Abstract
Folicular lymphoma (FL), the second most common subtype of non-Hodgkin lymphoma, shows considerable heterogeneity in its clinical behavior, representative of a biology that appears increasingly complex and diverse. As our knowledge of the molecular basis of FL increases, we strive for an integration between the bench and clinic that yields treatments based on our scientific understanding and biomarkers that allow us to prescribe treatment rationally.
In Section I, Dr. Randy Gascoyne describes the histologic, cytogenetic and biologic features of FL that underlie its clinical variability. Key aspects of the pathologic diagnosis of FL that have particular relevance to the clinician are highlighted. A proposed model for follicular lymphomagenesis and diffuse large B cell lymphoma transformation has emerged and continues to evolve as the molecular story unfolds. A biologic basis for clinical outcome in FL also appears to be forthcoming.
In Section II, Dr. Jane Winter addresses the complex process of selecting among the many treatment options for patients with FL. Previously a simple matter of deciding between oral or intravenous alkylators, clinicians and patients must now struggle to choose among vastly different approaches ranging from “watch and wait” to stem cell transplantation. The introduction of rituximab and radioimmunoconjugates is changing the treatment paradigm, but the optimal approach to integrating these and other new agents remains to be determined. At every decision point, the best approach is always a clinical trial.
In Section III, Dr. Koen Van Besien provides a well-documented update on outcomes associated with autologous and allogeneic stem cell transplantation for FL. The results of trials of autologous stem cell transplantation in first remission and recent data supporting a role for graft purging are discussed. Based on the premise that a graft-versus-lymphoma effect is operative in FL, reduced-intensity allogeneic transplantation is the preferred approach in many cases, and recently reported results are summarized. Criteria for patient selection and the optimal role of transplantation in the overall therapeutic plan for the patient with FL are presented.
I. Follicular Lymphoma: Pathology and Biology
Randy D. Gascoyne, MD*
British Columbia Cancer Agency, Dept. of Pathology, 600 W 10th Ave., Vancouver BC V5Z 4E6, Canada
Based on a large international study, follicular lymphoma (FL) represents the second most frequent lymphoma subtype; only diffuse large B cell lymphoma (DLBCL) is more common.1 Clinically most patients are elderly and present with advanced stage disease. Median survivals approximate 8–10 years, as the disease runs an inexorable course in most cases. The majority of patients are at risk for histologic progression to a process resembling DLBCL. The broad spectrum of clinical behavior in FL, together with the slope of the survival curve, provides compelling evidence for the marked clinical heterogeneity of this tumor. The range of presentations from cases that undergo spontaneous remissions to those that present with adverse cytogenetic features and run an aggressive clinical course attests to the clinical diversity of FL. Histologic, cytogenetic and biologic features of FL that lend credence to this underlying heterogeneity are the focus of the discussion that follows.
Pathology
Several key aspects in the diagnosis of FL are clinically relevant. The distinction of FL from reactive follicular hyperplasia (RFH) is paramount, as the latter represents a benign condition. A number of morphological features are of value in making this distinction; in particular, a low density of follicles per unit area, the presence of polarity within follicles and the lack of a monomorphic appearance within the follicles all favor RFH. A number of immunohistochemical tests are useful for distinguishing FL from RFH, including a high proliferation rate as assessed with MIB-1, absence of Bcl-2 staining within the follicle and absence of CD10 and/or Bcl-6 protein expression in the inter-follicular areas, all of which favor RFH.2 Using assays with limited sensitivity, the presence of B cell clonality or the finding of a BCL2 oncogene rearrangement are both useful features that support a diagnosis of FL.3 Gene expression studies can also be used to distinguish RFH from FL in most cases. FL is subdivided into cytological grades and variants as detailed in Table 1 . The distinction between grade 1 FL and grade 3 FL is based on the number of admixed centroblasts within the neoplastic follicles. Grade 1 is characterized by a predominance of small centrocytes with < 5 centroblasts per high power field (HPF). Grades 2 (> 5 centroblasts but < 15/HPF) and 3 have increasing numbers of centroblasts, with grade 3a having > 15 centroblasts per HPF. Grade 3 FL, previously referred to as follicular large cell lymphoma, is subdivided into two types. Grade 3a FL reveals a mix of centrocytes and centroblasts within follicles while 3b is characterized by sheets of large centroblasts and absent centrocytes. Whether or not this distinction has clinical significance is unclear.4 A small amount of data suggests that grade 3a FL is part of the spectrum of indolent grade 1 and 2 FL, while 3b FL may be more akin to de novo DLBCL.5 Lastly, the presence of diffuse areas within a FL appears to confer more aggressive clinical behavior.4 In addition, unusual cytological variants can be encountered that are not included in the World Health Organization classification, including cases with large centrocytes and others with small centroblasts; in some cases the latter may resemble the cytomorphology of Burkitt lymphoma and may on occasion be associated with MYC oncogene translocations.
An isolated cutaneous form of FL occurs uncommonly and characteristically involves the scalp. These cases are characterized by a lower frequency of t(14;18) and expression of Bcl-2 protein, yet the cells express CD10 and Bcl-6 in most cases. A recent study using polymerase chain reaction (PCR) and fluorescence in situ hybridization (FISH) suggests that primary cutaneous FL may not be related to primary nodal FL, as virtually all cases lack the t(14;18).5 A truly diffuse form of FL may be rarely encountered, but on closer inspection, most cases demonstrate vaguely follicular architecture that is underappreciated without the routine use of immunostains for follicular dendritic cells. The latter represent the antigen-presenting cells of the lymphoid follicle and possess long cytoplasmic processes that provide the scaffolding for the secondary follicle. When a diagnosis of true diffuse follicle center lymphoma is considered, the pathologist is encouraged to demonstrate co-expression of CD10 and Bcl-6 as well as presumptive evidence of the t(14;18).
Approximately 10% of FL cases reveal the presence of a zone of cells resembling marginal zone B cells, immediately surrounding neoplastic follicles. Importantly, a residual benign mantle zone is not seen, helping to distinguish FL from a marginal zone lymphoma. The area of marginal zone differentiation within a FL takes on a distinct morphology with cells having moderate amounts of pale cytoplasm; moreover, the immunophenotype is also different, with downregulation of CD10 and Bcl-6 expression by cells within the marginal zone compartment. This aspect of FL pathology serves to highlight the importance of microenvironment influencing both morphology and immunophenotype.
Finally, a rare form of FL may be seen referred to as “in-situ” FL. In these uncommon cases, scattered malignant follicles are identified within lymph nodes revealing mostly benign features. The malignant follicles are typically more monomorphic, but importantly strongly express Bcl-2 protein helping to identify them as neoplastic. Laser-capture microdissection together with PCR and sequencing suggests that these rare cases may represent early forms of FL, wherein malignant follicular B cells seed benign follicles.6 These and other data provide insights into the trafficking of malignant B cells in FL.
Unresolved issues in FL pathology remain and to some extent contribute to problems with reproducibility. Diffuse areas in FL are often not identified, in part resulting from underutilization of follicular dendritic cell stains.4,7 Similarly, follicular areas in suspected de novo DLBCL are underappreciated. Variability in the cytology of cells within follicles contributes to inconsistent reporting of FL grade. Follicular dendritic cells are commonly misidentified as centroblasts, resulting in higher grade and, in general, there is poor inter-observer reproducibility for the counting of centroblasts. All of these factors contribute to inconsistencies in the diagnosis and grading of FL biopsies, and to some extent raise questions regarding the validity of grading and clinical decision making based on grade.
Immunophenotype
In the majority of cases of FL the immunophenotype is uniform. The neoplastic B cells express the pan-B cell markers CD19, CD20, CD22 and CD79a. FL cells express antigens of the germinal center including CD10 and Bcl-6. Most cases of FL express Bcl-2 protein, which is highly correlated with the presence of the t(14;18) but may be expressed in cases with a clonal karyotype lacking the t(14;18) (discussed in detail below). Uncommon cases express CD43, and even less commonly CD5. The proliferative rate as assessed using Ki-67 is characteristically low in FL and is a useful criterion for distinguishing FL from RFH.
Cytogenetics
The t(14;18)(q32;q21) is the cytogenetic hallmark of FL, present in approximately 85% of grade 1 and 2 FL. The translocation results in the juxtaposition of the BCL2 oncogene into the IGH heavy chain locus on chromosome 14, leading to its constitutive expression. This molecular event is critical to follicular lymphomagenesis and is an early event, but may on its own be insufficient to produce FL. A marked heterogeneity of clonal evolution events characterizes FL at diagnosis and these alterations likely underlie the varied clinical behavior. The mean number of alterations is extremely variable, averaging 4.8, 6.5 and 19 for grades 1, 2 and 3 FL, respectively.8 Correlation of the cytogenetic alterations with gene expression data using purified FL cells suggests that although translocation mechanisms are critical to disease initiation, most of the clonal evolution events that underlie disease progression and disordered gene expression result from copy number alterations and not balanced translocations.9 Regions involved in these events include gains such as +X, +7, +12 or gain of 12q13-14, +18, and losses including del6q, del13q, 1p36-, 17p13- and del10q22-24.10 Recent studies of FL suggest that distinct pathways of clonal evolution may exist and point to specific temporal sequences that follow the acquisition of the t(14;18).11 How these pathways correlate with gene expression has yet to be studied.
