosis” of these patients, their higher median age, the unproven benefit of BMT and the potential toxicity of the treatment. Nonetheless, as our understanding of the pathogenesis of this disease improves and our ability to identify patients with aggressive disease and poor prognosis improves, the need for aggressive, potentially curative therapy becomes paramount. Also, as the mortality with transplantation decreases, as well as recent innovations like nonmyeloablative allogeneic transplants32 emerge, this modality will likely become an important facet in the treatment of some CLL patients.
The first report of BMT was by Michallet et al in 1988.33 Since then, multiple other investigators have shown the ability of patients with CLL to tolerate and respond to both allogeneic and autologous bone marrow transplants (reviewed in 34). In autologous transplant, the transplant mortality is less than 10% with the status of disease and the number of previous lines of treatments being the most important prognostic factors. Unfortunately, the survival plots do not show a plateau, with about 50% of patients relapsing at 4 years. However, durable remissions have been achieved in patients who received autologous grafts purged of leukemia35 and a survival advantage has been noted even in patients with poor prognostic factors.36
Allogeneic transplants, on the other hand, result in survival curves plateaus of about 40%. In a recent update, the European Bone Marrow Transplant Registry reported that OS and event-free survival at 10 years was 41% and 36.6% in a series of 54 patients who underwent allogeneic BMT from a matched sibling.37 Unfortunately, 48% of patients died from procedure-related causes, which is not surprising in light of the fact that 85% of the patients were refractory to chemotherapy at the time of transplant. The use of BMT early in the course of the disease dramatically reduces the procedure-related mortality38 and should be considered for patients with poor-risk features. However, most patients with CLL are over the age of 55 and thus usually not considered for allogeneic BMT with a standard preparative regimen. The realization that much of the efficacy of allogeneic transplantation came from the graft-versus-leukemia effect and the subsequent development of reduced intensity conditioning (RIC) regimens is particularly important to patients with CLL. Although the use of RIC regimens is still in relative infancy compared to standard regimens, early studies suggest that the efficacy is as good with RIC while the risks are significantly less. For example, in a study of 448 patients with CLL, 228 who received an RIC and 222 who received a standard myeloablative regimen, Dreger et al found that the hazard ratio for treatment-related mortality and OS were 0.5 ([95% CI 0.3–0.83]; P = .007) and 0.56 ([95% CI 0.37–0.86]; P = 0.007) respectively favoring RIC. Furthermore, no increased risk of relapse was seen.39 RIC regimens significantly broaden the utility of allogeneic BMT to patients well into their 70s and many institutions now favor RIC over standard regimens for nearly all of their patients.
Durable remissions have been achieved with BMT. Only time will tell whether these remissions will develop into survival advantages and possibly cures. The use of peripheral stem cells, nonmyeloablative (mini BMT) approaches and graft purging methods such as chemotherapy, B cell specific monoclonal antibodies, positive cell selection and others continue to improve upon the results and tolerability of transplantation and will likely make these procedures more widely available to CLL patients.
Complications of Therapy and Treatment Choices
An important consideration in deciding on an initial therapy is the potential morbidity and mortality of the therapy in both the short-term and long-term treatment plans for a patient. Infections and hemolytic anemia are the most common initial complications of the therapy. The nucleoside analogues and alemtuzumab can have prolonged effects on cellular immunity. Combination regimens are particularly problematic. However, the risk of infection can usually be managed. Many clinical trials of combination regimens with fludarabine have successfully utilized prophylactic antibiotics to prevent Pneumocystis carinii pneumonia.13,28 The depression in CD4 counts persists for at least 6 months after completing chemotherapy and thus so should prophylaxis. Similarly, in high-risk populations such as patients treated with the combination of fludarabine and cyclophosphamide, acyclovir and its analogues have been used to prevent herpes zoster. Re-activation of CMV is a prominent complication of alemtuzumab, especially when used in combination with fludarabine or for minimal residual disease. Prophylaxis is more cumbersome and less well established. However, surveillance for CMV using a PCR-based approach has been used successfully.31
The treatment of CLL in patients with a history of hemolytic anemia is highly controversial. The incidence of hemolytic anemia in patients treated with fludarabine or other nucleoside analogues may be increased (reviewed in 40). In randomized trials there do not appear to be huge differences in the incidence of this important complication.5 However, in patients that are direct antibody positive or have a history of autoimmune hemolytic anemia, the risk appears high enough that these patients have been excluded from some clinical trials,28 a paradigm useful in clinical practice.
