Abstract
Through the hard work of a large number of investigators, the biology of acute myeloid leukemia (AML) is becoming increasingly well understood, and as a consequence, new therapeutic targets have been identified and new model systems have been developed for testing novel therapies. How these new therapies can be most effectively studied in the clinic and whether they will ultimately improve cure rates are questions of enormous importance. In this article, Dr. Jacob Rowe presents a summary of the current state-of-the-art therapy for adult AML. His contribution emphasizes the fact that AML is not a single disease, but a number of related diseases each distinguished by unique cytogenetic markers which in turn help determine the most appropriate treatment. Dr. Jerald Radich continues on this theme, emphasizing how these cytogenetic abnormalities, as well as other mutations, give rise to abnormal signal transduction and how these abnormal pathways may represent ideal targets for the development of new therapeutics. A third contribution by Dr. Frederick Appelbaum describes how AML might be made the target of immunologic attack. Specifically, strategies using antibody-based or cell-based immunotherapies are described including the use of unmodified antibodies, drug conjugates, radioimmunoconjugates, non-ablative allogeneic transplantation, T cell adoptive immunotherapy and AML vaccines. Finally, Dr. John Dick provides a review of the development of the NOD/SCID mouse model of human AML emphasizing both what it has taught us about the biology of the disease as well as how it can be used to test new therapies. Taken together, these reviews are meant to help us understand more about where we are in the treatment of AML, where we can go and how we might get there.
I. Current Standard Therapy of Adult Acute Myeloid Leukemia
Jacob M. Rowe, MD*
Department of Hermatology and Bone Marrow Transplant, Rambam Medical Center, Haifa, 31096, Israel
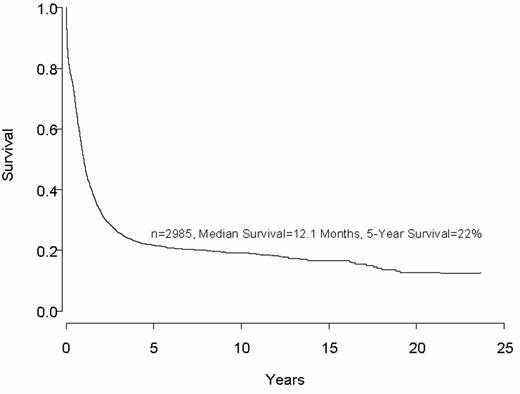
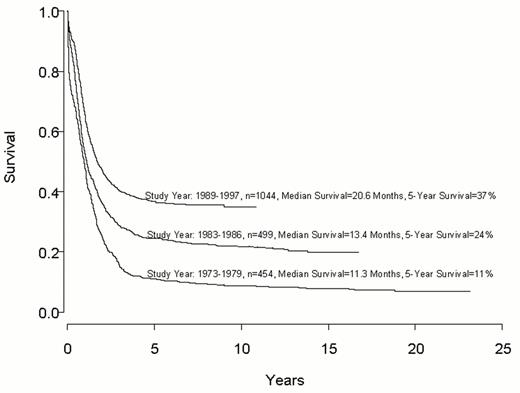
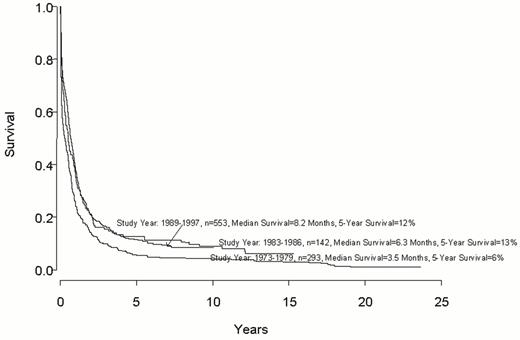
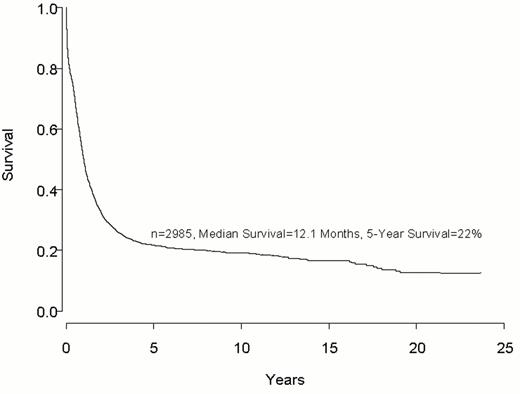
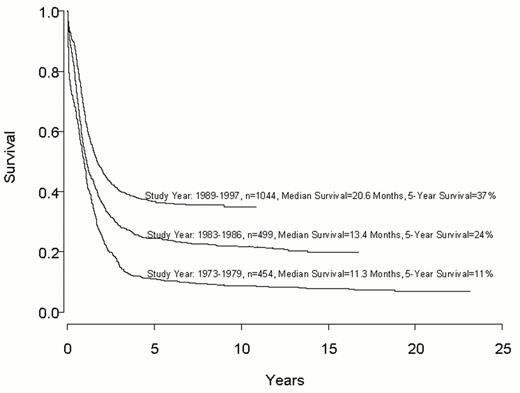
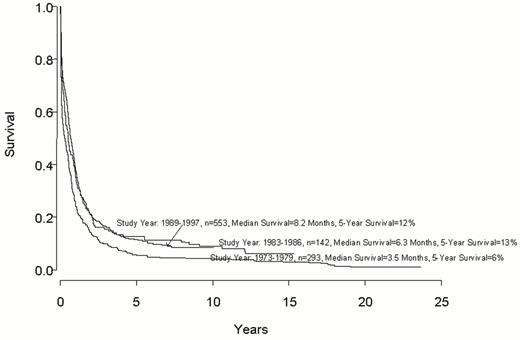
Despite important advances in the therapy of acute myeloid leukemia (AML) the majority of patients will die from their disease (Figure 1 ). Progress in therapy and supportive care over the past three decades has led to gradual improvement in the overall results, especially in adults up to age 55-60 years (Figure 2 ). However, very little progress has been made in the long-term survival of older adults with AML (Figure 3 ). Since the median age of patients with AML is 64 years, this older group of patients represents the majority with this disease, and the outcome of therapy remains frustratingly disappointing. This section will review current strategies for induction and post-remission therapy focusing on newly diagnosed younger adults. Possible strategies for older adults will then be discussed briefly.