The presence of the t(14;18), although characteristic of FL, is not specific, being found in 15%–20% of DLBCL and an almost equal percentage of mucosa-associated lymphoid tissue (MALT) lymphomas. Importantly, in MALT lymphomas the BCL2 oncogene is not involved but rather the translocations involve the MALT1 gene located 5 MB centromeric of the BCL2 gene.
FLs lacking the t(14;18) exist and reveal distinct patterns of chromosomal alterations.12 Translocations involving the BCL6 oncogene are overrepresented in this group and appear to travel with absent Bcl-2 protein expression. Moreover, Bcl-2 immunohistochemical studies suggest two subgroups with distinct cytogenetic, phenotypic and possibly clinical features; one with Bcl-2 protein overexpression unrelated to an IGH/BCL2 rearrangement and a second without Bcl-2 expression. Grade 3 FL is not a homogeneous entity, as recent cytogenetic data suggest at least two subgroups that appear to have a morphological correlate.7 Grade 3a FL with some residual centrocytes appears to represent the aggressive end of the clinical/morphological spectrum of indolent FL, closely related to grades 1 and 2 FL, and is characterized by the t(14;18). Grade 3b FL on the other hand is characterized by sheets of centroblasts without admixed centrocytes, is CD10-positive in only 50% of cases, is much less often associated with the t(14;18), often harbors the t(3;14) or variant involving the BCL6 oncogene and may be more closely related to de novo DLBCL. Cytogenetic studies suggest that within the category of grade 3b FL, the t(14;18) and t(3;14) are mutually exclusive.13 However, the clinical relevance of these distinctions remains controversial.
Molecular Genetics
In FL one of the IGH genes is involved in the t(14;18) and thus precludes involvement of this allele in a functional rearrangement. Similar to normal germinal center B cells, FL cells typically undergo a functional rearrangement of the remaining IGH locus, which shows frequent somatic mutations and evidence of ongoing mutation leading to intraclonal heterogeneity, suggesting persistent activity of the somatic hypermutation machinery. The t(14;18) is the cytogenetic equivalent of the BCL2 oncogene rearrangement that characterizes most cases of FL and is a critical event in lymphomagenesis. Transgenic mouse models suggest that the t(14;18) alone is insufficient to produce lymphoma. Presumably the constitutive expression of Bcl-2 accompanied by the inhibition of programmed cell death allows early FL cells to remain alive and therefore at risk for subsequent molecular alterations needed to produce a fully malignant phenotype.
Normal DNA structure is described as bimodal or B-DNA. This corresponds to the normal double-stranded DNA helix resulting from complementary base pairing. A non-B-DNA structure refers to areas of single stranded DNA where breakage would be more likely to occur. Previous dogma regarding the t(14;18) implicated the recombinase complex (RAG) and suggested that BCL2 translocations were the result of errors during primary VDJ recombination resulting from recombination recognition sequences (RRS) located on chromosome 18. In fact, this turns out not to be true. Although the RAG enzyme is involved, it is not the result of RRS on chromosome 18 but occurs because the major breakpoint region (MBR) within the 3′ untranslated region of the BCL2 gene forms a non-B-DNA structure that is recognized and cleaved by the RAG complex.14 Other breakpoints involved in the t(14;18) include the minor cluster region (MCR) located 20–30 kB downstream of the MBR and a more recently recognized region referred to as the intermediate cluster region (ICR) that lies between the MBR and the MCR. Proximity within the nucleus of chromosomes 14 and 18 may also play a role in promoting translocations. Breakpoints within the BCL2 region are clustered, but nonetheless span a large area of DNA. Thus, PCR strategies are associated with false-negative results, although when positive in a given case, they are useful for assessing minimal residual disease. Standard cytogenetic studies and locus-specific FISH techniques are much more sensitive.3
Biology
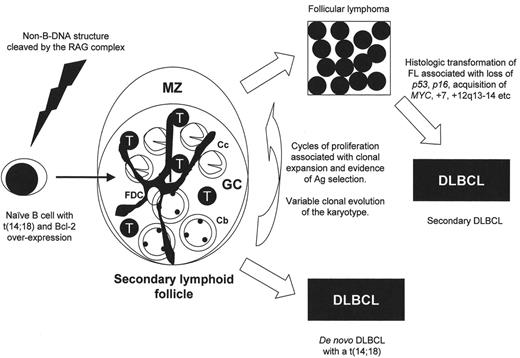
The weight of molecular evidence would favor that the t(14;18) occurs in an immature B cell within the bone marrow at a time when the nuclear enzyme terminal deoxynucleotidyl transferase (Tdt) is active and contact with antigen has not yet occurred. The neoplastic FL cell is left with the capacity to divide and differentiate, exit the bone marrow and seed peripheral lymph nodes primarily. The constitutive expression of Bcl-2 protein resulting from the t(14;18) gives these cells a growth advantage and maintains their extended survival. Now within the germinal center, a hostile microenvironment where the somatic hypermutation apparatus is active, the FL cells divide and acquire additional molecular alterations resulting in clonal evolution. The degree to which this occurs prior to clinical presentation is extremely variable, as noted earlier. The typical result following repeated cell cycles is characteristically FL. However, in some cases the presentation may be of a de novo DLBCL harboring a t(14;18) (Figure 1 ). The factors that dictate the diversity of cytogenetic alterations and presumed cytogenetic instability are largely unknown.
The development of FL recapitulates the formation of normal lymphoid follicles as evidenced by morphology and immunophenotype. Neoplastic follicles contain the same mix of germinal center B cells and accessory cells, the latter including T cells, macrophages, lymph node stromal elements and follicular dendritic cells (Figure 1 ). The latter cell type is not thought to be part of the clone, yet appears to be important in follicular lymphomagenesis, as these cells form an integral component of FL in all sites, including extranodal localizations and bone marrow metastases. A recent publication that raises questions about the nature of the tumor vasculature in FL immediately begs the same question concerning the follicular dendritic cells and other accessory cells in this tumor.15 The typical changes that herald transformation to DLBCL include a loss of the follicular dendritic cell meshwork, reduction in reactive T cells and an increase in centroblasts in proportion to centrocytes. The critical question is whether these non-neoplastic cells in FL are playing a role in immune surveillance, thus maintaining the lymphoma in check, or alternatively merely reflect the milieu required by the FL cells in order to survive.
The molecular events that characterize FL appear to represent a combination of background genetic alterations evident at the time of diagnosis and ongoing stochastic events that contribute to clonal evolution. Some data suggest that FL progression may be due to the outgrowth of minor clones present at diagnosis that gain a growth advantage over time.16 The comprehensive cytogenetic database of FL biopsies similarly suggests that the clinical course may be programmed at the time of diagnosis. Moreover, recent microarray gene expression profiling experiments (discussed below) suggest that the molecular features of FL at diagnosis dictate the aggressiveness of the disease and the survival of the patients. Alternatively, the analyses of paired samples of patients with FL and subsequent DLBCL suggest a role for on-going stochastic events occurring over time in the clonal evolution of FL. More than likely, a combination of these hypotheses will prove to be correct.
Inhibition of apoptosis resulting from the constitutive expression of Bcl-2 is critical to the pathogenesis of FL. Some data support the notion that for those cases without a t(14;18) and/or Bcl-2 expression, other molecules in the apoptotic signaling cascade may be involved (e.g., Bcl-XL, Bad).17 Importantly, inhibition of cell death rather than accelerated growth is the hallmark of FL development. Activation-induced cytidine deaminase (AID) has recently been shown to be required for somatic hypermutation and class-switch recombination and is expressed in a subset of FL. Its expression appears to correlate with the presence of ongoing mutation within the FL clone, a finding that would be of interest to correlate with survival.18
Initial gene expression studies in FL used purified malignant B cells and thus failed to appreciate the role of non-neoplastic cells in the biology of FL.19 Nonetheless, these studies produced a long list of genes that correlate well with chromosomal gains and losses in FL.9 More recent microarray studies using whole frozen tissues have provided important insights into the significance of non-neoplastic cells. In a study of 191 FL diagnostic biopsies, two gene expression signatures could be defined, both of which were not derived from the malignant B cells (S Dave et al, manuscript in preparation). One signature referred to as immune response 1 (IR1) contained many T cell signature genes and correlated with a survival advantage. The other signature, IR2, appeared to be associated with macrophages or perhaps follicular dendritic cells and predicted for inferior survival. These signatures did not appear to simply reflect absolute numbers of T cells and/or macrophages/follicular dendritic cells, as other characteristic pan-T cell or macrophage/follicular dendritic cell genes were not associated with survival. Interestingly, some of these genes overlap with genes thought to be associated with resistance to Rituximab treatment in FL. High-speed sorting of fresh FL biopsies confirmed the origin of the expressed genes and provided clear evidence that non-neoplastic cells in FL modulate the clinical course of the disease. These data, together with data from animal models of FL, suggest that T cells play an important role in follicular lymphomagenesis. Whether these immune response cells represent an active role for immune surveillance in FL remains unanswered, as does a clear explanation for the marked variation in immune cell signatures in FL. Genetic polymorphisms that govern immune responses in general may modulate the nature and extent of tumor-infiltrating host cells. Alternatively, the malignant cells in FL may dictate the character of the immune infiltrate reflecting nothing more than the degree of clonal evolution of the tumor cells.