The late consequences of initial treatment, most notably damage to normal bone marrow, are harder to incorporate in the initial management decisions of patients with CLL given the paucity of information. It is, however, clear that aggressive therapies that produce higher CRs also have deleterious side effects to bone marrow reserve. This side effect is probably most easily quantifiable in terms of the ability to collect autologous stem cells for transplantation. While autologous stem cell transplantation applies to a relatively small number of patients with CLL, the ability to collect stem cells reflects hematopoietic reserve. Although stem cells can still be collected when fludarabine is used judiciously,13 numerous groups have now reported that fludarabine-containing regimens impair the ability to collect autologous stem cells and perhaps even the ability of these stem cells to engraft.35,41 In addition, a concern about damage to normal hematopoietic precursors that results in an increased incidence of myelodysplasia has been raised with the combination of alkylating agents with fludarabine.42,43
Conclusion
Recent advances in risk stratification for patients with CLL have made it clear that one approach does not fit all patients with CLL. An approach to patients with different expected survival is depicted in Figure 9 . Important considerations include expected survival, age, and short- and long-term toxicities of a treatment regimen. The specific therapy chosen will vary from one patient to another and is a controversial subject as no outcome data are yet available to support the notion of this risk-adapted strategy. However, the therapy chosen for an 80-year-old patient with good-risk features and near normal life expectancy will be very different from a 40-year-old patient with a p53 abnormality and anticipated survival of less than 3 years. Fortunately, most patients with CLL have an anticipated survival of more than 3 years and lie somewhere between the two cited patients. For these patients, careful consideration must be given to the risks and benefits of a treatment choice as it relates to the prognosis of an individual patient.
III. Management of Patients with Fludarabine-Refractory CLL
John C. Byrd, MD*
The Ohio State University, B302 Starling Loving Hall, 320 West 10th Ave., Columbus OH 43210 This work was supported by the National Cancer Institute (P01 CA95426-01A1), the Sidney Kimmel Cancer Research Foundation, The Leukemia and Lymphoma Society of America and The D. Warren Brown Foundation.
For some patients CLL is an indolent disease that never progresses to require therapy. However, when patients become symptomatic with their CLL, the OS with older therapy is quite short, ranging from a mean of 1.5 to 6 years.1,2 Applying traditional therapies utilized in CLL such as chlorambucil or fludarabine results in palliative responses and a progression-free survival of 20 months in one study.3 Combination strategies with fludarabine and cyclophosphamide or fludarabine-based combinations with rituximab may extend PFS as noted in Section II, but no plateau is appreciated with any of these studies. At the time of relapse from initial response to fludarabine, 40% can be retreated and will respond again to the same regimen.4 Data with respect to retreatment with fludarabine and cyclophosphamide or fludarabine-based combinations with rituximab are currently not available. Ultimately, virtually all CLL patients who are treated become fludarabine-refractory.
Therapy for CLL for many years consisted only of alkylator-based therapy with or without corticosteroids. Until the approval of fludarabine for use in alkylator-resistant CLL in 1991, no salvage therapies existed for the treatment of CLL. This un-met medical need served as the basis for fludarabine’s United States Food and Drug Administration (FDA) approval for use to treat alkylator-resistant CLL. Soon after the approval of fludarabine, a new class of CLL patients emerged, those resistant to fludarabine. The definition for fludarabine-refractory disease generally utilized in publications and accepted by the FDA and other regulatory agencies is no response to therapy or initial response to fludarabine but subsequent progression within a 6-month period from completion of therapy.5 From 1991 to 2001, new therapies for fludarabine-refractory CLL were investigated in clinical trials, and several have emerged that can be effectively used for this patient population. This review will focus on the clinical and molecular features associated with fludarabine-refractory CLL and therapies available to treat these patients. Additionally, we will discuss new agents that are moving into clinical trials that have promise for the treatment of CLL.
Demographics and Clinical Features Associated with Fludarabine-Refractory CLL
Despite the greater than 13-year time period since fludarabine was approved, a paucity of data are available describing clinical features associated with this subset of CLL. One series published by the MD Anderson Cancer Center reported on 147 such patients followed over 13 years who either did not respond (138, 94%) or relapsed within 6 months (9, 6%) following completion of therapy with fludarabine.6 Valuable demographics were provided by the study. These patients had a median age of 60, a median of 3 prior therapies, and a majority had Rai high-risk disease. With initiation of different investigational or standard therapies, early death within 3 months was noted in 13% of patients and life-threatening infections in 58 (40%). Response to therapy was only 12% in those patients who had primary resistance to fludarabine and 22% in those who had previously had short responses. Responses were predominately noted with fludarabine-based combinations such as fludarabine and cyclophosphamide or cladribine and cyclophosphamide. Of interest, infection risk was lowest in patients who responded to therapy. For the entire group of patients, the median survival was 10 months.
A second retrospective study by our group examined demographics and infectious morbidity of fludarabine-refractory CLL and SLL patients who received alternative forms of commercially available chemotherapy or rituximab after they were classified as fludarabine-refractory.7 This study included 27 patients with a median age of 67 and a median of 3 prior therapies. Overall, 24 of 27 patients (89%) developed serious infections requiring intravenous antibiotics with a 17% frequency of hospital admission per month. Overall an 11.4% response to therapy was noted, predominantly in patients receiving rituximab. Median survival of this cohort was 13 months. Overall, both of these studies uniformly demonstrate fludarabine-refractory CLL patients have a high frequency of infections even in the absence of any treatment. Additionally, survival for this patient group is quite short, ranging from 9–13 months.