At some time during the first 10 years following a diagnosis of FL, approximately 30%–50% of patients will undergo histological transformation, often heralded by a change in the clinical course of the disease. Repeat biopsies characteristically show a change in the histologic appearance from FL to DLBCL, although less frequent patterns of transformation may be encountered. Like the cytogenetic alterations that characterize FL cells at diagnosis, the molecular events that underlie histologic transformation of FL are also diverse. A number of alterations are associated with transformation to DLBCL, including p53 mutations, loss of p16, upregulated MYC expression resulting from translocation or other mechanisms, +7, gain of 12q13-14, del(6q) and possibly mutations of BCL2 and/or BCL6 genes. Recent microarray studies have also suggested a role for MYC and MYC target genes and possible involvement of p38MAP kinase deregulation in FL transformation.20,21 High-resolution array comparative genomic hybridization (CGH) strategies will be important in helping to identify the myriad of molecular changes that underlie FL progression, but may require purified FL cells in order to accurately detect single copy genetic alterations. Important insights into pathogenetic mechanisms should result from these studies, particularly if these data are combined with genome-wide expression profiling.
II. Controversies in the Treatment of Follicular Lymphomas (Grades 1 and 2)
Jane N. Winter, MD*
Feinberg School of Medicine, Northwestern University, Div. of Hematology/Oncology, 676 N St. Clair, Suite 850, Chicago IL 60611-4538
Once a simple choice between “watch and wait” and alkylator therapy, decision-making for patients with FL and their physicians has become a complex process in which the pros and cons of many new effective treatment options must be weighed. Within a relatively short period of time, numerous new agents have become available. The current challenge is to devise optimal strategies for individual patients and to select the most pressing questions to be addressed in clinical trials.
Early Stage Disease
For the relatively small group of patients with stage I or II FL, radiotherapy (XRT) continues to be the standard of care based on studies showing long survivals and many patients without recurrence. The long-term outcome following involved field XRT (IF-XRT) at the Princess Margaret Hospital was recently updated showing cumulative relapse rates of 54% and 56% at 15 and 25 years, respectively, with only a 2% risk of relapse beyond 15 years.1 A recent report from Stanford University, however, suggests that deferring therapy until there are symptoms or other indications for treatment may yield survival that is comparable to that achieved with immediate XRT.2 In an effort to improve on the results of IF-XRT, the MD Anderson Cancer Center group added multiagent chemotherapy and report excellent disease control.3 A randomized trial comparing IF-XRT to combined modality therapy is ongoing. Use of functional imaging with positron emission tomography (PET) may improve the results of IF-XRT by more accurately identifying patients with localized disease. Radioimmunotherapy (RIT) is yet another approach that has the potential to improve outcomes in early stage FL by providing both local and systemic therapy with less toxicity but has not yet been studied in this setting.
Advanced Stage Disease
Watch and wait
Three randomized clinical trials have shown that the initiation of conventional therapy soon after diagnosis does not alter outcome in FL. The British National Lymphoma Investigation (BNLI) recently reported the results of a Phase III trial that randomized asymptomatic adults to either daily chlorambucil or observation.4 With a median follow-up of 16 years, there has been no difference in overall or cause-specific survival. The median time to first systemic treatment for patients in the observation group was 2.6 years. For patients over the age of 70, the chances of not receiving chemotherapy or dying of lymphoma were 40% at 10 years. Complete remission rates were higher in the immediate treatment group, compared to patients who were observed and later treated (63% vs 27%), a difference that may be important if the ultimate goal is to administer post-remission therapy (e.g., vaccine) that is likely to be most effective in the presence of minimal residual disease. Carefully designed clinical trials are required to establish whether or not treatment with rituximab (R, Rituxan; Genentech Inc., South San Francisco, CA, and Biogen Idec Inc., Cambridge, MA), chemoimmunotherapy, or RIT will benefit the asymptomatic patient with advanced stage disease.
Chemotherapy
Studies comparing single-agent chemotherapy with multiagent therapy in patients with advanced stage FL have not shown meaningful differences in clinical endpoints, including survival. A recently reported trial by the Cancer and Leukemia Group B (CALGB) compared single-agent daily oral cyclophosphamide with CHOP (cyclophosphamide, hydroxydaunomycin, oncovin, prednisone)–Bleo5 and showed no benefit to the more aggressive therapy. An unplanned analysis of the small subgroup with follicular, mixed lymphoma, however, demonstrated an improved failure-free survival and overall survival (OS) with combination chemotherapy. The achievement of durable remissions in patients with follicular mixed lymphoma (grade 2) is consistent with reports from the National Cancer Institute (NCI), also in small numbers of patients. Others, however, have failed to document a difference in clinical behavior between grades 1 and 2 FL or a benefit to more intensive therapy for grade 2 disease. Expert hematopathologists rarely agree when trying to distinguish between grades 1 and 2, further complicating matters. It is likely that molecular profiling, rather than morphology or clinical parameters, will soon be used to identify subgroups of patients most likely to benefit from specific treatments. The recently reported FL international prognostic index was derived using clinical data from patients diagnosed between 1985 and 1992 and may no longer be the best clinically based predictor of outcome in the face of new therapies.6
Fludarabine, identified in the 1980s as an active agent in FL, has been incorporated into combination regimens with high response rates including molecular remissions. Although toxicity was satisfactory in a Phase I/II trial combining fludarabine with cyclophosphamide, the toxicity associated with this combination was unacceptable in an Eastern Cooperative Oncology Group (ECOG) Phase III comparison with CVP (cyclophosphamide, vincristine, prednisone), forcing the premature closure of the fludarabine-containing arm.7 Other regimens containing fludarabine have not been associated with the toxicity observed in the ECOG trial, but have not been shown to prolong the duration of remission when compared to other multiagent regimens. Prior therapy with purine analogues has also been associated with poor mobilization of peripheral blood progenitor cells in patients destined for transplant, further complicating decision-making regarding its use as initial therapy in patients who may require autologous hematopoietic stem cell transplant in the future.
Interferon-α
Interferon (IFN)-α has been studied extensively in FL patients both in combination with chemotherapy and as maintenance therapy with varying results. In most studies, IFN-α was associated with a prolongation of remission, but not OS. In a recently reported large Phase III trial conducted by Southwestern Oncology Group (SWOG), patients with indolent lymphomas were randomized to IFN-α or observation following induction with an intensive anthracycline-containing regimen and in some cases radiotherapy.8 Neither progression-free survival nor OS was affected by postremission therapy. Although results of randomized trials in FL and toxicities associated with IFN-α have led many clinicians to abandon its use altogether, a large Phase III study is currently ongoing in Germany testing standard versus intensive dose maintenance.9 In addition, investigators at the MD Anderson Cancer Center are studying IFN-α in regimens including R and fludarabine.
Rituximab
Clinical trials investigating treatment with rituximab (R) in relapsed or refractory indolent lymphomas have shown overall response rates (ORR) of nearly 50% with median response durations of approximately 1 year. This success in previously treated patients has led to trials studying its use in untreated individuals. In the chemotherapy naïve, ORR are significantly higher (67% vs 46%, P = 0.0097) than in previously treated individuals.10
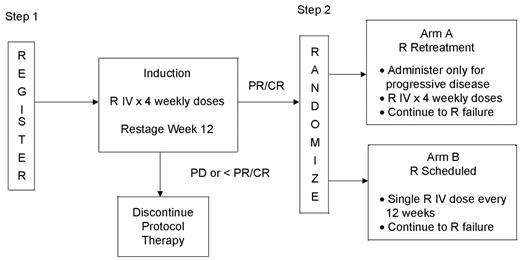
Maintenance R, repeated doses given at designated intervals after completion of 4 weekly treatments, has been studied in Phase III trials. In a trial conducted by the Swiss Group for Clinical Cancer Research (SAAK), the median event-free survival was significantly longer in the maintenance arm than the “no treatment” arm (23 vs 12 months, P = 0.024), with this difference being more pronounced in the previously untreated subset (36 vs 19 months, P = 0.009).10 In a separate randomized trial, previously treated patients with responsive or stable disease following four weekly doses of R were randomized to either receive repeated doses on schedule or at the time of disease progression.11 In this trial, PFS was significantly better in patients receiving maintenance, although the overall duration of benefit from R was the same in both arms. The total number of doses of R administered was also greater in the maintenance arm. In neither study were there differences in OS. A similar trial is ongoing in ECOG in previously untreated asymptomatic patients with low disease burden (Figure 2 ). Patients on the scheduled arm will receive only a single dose every 12 weeks, until progression, while those on the “retreatment” arm will be treated for progressive disease with four weekly doses until they no longer respond to R. Pharmacokinetics will be studied in this trial in an attempt to correlate clinical outcome with serum levels. Differences in maintenance schedules may underlie differences in outcome between trials and these may relate to pharmacokinetics. Studies such as this one will help to determine the optimal approach to administration of R.
For patients who relapse following chemotherapy and then respond to R, retreatment yields responses in fewer than half. Curiously, second remissions may last longer than the first. R acts through multiple mechanisms, and similarly, the basis for R resistance is likely to be multifactorial. For patients refractory to R, treatment with interleukin-2 to enhance antibody-dependent cellular cytotoxicity is being studied clinically. Other approaches to both overcoming resistance and improving the effectiveness of immunotherapy are under investigation. Although the majority of R failures will still respond to RIT (see below), the impact of previous R therapy on results of subsequent treatment with chemoimmunotherapy requires study. “Preemptive” use of R in asymptomatic patients could potentially lead to resistance early in the patient’s clinical course. The rate of transformation among patients treated with R or RIT will need to be monitored. Long-term follow-up of patients will be a crucial part of all clinical trials containing novel therapies.
Chemoimmunotherapy
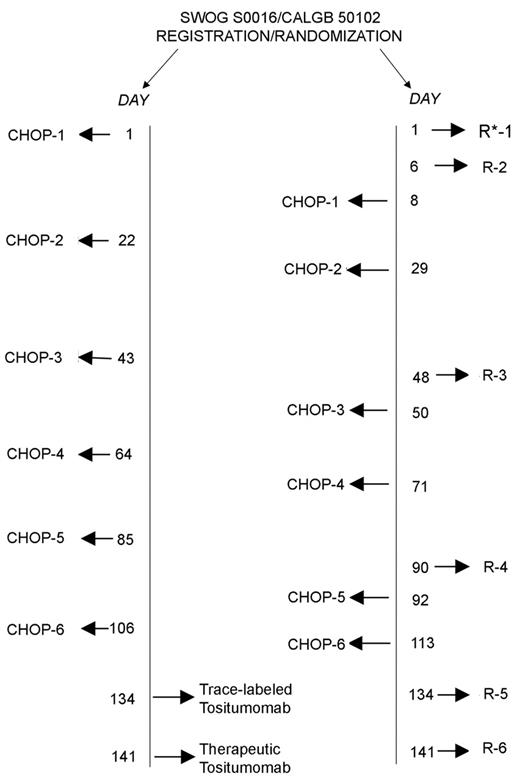
High response rates in multiply relapsed patients have led to the incorporation of R into frontline treatment programs. When combined with CHOP in a mixture of untreated (n = 30) and previously treated (n = 8) patients with indolent lymphomas, response rates were high (ORR 100%, complete response [CR] 58%) and remissions durable.12 The median time to progression was 82 months, and half of the patients continued in remission at the time of the last update. In virtually every randomized trial reported to date, the addition of R to chemotherapy increases time to treatment failure/progression. These include Phase III trials in previously untreated patients comparing R-CHOP to CHOP9 and R-CVP to CVP,13 and in both mantle cell and FL comparing FCM (fludarabine, cyclophosphamide, mitoxantrone) to R-FCM.14 Overall, chemoimmunotherapy appears to be superior to chemotherapy alone, for both chemotherapy-naïve patients and those who have been previously treated. The large US Intergroup trial comparing R-CHOP to CHOP followed by I-131-tositumomab (Bexxar; Corixa, Seattle, WA, and GlaxoSmith Kline, Philadelphia, PA) will hopefully confirm the durability of remissions induced with chemoimmunotherapy (Figure 3 ).
In patients induced with chemotherapy, R has been used as consolidation or maintenance with apparent benefit.7 In a Phase III trial, maintenance R following CVP improved PFS compared to observation (58% vs 34% at 4.5 years). An appropriately designed clinical trial is needed to compare the use of R as part of induction therapy (e.g., in R-CHOP) to R used only as post-remission therapy. New antibody reagents such as anti-CD22 (epratuzumab) and anti-CD80 are being studied individually and in combination with R, and are likely to become the latest members of the armamentarium.
Radioimmunotherapy
Radioimmunoconjugates have the capacity to deliver high doses of irradiation to disease that is widespread, while having only limited effects on normal tissues. The radiolabeled anti-CD20 antibodies, Y-90- ibritumomab tiuxetan (Zevalin; Biogen Idec, Inc., Cambridge, MA) and I-131-tositumomab, both deliver ionizing irradiation to target cells and their neighbors but also retain their capacity for mediating complement-mediated cytotoxicity, antibody-dependent cellular cytotoxicity, and apoptosis.15,16 Both radiopharmaceuticals have proven relatively easy to administer, safe and highly effective. They provide an alternative treatment modality that has the potential for combination with chemotherapy and other immunotherapeutic approaches.
Yttrium-90-ibritumomab tiuxetan, a high-energy β-emitter, has been extensively studied in previously treated patients. In the pivotal trial, the overall response rate for relapsed or refractory follicular or transformed CD20+ B cell non-Hodgkin’s lymphoma (NHL) was 80% (CR 30%) with a median duration of response of 14 months.17 Y-90-ibritumomab tiuxetan is effective in patients with bulk disease and multiple prior therapies, although response rates are higher in patients treated earlier in their disease course. Response rates in patients refractory to R are high (74% ORR), but the median duration of response is relatively short (6.4 months; range 0.5–25+ months). Y-90-ibritumomab tiuxetan is associated with predictable short-lived myelosuppression occurring 7–9 weeks post-therapy. Long-term follow-up of treated patients shows no excess risk of secondary leukemia or myelodysplasia. It is easily dosed on a body weight basis (0.4 mCi/kg) with a dose reduction (0.3 mCi/kg) for those with mild thrombocytopenia (< 100,000/μL). Subsequent treatment with chemotherapy is not precluded by prior RIT.16 Studies are underway to establish the efficacy of programs in which Y-90-ibritumomab tiuxetan is used as first-line therapy or to consolidate first responses achieved with chemotherapy, R, or the combination.
Iodine-131-tositumomab is both a γ- and β-emitter which is individually dosed according to dosimetry to deliver 75 cGy total body irradiation.15,18 Like Y-90-ibritumomab tiuxetan, it has proven effective in heavily pretreated relapsed and refractory patients. In the multicenter pivotal trial, heavily pretreated patients with refractory low-grade or transformed NHL had an ORR of 65% (20% CR) with a median duration of response of 6.5 months.18 This compares favorably to a response rate of only 28% to the patients’ previous chemotherapy regimens and illustrates the impact of alternative treatment strategies in chemoresistant patients. Predictable myelosuppression is also associated with I-131-tositumomab administration. Myelodysplasia and acute leukemia have occurred following RIT, but only in patients who were previously treated with chemotherapy, and thereby already at risk. Among previously untreated patients, the ORR is 95% with a complete response rate of 75%. The 5-year progression-free survival rate has been reported to be 62.3%.19 In a Phase II trial, the percentage of CR/CR unconfirmed increased from 39% following CHOP chemotherapy to 60% following I-131-tositumomab consolidation.20 At 2 years, approximately 80% of treated patients remain disease free. The relative ease of treatment and potential durability of these treatments would argue for their introduction early in the therapy of patients with FL. The US Intergroup is now comparing 6 cycles of CHOP chemotherapy followed by I-131-ibritumomab with R-CHOP in previously untreated patients with indolent lymphoma (Figure 3 ). It remains to be seen whether or not OS can be prolonged through the use of RIT as part of initial therapy. Thus far, it appears that subsequent therapy with chemotherapy and stem cell transplant is possible in patients who fail treatment with RIT (Figure 4 ).
Radioimmunoconjugates targeting cell surface antigens such as CD22 (Y-90-epratuzumab) and HLA-DR 10-β (Lym-1) have also shown promise. Whether cocktails of two or more radioimmunoconjugates can be used simultaneously or in sequence will require careful investigation. New strategies to improve the targeting of radioimmunoconjugates are also being studied.
Vaccines
Lymphoma-specific idiotypes serve as tumor-specific antigens that form the basis for vaccine therapy in FL. In studies conducted at Stanford, immunized patients who mounted an anti-Id response experienced longer remissions than those who failed to generate an immune response to vaccination. In a subsequent trial at the NCI, patients who achieved complete remissions with chemotherapy but had molecular evidence of minimal residual disease had disappearance of residual malignant cells from the peripheral blood after vaccination. Three Phase III trials to evaluate the efficacy of anti-idiotype vaccination in FL are in progress. Cytoreduction is first achieved with either chemotherapy or R, followed by a series of vaccinations. While Phase III trials to validate the concept of vaccination in FL go forward, alternative methodologies, including DNA vaccines and dendritic cell based vaccinations are also being studied.
Novel agents
A long list of targeted therapies including bortezomib and Bcl-2 antisense are under clinical investigation in FL. They are most likely to be used in the future as part of multiagent and possibly multimodality therapy that incorporates other strategies.
Summary
In the past, FL was seen as a disease process that was most often responsive to treatment but ultimately unremitting, for which palliation was the general approach. Some of the new strategies, including RIT and chemoimmunotherapy, are associated with high response rates and durable remissions, but whether any of these therapies has the potential to cure patients or alter OS is unknown. There are many other unanswered questions including how to integrate new information regarding biology and prognosis into treatment planning, when to initiate treatment, how to sequence therapy, and when to transplant. Only well-designed clinical trials can answer these questions. The cooperation of the entire international hematology community will be required for this purpose.
III. Autologous and Allogeneic Stem Cell Transplantation in Follicular Lymphoma
Koen Van Besien, MD*
University of Chicago, Section of Hematology/Oncology, 5841 So. Maryland Ave., Room 1 209, MC 2115, Chicago IL 60637-1470 Acknowledgments: The author thanks Drs. Hillard Lazarus and William Tse for help during the preparation of this manuscript and of a more extensive review published in Bone Marrow Transplantation.1
After initial treatment and first recurrence, the natural history of FL is a continuum of repeated chemosensitive relapses of progressively shorter duration and ultimately death from progressive disease. Conventional chemotherapy has limited or no impact on survival, and the long-term benefit of newer treatments such as monoclonal antibodies, chemo-immunotherapy, or radioimmunotherapy is as yet unknown. High-dose chemotherapy with autologous stem cell transplantation (ASCT) or allogeneic stem cell transplantation (alloSCT) represents an alternative treatment approach. Its advantages include the durability of responses and potential impact on survival, while the major disadvantages are immediate and long-term toxicities.