Understanding the molecular features present in fludarabine-refractory CLL that mediate resistance to therapy remains a high priority. P53 mutations and deletions are one such feature, being present in only 5%–10% of patients at diagnosis8 but in 40%–50% at the time fludarabine-refractory disease is present.9,10 Identifying the presence or absence of p53 mutations remains quite important to picking therapy even in the absence of fludarabine-refractory status. Indeed, p53 mutations and deletions predict for lack of response to monotherapy with fludarabine, chlorambucil, and rituximab.11–,13 Furthermore, p53 mutations and deletions also predict for markedly inferior survival as compared to those patients who have wild type p53.14 The biologic significance of other molecular markers and events associated with progression to refractory CLL has not been adequately explored. Such studies will be important if new, effective therapies are to be developed.
Alternative Nucleoside Analogues
During the 1990s significant debate existed over the crossresistance between fludarabine, cladribine and pentostatin. A preliminary report that noted responses to cladribine in patients with fludarabine-refractory CLL prompted great interest;15 however, subsequent experience from several other groups failed to confirm these results and demonstrated considerable toxicity from cladribine due to myelosuppression and infections.16,17 The majority of patients treated on these trials had significant baseline cytopenias and advanced disease. To address this question, the Cancer and Leukemia Group B (CALGB) performed a Phase II study in fludarabine-refractory CLL.18 Twenty-eight patients were enrolled on this study with 13 having intermediate (Rai stage I or II) and 15 high (Rai stage III and IV) risk stages. No patient had a complete remission, but 9 (32%; 95% confidence interval, 15%–49%) attained a partial remission using the NCI criteria (1996).19 Responses were noted predominately in patients without anemia or thrombocytopenia (7 of 13, 54% with PR) versus only 2 of 15, 13% with PR, in patients with modest thrombocytopenia or anemia. The median time to relapse for responders was 12 months, while median PFS for the entire group was 9 months. Of interest, the median OS for this highly selected group of patients was 2.2 years. Further supporting the selective nature of the eligibility is that a large cooperative group required 4 years to enroll the 28 patients. Despite the modest benefit in terms of response, this regimen was quite toxic with grade 3–5 neutropenia (75%), thrombocytopenia (68%), and infections (43%) noted. Trials with clofarabine using the 5 consecutive day schedule of administration have also demonstrated minimal activity in this patient population.20 Overall, the use of an alternative nucleoside analogue such as cladribine or pentostatin in the setting of fludarabine-refractory CLL is not recommended.
Rituximab
The activity of rituximab in previously treated CLL is highly dependent upon schedule/dose of administration and has been reviewed previously.21 Dosed at the standard 375 mg/m2 weekly for 4–8 weeks as would be done in lymphoma, very modest activity is observed. Based upon the pharmacokinetic considerations of rapid antibody clearance in SLL, investigators have taken two different strategies to improve efficacy. In one single institution study, 50 patients with previously treated CLL (n = 40) or other B cell leukemias (n = 10) received weekly rituximab dose-escalated from 500 mg/m2 to 2250 mg/m2.22 Although no CLL patient achieved complete response, the overall response rate was 40%, and a statistically significant dose-response relationship was observed; 22% of patients treated with 500–850 mg/m2 responded, compared to 75% of patients treated with 2250 mg/m2. The overall response rate was 36% for CLL and 60% for other B cell leukemias; median response duration was 8 months. Eight of 12 patients (67%) at 2250 mg/m2 developed grade 2 toxicity, primarily fatigue, but no grade 3 or 4 toxicity was observed. However, for the patients who were fludarabine-refractory in this trial, the response rate was 20%.
Our group treated 33 patients with relapsed or refractory SLL/CLL with thrice weekly rituximab (375 mg/m2) for 4 weeks.23 Patients received 100 mg over 4 hours on the first day of therapy and 375 mg/m2 thereafter. This “stepped up” dosing schedule was designed to minimize infusion-related toxicity. The overall response rate was 45% (complete response 3%), and median response duration was 10 months. While response rate was 42% in the fludarabine-refractory patients, the response duration was only 6 months. Examination of molecular features in this trial demonstrated that the CLL patients with del(17p13.1) did not respond to therapy.13 An ongoing follow-up study by our group demonstrated absence of response in a second series of 8 del(17p13.1) patients (personal communication, JC Byrd). In general, it is the author’s approach not to consider rituximab monotherapy for fludarabine-refractory CLL unless the patient lacks a del(17p13.1) on interphase cytogenetics and is not a candidate for more aggressive therapy.
Fludarabine and Cyclophosphamide
A number of trials throughout the 1990s studied the combination of fludarabine and alkylating agents. Preclinical data24 suggest synergistic interaction between DNA damaging agents and the purine analogues. Such synergy likely occurs as a consequence of alkylator-induced DNA damage and subsequent inhibition of DNA repair by the nucleoside analogues. Combination studies with alkylating agents and each of the purine analogues have been performed. Early clinical studies demonstrated that myelosupression was more problematic and compromised the total administered dose of each agent.
Recent Phase II reports on fludarabine and cyclophosphamide with or without filgrastim support have been published. The MD Anderson experience included the largest series of fludarabine-refractory patients.25 O’Brien et al describe a total of 128 patients with CLL who were treated with fludarabine 30 mg/m2 intravenously daily for 3 days and cyclophosphamide at either 500 mg/m2 daily for 3 days (n = 11), 350 mg/m2/d for 3 days (n = 26), or 300 mg/m2 daily for 3 days (n = 91). The cyclophosphamide dose was decreased because of myelosuppression in the early part of the study. Patients were divided into four groups based on the expectation for response to single-agent fludarabine, including previously untreated patients (n = 34), patients previously treated with alkylating agents (n = 20), patients successfully treated with alkylating agents and fludarabine but relapsing (n = 46) and patients refractory to fludarabine with or without alkylating agents (n = 28). Fludarabine and cyclophosphamide produced 80% response rates in all patients not refractory to fludarabine at the start of therapy; however, it had a 38% response rate in patients who were refractory to fludarabine. Twenty-eight patients who were refractory to an alkylating agent + fludarabine had an overall response rate of 39%, with 3% complete response, 13% nodular partial response, and 26% partial response. The median time to progression in patients who had previously received fludarabine and alkylating agents was 20 months. However, the estimated median survival was 12 months for those patients who were fludarabine-refractory. Infections or fever of unknown origin occurred in almost half of all patients. Documented sepsis or pneumonia was noted in 25% of patients at some time during the therapy. Fever of unknown origin, frequently associated with neutropenia and requiring hospitalization, was observed in another 25% of patients. Significant infections were seen in 48% of fludarabine-refractory patients compared with 18% of patients not refractory to fludarabine with or without alkylating agents. Thus, while the combination of fludarabine and cyclophosphamide does produce palliation in a subset of patients with refractory CLL, the toxicities are significant. Because of this, pursuit of the potentially less myelosuppressive pentostatin in combination with cyclophosphamide has occurred, with preliminary data being quite positive in one single center Phase II study.26 Further pursuit of this strategy in larger Phase II trials is warranted to confirm these findings.
Fludarabine, Cyclophosphamide and Rituximab
Rituximab has several biologic properties in vivo in primary CLL cells including downmodulation of anti-apoptotic proteins that mediate resistance to fludarabine and alkylator-based therapy. In symptomatic, but untreated CLL, the addition of rituximab to fludarabine or fludarabine and cyclophosphamide improves CR rate, PFS and OS as compared to historical control studies that did not use rituximab. In relapsed CLL, a large 177 patient study from MD Anderson Cancer Center examining fludarabine, cyclophosphamide, and rituximab was reported.27 This study has demonstrated that 73% of patients responded with 25% attaining a CR. For the 37 patients who had fludarabine-refractory disease, a 5% complete response rate and 59% overall response was observed. Response duration for this group was not reported. Toxicity including grade 3 and 4 neutropenia was noted in 81% of patients while infections requiring hospitalization and antibiotic therapy occurred with 5% of the cumulative treatment courses. AML or MDS following therapy with this regimen was noted in 3% patients. Given the success of this combination, it is reasonable to consider this in fludarabine-refractory CLL patients who have bulky lymphadenopathy who are not good candidates for alemtuzumab (personal communication).
Alemtuzumab
Alemtuzumab (Campath-1H) is a humanized anti-CD52 monoclonal antibody that effectively fixes complement and depletes normal lymphocytes and lymphoma cells.28–,30 CD52 is a 21–28 kD glycopeptide expressed on the surface of nearly all human lymphocytes, monocytes and macrophages.31– 33 The function of CD52 is uncertain.
Alemtuzumab was approved for marketing in fludarabine-refractory CLL based upon treatment efficacy in 93 heavily pretreated, fludarabine-refractory CLL patients.5 This trial demonstrated an intent-to-treat response rate of 33%, although only 2% of patients achieved complete response. Median time to progression for responders was 9.5 months, with a median overall survival of 16 months for all patients and 32 months for responders. While the median peripheral blood CLL count decreased by more than 99.9%, alemtuzumab was less effective against nodal disease with only 12% of patients responding who had lymph nodes 5 cm or greater. Additionally, patients with an Eastern Cooperative Group performance status of 2 did markedly worse than patients with no or minimal symptoms from their disease. As a result of this pivotal CAM211 study, alemtuzumab was approved for the treatment of fludarabine-refractory CLL in the US. The activity of alemtuzumab in CLL was confirmed by a multi-institutional study in 136 patients with fludarabine-refractory B-CLL who received 30 mg thrice weekly for up to 12 weeks on a compassionate basis.34 The overall response rate was 40% (CR rate 7%), and the median PFS and OS of responders were 7.3 and 13.4 months, respectively. Currently several groups are combining alemtuzumab with fludarabine35 or rituximab36 as part of clinical trials.