Autologous Stem Cell Transplantation: Indications and Results
Although ASCT in follicular NHL has been studied most extensively in the treatment of patients with recurrent disease, it also has been used as consolidation of first remission and in the treatment of patients with transformed lymphoma.
ASCT for recurrent disease
Chemotherapy at conventional dose for the treatment of patients with recurrent follicular NHL is likely to produce consecutive remissions of shorter duration each time. Several Phase II studies suggest, however, that salvage treatment followed by consolidation with ASCT can result in prolonged disease-free survival (DFS).1;,2 Freedman and colleagues reported the largest single institution experience. A total of 153 patients were treated with ASCT using autologous bone marrow purged in vitro with anti-B cell monoclonal antibody. At a median follow-up of 5 years (range 2–13 years), the estimated 8-year DFS and OS were estimated as 42% and 66%, respectively.3Table 2 lists other major Phase II trials that showed similar results using different hematopoietic cell sources, purging methods, conditioning regimens, and follow-up periods. All trials suggested an improved median duration of progression-free survival (PFS) compared to historic controls treated with conventional chemotherapy and prolonged PFS in a fraction of patients. On the other hand, with prolonged follow-up, recurrence rates of over 50% were generally observed and questions remain as to whether the durable responses were due to a treatment effect versus patient selection.
The European Bone Marrow Transplantation Registry (EBMTR)–sponsored CUP trial (conventional Chemotherapy, Unpurged autograft, Purged autograft) conducted between 1993 and 1997 is the only prospective randomized trial to address the role of ASCT in prolonging PFS and overall survival in patients with follicular NHL.4 Due to slow accrual, the trial was closed after enrollment of 140 of a planned 250 patients. Sixty-five percent of patients were in first relapse, the remainder in subsequent relapses. Patients were given three initial cycles of chemotherapy (usually a CHOP-like regimen), and 89 patients who attained at least a partial response were randomized to one of three treatment arms: further conventional chemotherapy, ASCT using purged autografts or ASCT using unpurged autografts. Too few patients were entered to assess the effect of ex-vivo purging. PFS and OS at 2 years after transplant for both patient groups randomized to ASCT were 55% and 71% compared to 26% and 46% for those receiving conventional chemotherapy, respectively. Hazard ratios for survival and PFS were 0.3 and 0.4, respectively, when comparing conventional chemotherapy with ASCT. These data are highly significant statistically and demonstrate that ASCT provides an important survival benefit in patients with chemosensitive recurrences of follicular NHL and should currently be considered a treatment of choice in this situation.
ASCT as consolidation of first remission
ASCT has also been used for low-grade NHL patients in first complete or partial remission in order to prolong or render such remissions permanent. Several Phase II trials are summarized in Table 3 . Most of them found very prolonged PFS in a substantial proportion of patients and favorable outcomes compared with historical controls. This led several groups to develop multicenter, randomized studies comparing ASCT consolidation versus IFN maintenance or observation. The German Low Grade Lymphoma Study Group randomized patients younger than 60 years of age with chemosensitive indolent NHL (mostly follicular NHL) in first partial or complete remission to ASCT versus maintenance interferon therapy.5 Two hundred forty patients with FL were evaluable for the comparison of ASCT versus IFN maintenance. At a median follow-up period of 4.2 years, 31 relapses (27.2%) were observed in the ASCT study arm and 76 relapses (60.3%) in patients receiving IFN maintenance. In addition, 5 deaths occurred in remission (4 patients in the ASCT study arm and 1 in the IFN group). Accordingly, the PFS was significantly different in the two study arms. In patients receiving ASCT, the PFS was 79.1% after 2 years (95% confidence interval 71.4% to 86.9%) and 64.7% after 5 years (95% confidence interval 54.6% to 74.8%) in comparison to only 52.7% (95% confidence interval 43.8% to 61.7%) after 2 years and 33.3% (95% confidence interval 24.3% to 42.3%) after 5 years in the IFNα study arm respectively (P < 0.0001).
The French Groupe Ouest Est Leucémies Aiguës Myéloblastiques (GOELAM) group also reported in preliminary fashion that ASCT decreased recurrence rates but did not have any effect on long-term survival.6 This may be due to the fact that patients undergoing ASCT appeared at increased risk for the development of myelodysplastic syndrome (MDS). In the absence of a demonstrated survival benefit, we do not routinely recommend ASCT in first remission for follicular NHL patients.
Technical Considerations
Several technical aspects of ASCT such as method of stem cell collection, ex vivo purging, conditioning regimen, and aspects of supportive care may have a considerable influence on the outcome of transplant and ideally should be investigated in randomized studies. For a variety of reasons these aspects of care generally have not been addressed in this fashion. Definitive answers to questions of the optimal preparative regimen or optimal stem cell sources therefore are not possible. Some recommendations can be provided by comparison of individual study results, case-control studies and perhaps best from multivariable analysis of large observational data sets.
Conditioning
Transplant conditioning regimens for ASCT in follicular NHL can be classified into three variants: total-body irradiation (TBI)–containing; high-dose BCNU-based (BEAC, BEAM, CBV); and busulfan-based (BuCy, BuEtoposide). Largely for historic reasons, TBI–containing or BCNU-based regimens are used most commonly in follicular NHL. An IBMTR analysis indicated that use of TBI might be associated with an increased treatment-related mortality (TRM).7 On the other hand, TBI in the preparative regimen was also associated with decreased rates of disease recurrence. This observation, while far from definitive, suggests that TBI is a particularly effective but rather toxic treatment for FL and provides a rationale for the further development of radiolabeled monoclonal antibodies as part of conditioning regimens for transplant. A Phase II study of I131-labeled anti-CD20 (tositumomab) combined with high-dose chemotherapy suggests an improved overall survival compared with historical controls receiving TBI.8
Purging
Most patients with follicular NHL present in advanced disease stage and often with morphologic evidence of marrow involvement. Even in patients with histologically normal marrow, occult marrow involvement usually can be demonstrated by PCR. Further, occult lymphoma cells contaminating the stem cell infusate likely contribute to relapse after autologous transplantation. Ex vivo technologies to decrease tumor contamination include ‘positive’ selection (i.e., the in vitro enrichment of the graft for CD34 cells, a marker presumably lacking on lymphoma cells) as well as ‘negative’ selection (i.e., the removal of tumor cells by exposure to lymphoma-specific antibodies).
The group from the Dana Farber Cancer Institute found that successful in vitro purging was associated with dramatically improved freedom from relapse in follicular NHL patients undergoing ASCT.3 Furthermore, Ladetto et al showed that successful in vivo purging (i.e., the generation of PCR-negative harvests after intensive induction chemotherapy) was associated with improved PFS.9 Fouillard et al, by using various purging methods, also found a correlation between graft purging and outcome.10 Only one group failed to find a correlation between PCR status of the infusate and recurrence.11 The obvious corollary of these findings is that occult lymphoma cells contribute to recurrence in tumor-contaminated grafts. However, the absence of a control group makes it impossible to rule out the alternative explanation, namely that the ability to achieve a tumor-free graft is a surrogate for chemotherapy sensitivity. The CUP trial attempted to address this issue in a prospective fashion, but low patient numbers limited the study’s power. Indirect evidence of the role of a tumor-free graft comes from registry analysis. In an IBMTR study of follicular NHL, stem cell purging was identified as an independent predictor for PFS and OS.7 Also a case-control study by Bierman et al showed that syngeneic transplants had a lower recurrence rate than purged autologous transplant, which in turn had a lower recurrence rate than unpurged autologous transplant.12 We interpret these data as indicative of the importance of providing a tumor-free graft. Current technology limits the efficacy of graft purging and further research in this area is indicated. Recently, rituximab has been used as an “in vivo purging agent.”13 Pilot studies indicate that rituximab administration eliminates PCR-detectable cells in a significant proportion of hematopoietic cell harvests but may be associated with late neutropenia and serious infection.14 Whether transplant outcome will be improved by collecting PBSC after exposure to rituximab remains to be demonstrated.
Late toxicities of ASCT
Although early morbidity is considerable, advances in supportive care have significantly reduced the immediate TRM associated with ASCT, even in elderly patients. On the other hand, late treatment-related side effects, such as MDS or secondary acute myeloid leukemia (sAML) occur with a 5- to 15-fold increased incidence.15,16 The etiology of posttransplant MDS is complex and relates to a combination of host-related factors, prior treatment, and conditioning. The outcome for patients who develop treatment related MDS/AML usually is very poor.
Allogeneic Stem Cell Transplantation
Myeloablative transplantation
The exact role of allogeneic stem cell transplant (alloSCT) in follicular NHL remains somewhat difficult to define (Table 4 ). Allotransplant initially was used in patients thought not to be candidates for ASCT due to extent of disease or marrow involvement. Several retrospective studies suggest that alloSCT is associated with a very low relapse rate and might be a curative treatment for FL. On the other hand, the benefit of alloSCT was offset by high TRM. The IBMTR reported data on 904 follicular NHL patients who underwent either ASCT or alloSCT between 1990 and 1999.7 A total of 176 (19%) received alloSCT, 131 (14%) received purged ASCT, and 597 (67%) received unpurged ASCT. The 5-year TRM rates were 30%, 14%, and 8% and recurrence rates were 21%, 43%, and 58% after alloSCT, purged ASCT, and unpurged ASCT, respectively. Furthermore, the 5-year probabilities of survival were 51%, 62%, and 55% after alloSCT, purged ASCT, and unpurged ASCT. This communication confirms the high TRM yet low recurrence rate with alloSCT and overall similar long-term survival compared to patients undergoing ASCT. These data do not allow practitioners to make decisions for the use of alloSCT versus ASCT, and treatment recommendations continue to be guided by physician judgment. Over the past decade, advances in supportive care and better patient selection have resulted in improved outcomes for alloSCT. Also, the risk of secondary MDS after alloSCT is negligible. We currently recommend alloSCT for recurrent NHL patients under age 50 years who have an HLA-identical sibling. Use of a TBI-based conditioning may be preferred due to the decreased recurrence rate associated with that modality.