One intriguing finding with alemtuzumab is its ability to eliminate CLL disease with p53 mutations or deletions. This was first reported by Stilgenbauer and Dohner, who demonstrated a complete response in a single patient with del(17)(p13.1) receiving alemtuzumab.37 Based upon this intriguing observation, our group sought to determine if alemtuzumab was an effective therapy in this patient group.10 Thirty-six patients with fludarabine-refractory CLL were treated with alemtuzumab, 15 of whom (42%) had p53 mutations or deletions. Clinical responses in patients with p53 mutations and/or deletions were noted in 6 of 15 (40%) versus 4 of 21 (19%) of patients without p53 abnormalities. The median response duration for this subset of patients was 8 months (range 3–17 months). These data confirm the results of Stilgenbauer and Dohner and suggest that alemtuzumab may be an effective therapy for CLL patients with p53 mutations or deletions. This also serves to contrast the divergent response patterns observed with monoclonal antibodies, as CLL with p53 deletions is not responsive to rituximab therapy.13
The major complications of alemtuzumab therapy include infections, infusion toxicity, and myelosuppression, which have limited its implementation into wide practice.38,39 With respect to infusion-related toxicity, subcutaneous administration has demonstrated promise in Phase II trials of previously untreated patients, but may have to be administered for a more prolonged period and have different pharmacokinetics. This option should therefore not be considered outside of a clinical trial until more data are available. Patients receiving alemtuzumab should receive prophylaxis for Pneumocystis carinii and Varicella zoster virus. In addition, patients should also be monitored carefully for cytomegalovirus reactivation during and immediately after therapy (for a minimum of 2 months after therapy).
Where does alemtuzumab fit into therapy of fludarabine-refractory CLL? This author considers this therapy for patients with a performance status of 0–1 who do not have lymph nodes exceeding 5 cm. In general alemtuzumab should be avoided in patients with active infections or who have a contraindication to prolonged immunosuppression.
New Therapies in Clinical Trials
Many new treatments are in clinical trials for CLL. Most promising of these includes the cyclin-dependent kinase inhibitor flavopiridol using a modified schedule of administration where promising early results have been observed. In addition, several other monoclonal antibodies including engineered anti-CD20 antibodies, anti-HLA-DR antibodies, anti-CD40 antibodies, TRAIL receptor DR4 and DR5 directed antibodies, and antibody-like molecules targeting CD37 are also entering early Phase I trials. Finally a recent trial with the interleukin (IL)-2 receptor ligand immunotoxin Ontak was reported in 18 fludarabine-refractory patients where 2 of 18 (11%) patients had partial responses.40 Significant constitutional symptoms were noted in a large proportion of these patients. Based upon these results a confirmatory study is ongoing at this time with this agent. Given the cost, toxicity, and marginal efficacy of this therapy, use of Ontak should be confined to ongoing Phase II trials. Indeed, it is through performance of clinical trials of promising new therapies in this patient population, with subsequent confirmation in less treated patients, that therapeutic options of CLL will ultimately improve.
Incidence of genomic aberrations and VH mutation status in one large single center fluorescence in situ hybridization (FISH) study compared with preliminary results from prospective multicenter trials of the German Chronic Lymphocytic Leukemia (CLL) Study Group (GCLLSG) for different clinical situations.
| Study . | 13q- . | 13q-single . | 11q- . | +12q . | 17p- . | 6q- . | VH Unmutated . | VH Mutated . |
|---|---|---|---|---|---|---|---|---|
| * Single center cohort of CLL patients distributed over all stages7,12 | ||||||||
| ** CLL1 trial of the GCLLSG for untreated Binet A patients with no classical indication for treatment | ||||||||
| *** CLL4 trial (randomized F vs FC) of the GCLLSG for untreated Binet B/C patients up to 65 years of age with indication for treatment | ||||||||
| **** CLL3 trial (early myeloablative radio-chemotherapy and autologous transplantation) of the GCLLSG for Binet B/C patients up to 60 years of age with maximum one line of prior therapy | ||||||||
| ***** CLL2H trial (subcutaneous alemtuzumab) of the GCLLSG for fludarabine-refractory patients with indication for treatment | ||||||||
| For details of the GCLLSG trials see also www.dcllsg.de | ||||||||
| Single center* | 55% | 36% | 18% | 16% | 7% | 7% | 56% | 44% |
| CLL1** | 59% | 40% | 10% | 13% | 4% | 2% | 41% | 59% |
| CLL4*** | 53% | 34% | 21% | 11% | 3% | 9% | 69% | 31% |
| CLL3**** | 52% | 27% | 22% | 12% | 3% | 6% | 68% | 32% |