Reduced-intensity transplantation
Reduced-intensity conditioning regimens, considered less toxic, are being used increasingly often (Table 5 ). The underlying hypothesis of such a strategy is that fewer patients will develop TRM and graft-versus-leukemia (GVL) effects will be effective in curing NHL. Interesting preliminary results indicate that this premise may be the case,17 and the potency of GVL effects is underscored by the sometimes spectacular responses to donor lymphocyte infusion.18 Still, the considerable risks and toxicity associated with chronic GVHD cannot be underestimated.19 Paradoxically some of the best reported results involve in-vivo or in-vitro T cell–depleted transplants.20 Also, there is a certain arbitrariness to the definition of “reduced intensity” and a plethora of conditioning variants that renders interpretation of data difficult. The value of reduced-intensity alloSCT hopefully will be answered by a recently activated trial conducted by the Blood and Marrow Transplant Clinical Trials Network comparing ASCT versus alloSCT in follicular NHL patients.
Future Directions
The limitations of ASCT transplantation include a high incidence of disease recurrence and risk of secondary MDS. While it can be considered the standard of care for patients with recurrent FL, it constitutes a very mediocre standard. Future improvements likely will focus on incorporation of targeted monoclonal antibodies into the preparative regimen and posttransplant period, novel stem cell purging strategies, and administration of additional treatments in the posttransplant period. Studies in alloSCT will focus on minimizing treatment-related complications that likely will include newer GVHD prevention approaches, use of new graft-versus-lymphoma maneuvers, and incorporation of more advanced techniques of donor lymphocyte infusion.
World Health Organization classification of follicular lymphoma (FL).
| Follicular Lymphoma: Grading & Variants . |
|---|
| Abbreviations: HPF, high power field |
| Grade 1, 0–5 centroblasts/HPF |
| Grade 2, 6–15 centroblasts/HPF |
| Grade 3, > 15 centroblasts/HPF |
| 3a, > 15 centroblasts, but centrocytes are still present |
| 3b, centroblasts form solid sheets with no residual centrocytes |
| Variants: |
| Cutaneous follicle center lymphoma |
| Diffuse follicle center lymphoma |
| Grade 1, 0–5 centroblasts/HPF |
| Grade 2, 6–15 centroblasts/HPF |
| Follicular Lymphoma: Grading & Variants . |
|---|
| Abbreviations: HPF, high power field |
| Grade 1, 0–5 centroblasts/HPF |
| Grade 2, 6–15 centroblasts/HPF |
| Grade 3, > 15 centroblasts/HPF |
| 3a, > 15 centroblasts, but centrocytes are still present |
| 3b, centroblasts form solid sheets with no residual centrocytes |
| Variants: |
| Cutaneous follicle center lymphoma |
| Diffuse follicle center lymphoma |
| Grade 1, 0–5 centroblasts/HPF |
| Grade 2, 6–15 centroblasts/HPF |
Autologous transplantation performed in relapsed disease or partial remission (based on Reference1).
| Author (No. Pts.) . | Conditioning . | In Vitro Purging . | Stem Cell Source . | Median Follow Up (mo.) . | PFS . | OS . | Early TRM* . | Comments . |
|---|---|---|---|---|---|---|---|---|
| *Early treatment-related mortality (TRM), usually defined as occurring within 100 days after ASCT. | ||||||||
| Abbreviations: PFS, progression-free survival; OS, overall survival; MoAB, monoclonal antibody; VP, etoposide; BM, bone marrow; PB, peripheral blood; NS, not stated; BEAM, BCNU/etoposide/cytarabine/melphalan; BEAC, BCNU/etoposide/cytarabine/cyclophosphamide; Bu/Cy, busulfan/cyclophosphamide; MDS, myelodysplastic syndrome; NHL, non-Hodgkin’s lymphoma; XPB; C′, complement; ASCT, autologous stem cell transplantation. | ||||||||
| Colombat (42) | Cy/TBI or BEAM | purging in 40% BM pts | BM 88%, PB 12% | 43 | 58% @ 43 mo | 83% @ 43 mo | ||
| Freedman (153) | Cy/TBI | Anti B MoAB + C′ | BM | 96 | 42% @ 8 yr | 66% @ 8 yr | <1% | 18 secondary malignancies (12 MDS) |
| Bierman (100) | Cy/TBI or BEAM | none | BM 13%, PB 87% | 31 | 44% @ 48 mo | 65% @ 48 mo | 8% | 2 secondary MDS |
| Apostolidis (99) | Cy/TBI | Anti B MoAB + C′ | BM | 66 | 63% @ 66 mo | 69% @ 66 mo | 4% | 12 secondary hematologic malignancies |
| Voso (41) | Cy/TBI 80% BEAM 20% | CD34+ selection in some pts | PB | 44 | 43% @ 44 mo | 72% @ 44 mo | 4% | 5% secondary malignancies |
| Bastion (48) | Cy/TBI | none | PB | 21 | 53% @ 21 mo | 86% @ 21 mo | 1 secondary MDS | |
| Weaver (49) | Bu/Cy or | none BEAC | PB | 42 | 35% @ 42 mo | 55% @ 42 mo | 8% | BuCy equivalent to BEAC |
| Berglund (22) | BEAC +/−TBI | MoAB in 9 pts. | BM | 74 | 72% @ 74 mo | 81% @ 74 mo | 50% transformed NHL | |
| Brice (83) | TBI 71%, BEAM 29% | CD34 22% | PB 73%, BM 27% | 44 | 42% @ 60 mo | 58% @ 60 mo | 29% transformed NHL | |
| Verdonck (18) | Cy/TBI | none | BM | 36 | 22% @ 24 mo | 33% @ 36 mo | ||
| Cervantes (34) | Cy/TBI 91%, | Anti B MoAB + C’ BEAM 9% | BM 70%, PB 27%, both 3% | 40 | 18% @ 24 mo | 37% @ 60 mo | 4% | |
| Cao (49) | Cy/TBI/VP 78% Cy/BCNU/VP 22% | Anti B MoAB | BM 39% PB 61% | 66 | 44% @ 48 mo | 60% @ 48 mo | ||
| Van Besien (597) | TBI 31% Non-TBI 69% | None | BM 15% PB 85% | 41 | 31% | 55% | 4% | TRM increases to 8% @ 5 yr |
| Van Besien (131) | TBI 34% Non-TBI 66% | MoAB 11% CD34+ selection 21% in vitro chemotherapy 68% | BM 76% PB 24% | 49 | 39% | 62% | 8% | TRM increases to 14% @ 5 yr |
| Schouten (65) | Cy/TBI | Anti B MoAB 50% | BM | 69 | 52% | 72% | Randomized study | |
| Molina (58) | Cy/VP and either BCNU or TBI | none | PB | 62 | 42% @ 60 mo | 67% @ 60 mo | 2% | 2 secondary MDS |
| Seyfarth (22) | Cy/TBI 38% BEAM or Bu/Cy 62% | CD34+ selection in 95% | PB | 48 | 38% @ 48 mo | 73% @ 48 mo | ||
| Stein (36) | Cy/VP/TBI 75% CBV 25% | none | BM 93% PB 7% | NS | NS | 53% | 15% | 1 secondary MDS |
| Author (No. Pts.) . | Conditioning . | In Vitro Purging . | Stem Cell Source . | Median Follow Up (mo.) . | PFS . | OS . | Early TRM* . | Comments . |
|---|---|---|---|---|---|---|---|---|
| *Early treatment-related mortality (TRM), usually defined as occurring within 100 days after ASCT. | ||||||||
| Abbreviations: PFS, progression-free survival; OS, overall survival; MoAB, monoclonal antibody; VP, etoposide; BM, bone marrow; PB, peripheral blood; NS, not stated; BEAM, BCNU/etoposide/cytarabine/melphalan; BEAC, BCNU/etoposide/cytarabine/cyclophosphamide; Bu/Cy, busulfan/cyclophosphamide; MDS, myelodysplastic syndrome; NHL, non-Hodgkin’s lymphoma; XPB; C′, complement; ASCT, autologous stem cell transplantation. | ||||||||
| Colombat (42) | Cy/TBI or BEAM | purging in 40% BM pts | BM 88%, PB 12% | 43 | 58% @ 43 mo | 83% @ 43 mo | ||
| Freedman (153) | Cy/TBI | Anti B MoAB + C′ | BM | 96 | 42% @ 8 yr | 66% @ 8 yr | <1% | 18 secondary malignancies (12 MDS) |
| Bierman (100) | Cy/TBI or BEAM | none | BM 13%, PB 87% | 31 | 44% @ 48 mo | 65% @ 48 mo | 8% | 2 secondary MDS |
| Apostolidis (99) | Cy/TBI | Anti B MoAB + C′ | BM | 66 | 63% @ 66 mo | 69% @ 66 mo | 4% | 12 secondary hematologic malignancies |
| Voso (41) | Cy/TBI 80% BEAM 20% | CD34+ selection in some pts | PB | 44 | 43% @ 44 mo | 72% @ 44 mo | 4% | 5% secondary malignancies |
| Bastion (48) | Cy/TBI | none | PB | 21 | 53% @ 21 mo | 86% @ 21 mo | 1 secondary MDS | |
| Weaver (49) | Bu/Cy or | none BEAC | PB | 42 | 35% @ 42 mo | 55% @ 42 mo | 8% | BuCy equivalent to BEAC |
| Berglund (22) | BEAC +/−TBI | MoAB in 9 pts. | BM | 74 | 72% @ 74 mo | 81% @ 74 mo | 50% transformed NHL | |
| Brice (83) | TBI 71%, BEAM 29% | CD34 22% | PB 73%, BM 27% | 44 | 42% @ 60 mo | 58% @ 60 mo | 29% transformed NHL | |
| Verdonck (18) | Cy/TBI | none | BM | 36 | 22% @ 24 mo | 33% @ 36 mo | ||
| Cervantes (34) | Cy/TBI 91%, | Anti B MoAB + C’ BEAM 9% | BM 70%, PB 27%, both 3% | 40 | 18% @ 24 mo | 37% @ 60 mo | 4% | |
| Cao (49) | Cy/TBI/VP 78% Cy/BCNU/VP 22% | Anti B MoAB | BM 39% PB 61% | 66 | 44% @ 48 mo | 60% @ 48 mo | ||
| Van Besien (597) | TBI 31% Non-TBI 69% | None | BM 15% PB 85% | 41 | 31% | 55% | 4% | TRM increases to 8% @ 5 yr |
| Van Besien (131) | TBI 34% Non-TBI 66% | MoAB 11% CD34+ selection 21% in vitro chemotherapy 68% | BM 76% PB 24% | 49 | 39% | 62% | 8% | TRM increases to 14% @ 5 yr |
| Schouten (65) | Cy/TBI | Anti B MoAB 50% | BM | 69 | 52% | 72% | Randomized study | |
| Molina (58) | Cy/VP and either BCNU or TBI | none | PB | 62 | 42% @ 60 mo | 67% @ 60 mo | 2% | 2 secondary MDS |
| Seyfarth (22) | Cy/TBI 38% BEAM or Bu/Cy 62% | CD34+ selection in 95% | PB | 48 | 38% @ 48 mo | 73% @ 48 mo | ||
| Stein (36) | Cy/VP/TBI 75% CBV 25% | none | BM 93% PB 7% | NS | NS | 53% | 15% | 1 secondary MDS |
Autologous transplantation performed in first complete remission (based on reference1).
| Author (No. Pts.) . | Conditioning . | In vitro Purging . | Stem Cell Source . | Median Follow Up (mo.) . | PFS . | OS . | Early TRM* . | Comments . |
|---|---|---|---|---|---|---|---|---|
| *Early TRM, treatment-related mortality usually defined as occurring within 100 days after autologous stem cell transplantation (ASCT). | ||||||||
| Abbreviations: PFS, progression-free survival; OS, overall survival; MoAB, monoclonal antibody; VP, etoposide; BM, bone marrow; PB, peripheral blood; Cy, cyclophosphamide; TBI, total body irradiation: BEAM, BCNU/etoposide/cytarabine/melphalan; FL, follicular lymphoma; MDS, myelodysplastic syndrome; PCR, polymerase chain reaction; C′, complement | ||||||||
| Freedman (77) | Cy/TBI | Anti B MoABb + C′ | BM | 45mo | 63% @ 3 yr | 89% @ 3 yr | 3% | 5 secondary hematologic malignancies. |
| Horning (37) | Cy/TBI/VP | Anti B MoAB + C′ | BM | 78mo | 60% @ 10 yr | 86% @ 10 yr | 5% | 2 secondary hematologic malignancies. |
| Corradini (40) | Mitoxantrone/ Melphalan | none | PB | 90mo | 57% @ 12 yr | 60% @ 12 yr | 1% | 11 pts had transformed FL. 3 secondary hematologic malignancies. |
| Voso (70) | Cy/TBI | CD34+ selection (10%); None (90%) | PB | 44mo | 78% @ 44 mo | 86% @ @ 44 mo | 5% | |
| Lenz (140) | TBI/Cy | none | PB | 53mo | 65% @ 60 mo | NS | 1% | Randomized trial; 6.6% secondary MDS in transplant group. |
| Colombat (27) | Cy/TBI | CD34+ selection or Anti B MoAB + C′ | PB | 72 mo | 55% @ 72 mo | 64% @ 72 mo | Randomized trial. MDS increased in transplant group. | |
| Seyfarth (33) | Cy/TBI 79% BEAM 21% | none | PB | 48 mo | 76% @ 48 mo | 92% @ 48 mo | ||
| Bociek (43) | not stated | none | PB | 36 mo | 36% @ 60 mo | 63% @ 60 mo | ||
| González- Barca (15) | Cy/TBI | immunomagnetic purging | BM 9 PB 6 | 56 mo | 83% @ 56 mo | NS | 13% | 6/8 BM PCR negative for BCL-2. |
| Linassier (42) | TBI 48% non-TBI 52% | Immunomagnetic purging 36% | BM 88% PB 12% | 43mo | 66% @ 43 mo | 83% @ 43 mo | No prognostic factors identified. | |
| Author (No. Pts.) . | Conditioning . | In vitro Purging . | Stem Cell Source . | Median Follow Up (mo.) . | PFS . | OS . | Early TRM* . | Comments . |
|---|---|---|---|---|---|---|---|---|
| *Early TRM, treatment-related mortality usually defined as occurring within 100 days after autologous stem cell transplantation (ASCT). | ||||||||
| Abbreviations: PFS, progression-free survival; OS, overall survival; MoAB, monoclonal antibody; VP, etoposide; BM, bone marrow; PB, peripheral blood; Cy, cyclophosphamide; TBI, total body irradiation: BEAM, BCNU/etoposide/cytarabine/melphalan; FL, follicular lymphoma; MDS, myelodysplastic syndrome; PCR, polymerase chain reaction; C′, complement | ||||||||
| Freedman (77) | Cy/TBI | Anti B MoABb + C′ | BM | 45mo | 63% @ 3 yr | 89% @ 3 yr | 3% | 5 secondary hematologic malignancies. |
| Horning (37) | Cy/TBI/VP | Anti B MoAB + C′ | BM | 78mo | 60% @ 10 yr | 86% @ 10 yr | 5% | 2 secondary hematologic malignancies. |
| Corradini (40) | Mitoxantrone/ Melphalan | none | PB | 90mo | 57% @ 12 yr | 60% @ 12 yr | 1% | 11 pts had transformed FL. 3 secondary hematologic malignancies. |
| Voso (70) | Cy/TBI | CD34+ selection (10%); None (90%) | PB | 44mo | 78% @ 44 mo | 86% @ @ 44 mo | 5% | |
| Lenz (140) | TBI/Cy | none | PB | 53mo | 65% @ 60 mo | NS | 1% | Randomized trial; 6.6% secondary MDS in transplant group. |
| Colombat (27) | Cy/TBI | CD34+ selection or Anti B MoAB + C′ | PB | 72 mo | 55% @ 72 mo | 64% @ 72 mo | Randomized trial. MDS increased in transplant group. | |
| Seyfarth (33) | Cy/TBI 79% BEAM 21% | none | PB | 48 mo | 76% @ 48 mo | 92% @ 48 mo | ||
| Bociek (43) | not stated | none | PB | 36 mo | 36% @ 60 mo | 63% @ 60 mo | ||
| González- Barca (15) | Cy/TBI | immunomagnetic purging | BM 9 PB 6 | 56 mo | 83% @ 56 mo | NS | 13% | 6/8 BM PCR negative for BCL-2. |
| Linassier (42) | TBI 48% non-TBI 52% | Immunomagnetic purging 36% | BM 88% PB 12% | 43mo | 66% @ 43 mo | 83% @ 43 mo | No prognostic factors identified. | |
Selected studies using myeloablative conditioning and allogeneic transplantation for follicular lymphoma (based on reference1).