| CLL2H***** | 48% | 14% | 32% | 18% | 27% | 9% | 81% | 19% |
| Study . | 13q- . | 13q-single . | 11q- . | +12q . | 17p- . | 6q- . | VH Unmutated . | VH Mutated . |
|---|---|---|---|---|---|---|---|---|
| * Single center cohort of CLL patients distributed over all stages7,12 | ||||||||
| ** CLL1 trial of the GCLLSG for untreated Binet A patients with no classical indication for treatment | ||||||||
| *** CLL4 trial (randomized F vs FC) of the GCLLSG for untreated Binet B/C patients up to 65 years of age with indication for treatment | ||||||||
| **** CLL3 trial (early myeloablative radio-chemotherapy and autologous transplantation) of the GCLLSG for Binet B/C patients up to 60 years of age with maximum one line of prior therapy | ||||||||
| ***** CLL2H trial (subcutaneous alemtuzumab) of the GCLLSG for fludarabine-refractory patients with indication for treatment | ||||||||
| For details of the GCLLSG trials see also www.dcllsg.de | ||||||||
| Single center* | 55% | 36% | 18% | 16% | 7% | 7% | 56% | 44% |
| CLL1** | 59% | 40% | 10% | 13% | 4% | 2% | 41% | 59% |
| CLL4*** | 53% | 34% | 21% | 11% | 3% | 9% | 69% | 31% |
| CLL3**** | 52% | 27% | 22% | 12% | 3% | 6% | 68% | 32% |
| CLL2H***** | 48% | 14% | 32% | 18% | 27% | 9% | 81% | 19% |
Relation of VH mutation status and genomic aberrations in 300 chronic lymphocytic leukemia (CLL) cases.7
| Aberration . | VH Mutated . | VH Unmutated . | P-value# . |
|---|---|---|---|
| . | (homology < 98%) n = 132 (44%) . | (homology > 98%) n = 168 (56%) . | . |
| # Fisher’s exact test | |||
| Clonal aberrations | 80% | 84% | .37 |
| 13q deletion | 65% | 48% | .004 |
| 13q deletion single | 50% | 26% | <.001 |
| Trisomy 12 | 15% | 19% | .44 |
| 11q deletion | 4% | 27% | <.001 |
| 17p deletion | 3% | 10% | .03 |
| 17p or 11q deletion | 7% | 35% | <.001 |
| Aberration . | VH Mutated . | VH Unmutated . | P-value# . |
|---|---|---|---|
| . | (homology < 98%) n = 132 (44%) . | (homology > 98%) n = 168 (56%) . | . |
| # Fisher’s exact test | |||
| Clonal aberrations | 80% | 84% | .37 |
| 13q deletion | 65% | 48% | .004 |
| 13q deletion single | 50% | 26% | <.001 |
| Trisomy 12 | 15% | 19% | .44 |
| 11q deletion | 4% | 27% | <.001 |
| 17p deletion | 3% | 10% | .03 |
| 17p or 11q deletion | 7% | 35% | <.001 |
Probability of survival from the date of diagnosis among patients with mutated (VH homology < 98%) and unmutated (VH homology ≥ 98%) VH status.7
A) The estimated median survival time for the VH homology ≥ 98 and < 98% groups were 79 months and 152 months, respectively.
B) When only patients diagnosed at Binet stage A were evaluated the estimated median survival times for the VH homology ≥ 98% and VH homology < 98% groups were 79 months vs 152 months.
Probability of survival from the date of diagnosis among patients with mutated (VH homology < 98%) and unmutated (VH homology ≥ 98%) VH status.7
A) The estimated median survival time for the VH homology ≥ 98 and < 98% groups were 79 months and 152 months, respectively.
B) When only patients diagnosed at Binet stage A were evaluated the estimated median survival times for the VH homology ≥ 98% and VH homology < 98% groups were 79 months vs 152 months.
Prognostic relevance of genomic aberrations in chronic lymphocytic leukemia (CLL).12
A) Probabilities of disease progression as assessed by the treatment-free interval in the 5 dominant categories of genomic aberrations. The median treatment-free intervals for the 17p deletion (n = 23), 11q deletion (n = 56), 12q trisomy (n = 47), normal karyotype (n = 57), and 13q deletion (single abnormality; n = 117) groups were 9, 13, 33, 49, and 92 months, respectively.
B) Estimated survival probabilities from the date of diagnosis in 325 CLL patients divided into the 5 categories defined in a hierarchical model of genomic aberrations in CLL.12 The median survival times for the 17p deletion (n = 23), 11q deletion (n = 56), 12q trisomy (n = 47), normal karyotype (n = 57), and 13q deletion (as single abnormality; n = 117) groups were 32, 79, 114, 111, and 133 months, respectively.
Prognostic relevance of genomic aberrations in chronic lymphocytic leukemia (CLL).12
A) Probabilities of disease progression as assessed by the treatment-free interval in the 5 dominant categories of genomic aberrations. The median treatment-free intervals for the 17p deletion (n = 23), 11q deletion (n = 56), 12q trisomy (n = 47), normal karyotype (n = 57), and 13q deletion (single abnormality; n = 117) groups were 9, 13, 33, 49, and 92 months, respectively.
B) Estimated survival probabilities from the date of diagnosis in 325 CLL patients divided into the 5 categories defined in a hierarchical model of genomic aberrations in CLL.12 The median survival times for the 17p deletion (n = 23), 11q deletion (n = 56), 12q trisomy (n = 47), normal karyotype (n = 57), and 13q deletion (as single abnormality; n = 117) groups were 32, 79, 114, 111, and 133 months, respectively.