| Author (No. Pt.) . | Conditioning . | Stem Cell Source . | Median Follow-up (mo) . | PFS . | OS . | TRM* . | Relapse . | Comments . |
|---|---|---|---|---|---|---|---|---|
| * treatment-related mortality occurring in the first year after transplant | ||||||||
| Abbreviations: PFS, progression-free survival; OS, overall survival; VP, etoposide; BM, bone marrow; PB, peripheral blood; CR, complete remission; DLI, donor lymphocyte infusion; Bu/Cy±MEL, busulfan/cyclophosphamide with/without melphalan; NS, not stated specifically; DexaBEAM, dexamethasone/BCNU/etoposide/cytarabine/melphalan; TRM, treatment-related mortality | ||||||||
| Van Besien (113) | TBI 84% Non-TBI 16% | BM | 25 | 49 @ 36mo | 49 @ 36mo | 40% @ 36mo | 16% | Transplants between 1984–1995. One third with small lymphocytic lymphoma |
| Peniket (231) | varied regimens | NS | 60 | 43% @ 48mo | 51% @ 48mo | 38% @ 48mo | 25% | Case-matched study comparing allo with auto. Allo has higher TRM but low relapses |
| Van Besien (176) | TBI 68% Non-TBI 32% | BM 77% PB 23% | 36 | 45% @ 60mo | 51% @ 60mo | 24% @ 12mo | Transplants between 1990–1999 | |
| Mandigers (15) | Cy/TBI | BM | 36 | 67% | 33% | 13% | T cell depleted transplant | |
| Stein (15) | Cy/TBI 93% | BM 93% PB 7% | 60 | NS | 15% | 53% | 33% | |
| Verdonck (15) | Cy/TBI | 25 | 70% | 70% | 27% | 0 | T cell depletion | |
| Toze (16) | NS | BM 100% | 29 | 56% | 25% | 0 | 4 unrelated donors and 12 sibling donors | |
| Forrest (24) | Bu/Cy±MEL 92% | BM 16 PB 8 | 28 | 78% @ 28 mo | 78% @ 28 mo | 21% | 0 | No relapses noted |
| Author (No. Pt.) . | Conditioning . | Stem Cell Source . | Median Follow-up (mo) . | PFS . | OS . | TRM* . | Relapse . | Comments . |
|---|---|---|---|---|---|---|---|---|
| * treatment-related mortality occurring in the first year after transplant | ||||||||
| Abbreviations: PFS, progression-free survival; OS, overall survival; VP, etoposide; BM, bone marrow; PB, peripheral blood; CR, complete remission; DLI, donor lymphocyte infusion; Bu/Cy±MEL, busulfan/cyclophosphamide with/without melphalan; NS, not stated specifically; DexaBEAM, dexamethasone/BCNU/etoposide/cytarabine/melphalan; TRM, treatment-related mortality | ||||||||
| Van Besien (113) | TBI 84% Non-TBI 16% | BM | 25 | 49 @ 36mo | 49 @ 36mo | 40% @ 36mo | 16% | Transplants between 1984–1995. One third with small lymphocytic lymphoma |
| Peniket (231) | varied regimens | NS | 60 | 43% @ 48mo | 51% @ 48mo | 38% @ 48mo | 25% | Case-matched study comparing allo with auto. Allo has higher TRM but low relapses |
| Van Besien (176) | TBI 68% Non-TBI 32% | BM 77% PB 23% | 36 | 45% @ 60mo | 51% @ 60mo | 24% @ 12mo | Transplants between 1990–1999 | |
| Mandigers (15) | Cy/TBI | BM | 36 | 67% | 33% | 13% | T cell depleted transplant | |
| Stein (15) | Cy/TBI 93% | BM 93% PB 7% | 60 | NS | 15% | 53% | 33% | |
| Verdonck (15) | Cy/TBI | 25 | 70% | 70% | 27% | 0 | T cell depletion | |
| Toze (16) | NS | BM 100% | 29 | 56% | 25% | 0 | 4 unrelated donors and 12 sibling donors | |
| Forrest (24) | Bu/Cy±MEL 92% | BM 16 PB 8 | 28 | 78% @ 28 mo | 78% @ 28 mo | 21% | 0 | No relapses noted |
Studies using reduced intensity conditioning and allogeneic transplant for follicular lymphoma (based on reference1).
| Author (No. Pts.) . | Conditioning . | Stem Cell Source . | Median Follow-up (mo) . | PFS . | OS . | * TRM . | Comments . |
|---|---|---|---|---|---|---|---|
| * treatment-related mortality occurring in the first year after transplant | |||||||
| **Personal communication MB Maris, Fred Hutchinson Cancer Research Center | |||||||
| Abbreviations: PFS, progression-free survival; OS, overall survival; PB, peripheral blood; Flud, fludarabine; BEAM, BCNU/etoposide/cytarabine/melphalan; GVHD, graft-versus-host disease; TRM, treatment-related mortality | |||||||
| Seattle** (9) | Flud/TBI 200 | PB | >12 | 7 | 8 | 0 | |
| Khouri (20) | Flud/CY | PB | 21 | 80% | 80% | 20% | 64% cumulative incidence chronic GVHD |
| Faulkner (28) | BEAM Campath | PB | 16 | 69% | 74% | 16% | No extensive chronic GVHD |
| Robinson (28) | Flud/Alkylator (92%) BEAM Campath 8% | PB | NS | 29% | 39% | 39% | Higher than expected TRM and relapse |
| Author (No. Pts.) . | Conditioning . | Stem Cell Source . | Median Follow-up (mo) . | PFS . | OS . | * TRM . | Comments . |
|---|---|---|---|---|---|---|---|
| * treatment-related mortality occurring in the first year after transplant | |||||||
| **Personal communication MB Maris, Fred Hutchinson Cancer Research Center | |||||||
| Abbreviations: PFS, progression-free survival; OS, overall survival; PB, peripheral blood; Flud, fludarabine; BEAM, BCNU/etoposide/cytarabine/melphalan; GVHD, graft-versus-host disease; TRM, treatment-related mortality | |||||||
| Seattle** (9) | Flud/TBI 200 | PB | >12 | 7 | 8 | 0 | |
| Khouri (20) | Flud/CY | PB | 21 | 80% | 80% | 20% | 64% cumulative incidence chronic GVHD |
| Faulkner (28) | BEAM Campath | PB | 16 | 69% | 74% | 16% | No extensive chronic GVHD |
| Robinson (28) | Flud/Alkylator (92%) BEAM Campath 8% | PB | NS | 29% | 39% | 39% | Higher than expected TRM and relapse |
Proposed model for follicular lymphogenesis and diffuse large B cell lymphoma (DLBCL) transformation.
The t(14;18) is a rare event occurring in naïve B cells and likely at a time when the RAG complex is active (primary VDJ recombination). These cells are relatively immortalized resulting from overexpression of the Bcl-2 protein and likely seed lymph nodes and reside within follicles. Without significant clonal evolution and independence, these follicular lympoma (FL) cells may require a microenvironment complete with follicular dendritic cells and reactive T cells. Repeated cycles of proliferation occur (despite the expression of Bcl-2), but at a low rate as is characteristic of follicular lymphoma (FL). With cell divisions the clone expands and secondary cytogenetic alterations occur. If critical cytogenetic events occur early in the evolution of this process, the cells may lose the need for a germinal center (GC) microenvironment and patients may present as de novo DLBCL. Alternatively, patients present with FL characterized by a diverse spectrum of cytogenetic alterations, the majority of which are copy number alterations (chromosomal gains and losses). The presence of FDCs and T cells within the follicle recapitulates the normal secondary follicle, but their presence in FL may be as immune response cells or alternatively, reflect the clonal evolution of the malignant B cells.
Abbreviations: FDC, follicular dendritic cell; GC, germinal center; MZ, mantle zone
Proposed model for follicular lymphogenesis and diffuse large B cell lymphoma (DLBCL) transformation.
The t(14;18) is a rare event occurring in naïve B cells and likely at a time when the RAG complex is active (primary VDJ recombination). These cells are relatively immortalized resulting from overexpression of the Bcl-2 protein and likely seed lymph nodes and reside within follicles. Without significant clonal evolution and independence, these follicular lympoma (FL) cells may require a microenvironment complete with follicular dendritic cells and reactive T cells. Repeated cycles of proliferation occur (despite the expression of Bcl-2), but at a low rate as is characteristic of follicular lymphoma (FL). With cell divisions the clone expands and secondary cytogenetic alterations occur. If critical cytogenetic events occur early in the evolution of this process, the cells may lose the need for a germinal center (GC) microenvironment and patients may present as de novo DLBCL. Alternatively, patients present with FL characterized by a diverse spectrum of cytogenetic alterations, the majority of which are copy number alterations (chromosomal gains and losses). The presence of FDCs and T cells within the follicle recapitulates the normal secondary follicle, but their presence in FL may be as immune response cells or alternatively, reflect the clonal evolution of the malignant B cells.
Abbreviations: FDC, follicular dendritic cell; GC, germinal center; MZ, mantle zone
Schema for E4402/CTSU E4402, “Randomized Phase III Trial Comparing Two Different Rituximab Dosing Regimens for Patients with Low Tumor Burden Indolent Non-Hodgkin’s Lymphoma.”
Abbreviations: R, rituximab; CR, complete response; PR, partial response; PD, progressive disease
Schema for E4402/CTSU E4402, “Randomized Phase III Trial Comparing Two Different Rituximab Dosing Regimens for Patients with Low Tumor Burden Indolent Non-Hodgkin’s Lymphoma.”
Abbreviations: R, rituximab; CR, complete response; PR, partial response; PD, progressive disease
Schema for Phase III randomized study of cyclophosphamide, doxorubicin, vincristine and prednisone (CHOP) with either rituximab or iodine I-131 tositumomab (monoclonal antibody anti-B1) in patients with newly diagnosed follicular non-Hodgkin’s lymphoma.
* Rituximab 375 mg/m2
Schema for Phase III randomized study of cyclophosphamide, doxorubicin, vincristine and prednisone (CHOP) with either rituximab or iodine I-131 tositumomab (monoclonal antibody anti-B1) in patients with newly diagnosed follicular non-Hodgkin’s lymphoma.
* Rituximab 375 mg/m2
How I treat folicular lymphoma (Grades 1 and 2). (A clinical trial is always the preferred approach!)
*Symptoms, cytopenias, rapid growth, of disease, potential organ compromise (e.g., hydronephrosis)
†Consider collection of peripheral blood progenitor cells for future transplant
How I treat folicular lymphoma (Grades 1 and 2). (A clinical trial is always the preferred approach!)
*Symptoms, cytopenias, rapid growth, of disease, potential organ compromise (e.g., hydronephrosis)
†Consider collection of peripheral blood progenitor cells for future transplant