Survival probabilities among patients in the following genetic categories: 17p- (17p deletion irrespective of VH mutation status), 11q- (11q deletion irrespective of VH mutation status), unmutated VH (homology ≥ 98% and no 17p or 11q deletion), and mutated VH (homology < 98% and no 17p or 11q deletion).7
A) Among all 300 patients estimated median survival times were: 17p- 30 months, 11q- 70 months, VH ≥ 98% 89 months. and VH < 98% not reached (54% survival at 152 months)B) In Binet A patients only (n = 189) estimated median survival times were: 17p- 36 months, 11q- 68 months, VH ≥ 98% 86 months months, and VH < 98% not reached (52% survival at 152 months).
Survival probabilities among patients in the following genetic categories: 17p- (17p deletion irrespective of VH mutation status), 11q- (11q deletion irrespective of VH mutation status), unmutated VH (homology ≥ 98% and no 17p or 11q deletion), and mutated VH (homology < 98% and no 17p or 11q deletion).7
A) Among all 300 patients estimated median survival times were: 17p- 30 months, 11q- 70 months, VH ≥ 98% 89 months. and VH < 98% not reached (54% survival at 152 months)B) In Binet A patients only (n = 189) estimated median survival times were: 17p- 36 months, 11q- 68 months, VH ≥ 98% 86 months months, and VH < 98% not reached (52% survival at 152 months).
Progression-free survival (PFS) assessed according to genetic markers in the multicenter prospective CLL1 trial of the GCLLSG26,28(see also www.dcllsg.de).
A) According to VH mutation status
B) According to genomic aberrations
Progression-free survival (PFS) assessed according to genetic markers in the multicenter prospective CLL1 trial of the GCLLSG26,28(see also www.dcllsg.de).
A) According to VH mutation status
B) According to genomic aberrations
Matched pair analysis based on the matching variables age, Binet stage, VH mutation status, and lymphocyte count between patients receiving autologous stem cell transplantation (autologous SCT) and conventional treatment (conventional).39
Survival from the time of diagnosis (n = 88) of all patients treated with autologous SCT (broken line) and conventional chemotherapy (solid line), respectively.
Matched pair analysis based on the matching variables age, Binet stage, VH mutation status, and lymphocyte count between patients receiving autologous stem cell transplantation (autologous SCT) and conventional treatment (conventional).39
Survival from the time of diagnosis (n = 88) of all patients treated with autologous SCT (broken line) and conventional chemotherapy (solid line), respectively.
Minimal residual disease (MRD) kinetics after autologous stem cell transplantation (SCT) and non-myeloablative allogeneic SCT.40
MRD levels (number of CLL specific DNA copies per total DNA copies in the sample compared to the reference [pre-therapeutic] sample) of patients after myeloablative conditioning and auto-SCT and after non myeloablative allogeneic SCT. Black-filled symbols (circles and triangles) denote MRD-positive samples whereas blank symbols denote the sensitivity of PCR-negative samples (calculated minimum MRD level, which would have been detected in this particular sample).
Minimal residual disease (MRD) kinetics after autologous stem cell transplantation (SCT) and non-myeloablative allogeneic SCT.40
MRD levels (number of CLL specific DNA copies per total DNA copies in the sample compared to the reference [pre-therapeutic] sample) of patients after myeloablative conditioning and auto-SCT and after non myeloablative allogeneic SCT. Black-filled symbols (circles and triangles) denote MRD-positive samples whereas blank symbols denote the sensitivity of PCR-negative samples (calculated minimum MRD level, which would have been detected in this particular sample).
An approach to the initial management of patients with symptomatic chronic lymphocytic leukemia (CLL).
An approach to the initial management of patients with symptomatic chronic lymphocytic leukemia (CLL).

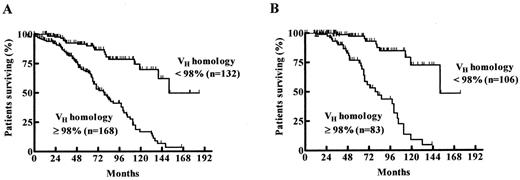
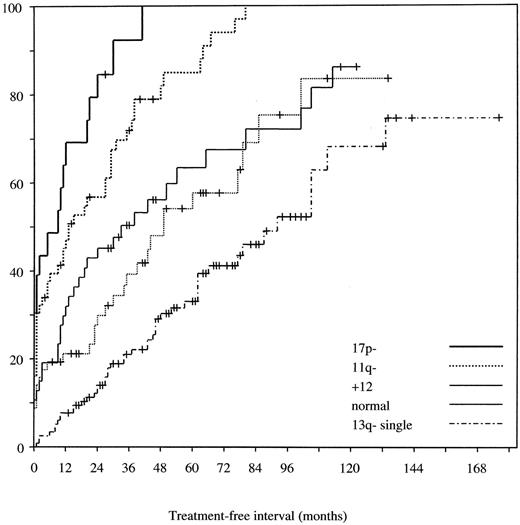
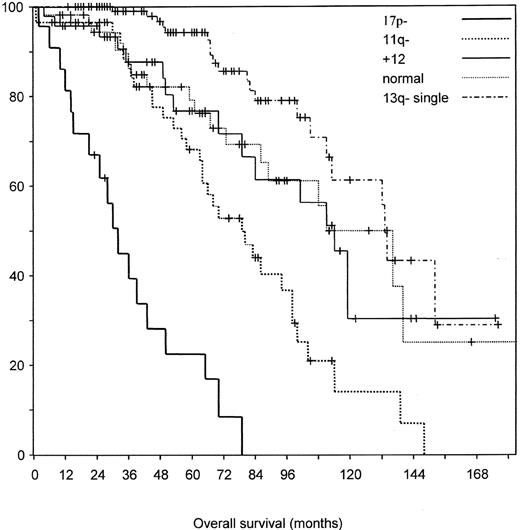
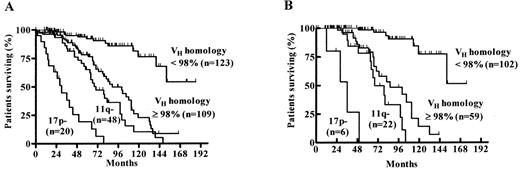
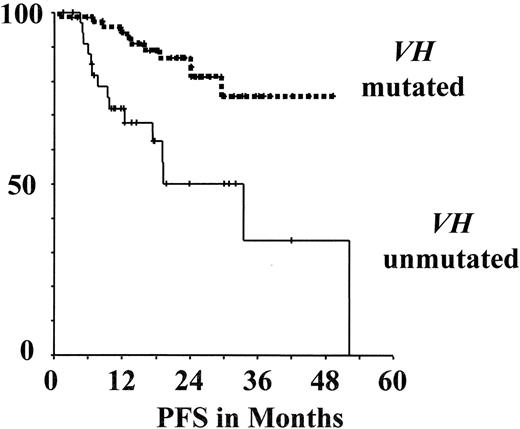
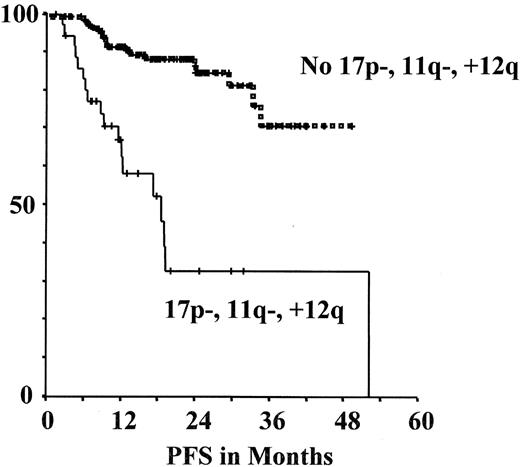
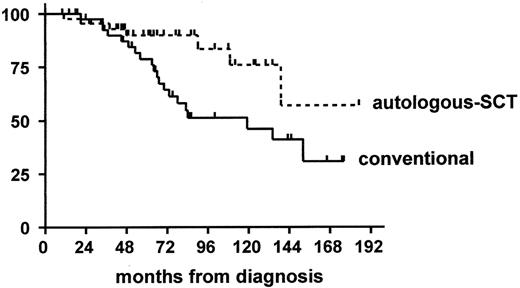
![Figure 8. Minimal residual disease (MRD) kinetics after autologous stem cell transplantation (SCT) and non-myeloablative allogeneic SCT.40. / MRD levels (number of CLL specific DNA copies per total DNA copies in the sample compared to the reference [pre-therapeutic] sample) of patients after myeloablative conditioning and auto-SCT and after non myeloablative allogeneic SCT. Black-filled symbols (circles and triangles) denote MRD-positive samples whereas blank symbols denote the sensitivity of PCR-negative samples (calculated minimum MRD level, which would have been detected in this particular sample).](https://ash.silverchair-cdn.com/ash/content_public/journal/hematology/2004/1/10.1182_asheducation-2004.1.163/4/m_byrd_fig8.jpeg?Expires=1765905315&Signature=nf0Vv2mZ-aTTTPuFveDqw2QuP9uFBQ4jyUTyOPmPow6sCG3F-8rIrXcsO0FcHxEeOMbR089ydxf0m~BNrkOqG4cItXcyL4F6w8i4xGfIjztzoa4v5cVoQDU8GV04GsHUT1NkzWA4Lz7Vr4GW6Q1cGLBC~WlYccSd2fDPirHxUjuje9QYWQhlSUfN5~8PnDEwGNxsyGA9SxmGHFnwDMx5KKiRZcm8ZwUmEsKVQM34gShvrmEGDerkGVBljlZumNrFUKWhRjuvDkrwu0orObwQgmhzH-6ZomNSZZi17OMHAW7A0NAgeHMMciKj1JqkLqYFlYdE0ZexnRWa0nsA-0zIBw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)