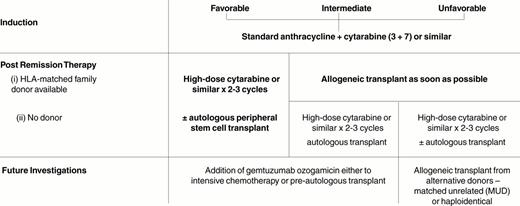
It is no longer appropriate to consider all subgroups of AML as a single entity. The most important prognostic factors determining the outcome of therapy are the acquired genetic changes in the leukemic cells; determined by conventional cytogenetic techniques, fluorescence in situ hybridization (FISH) analysis or polymerase chain reaction (PCR). Thus, the following discussion will focus on acute promyelocytic leukemia (APL) and all other AMLs divided into three recognized prognostic groups: those exhibiting favorable, intermediate or unfavorable cytogenetics at presentation (Table 1 ).
Acute Promyelocytic Leukemia—t(15;17)— PML-RARα
APL is the subtype of acute leukemia where the greatest progress has been made over the past decade. It is the most curable subtype of AML and the most important development leading to the dramatic improvement in survival has been the introduction of all-trans retinoic acid (ATRA). While the incorporation of ATRA has led to these remarkable results, differentiation therapy with ATRA is associated with unique toxicities not previously observed with conventional cytotoxic therapy.
Induction therapy
Historically, induction therapy for APL included an anthracycline and cytarabine. APL was known to be particularly sensitive to anthracyclines, in part due to significantly lower Pgp expression and other resistance markers in APL cells compared to other subtypes of AML.1 It may also be that for this reason the long-term results in APL with conventional chemotherapy have historically been significantly better than in other forms of AML.2
Several studies have confirmed that both daunorubicin and idarubicin, used as single agents, induce a CR in 60%-80% of patients.3,4 Importantly, a retrospective analysis by the Southwest Oncology Group (SWOG) showed an improved survival in patients with APL when the dose of anthracycline was increased (70 mg/m2 ) but without a change in cytarabine dose.5 It has now been established that ATRA is an important part of induction therapy for APL. The benefit of ATRA in induction may not necessarily be a dramatic change in the initial CR rate;6 rather, incorporation of ATRA has a major impact on the number of patients that may be cured in this disease.7– 9
Several studies have shown that when ATRA is combined with single-agent anthracycline the results are at least as good as when cytarabine is also added.4,10 It is likely that the standard form of induction therapy for APL will rely on ATRA with an anthracycline without the addition of cytarabine (Table 2 ). Despite theoretic considerations, there is no evidence for the superiority of any anthracycline in APL. Using ATRA and an anthracycline, a CR rate of greater than 80% may be reasonably expected. However, despite the remarkable impact of ATRA in the treatment of AML, the induction mortality remains approximately 10%, and acquired retinoid resistance contributes to relapse in approximately 20-30% of patients.7–,9,11
While the incidence of coagulopathy and bleeding have diminished significantly with ATRA therapy, the retinoic acid syndrome (RAS) is now the major toxicity associated with ATRA. Among patients treated with ATRA alone the incidence is approximately 15%. The mortality from this syndrome has declined over time, likely reflecting earlier recognition and institution of dexamethasone. Furthermore, the concurrent administration of chemotherapy with ATRA may decrease the incidence of RAS.13
Post-remission therapy
Although CR may be achieved using ATRA alone, most patients relapse with this therapy. Consolidation chemotherapy after CR is mandatory, although the best form of such therapy is unknown. In most studies consolidation chemotherapy has been anthracycline-based, and several studies have included high-dose cytarabine.7,9,12 However, the administration of lower doses of cytarabine appears to be just as efficacious,13 and a recent prospective study has suggested that patients do as well without cytarabine in either induction or consolidation.10 Thus, it is likely that, just as in induction, there is probably little role for cytarabine in consolidation, although this remains a subject of study in current clinical trials. Most centers administer at least two courses of post-remission therapy following induction with ATRA and anthracyclines, although, as in all types of AML, there are no prospective data establishing the number of courses of intensive post-remission consolidation.14 Clearly, the objective of post-remission therapy is complete eradication of the leukemic clone with lack of detection of PML-RARα by PCR. This remains critical as the persistence of such minimal residual disease predicts for relapse.9
Maintenance therapy
Randomized trials have suggested that maintenance therapy with ATRA is a critical component of therapy for APL.7,8 There has also been a suggestion that when ATRA maintenance is combined with low-dose chemotherapy this may further improve the long-term survival.8 Thus, it appears that patients with APL may benefit from maintenance ATRA, with or without continuous low-dose chemotherapy, particularly those patients who present with higher risk for recurrent disease.8
Investigational approaches
While the outlook for patients with APL has improved dramatically over the past decade, complacency is to be discouraged. Over 20% of patients presenting with APL will die from this disease. This includes some patients who relapse even after achieving PML-RARα molecular negativity after induction and consolidation therapy.9 Arsenic trioxide has been established as an important agent for the treatment of ATRA-resistant APL. Whether this should be incorporated into the therapy of newly diagnosed APL is being currently investigated in clinical trials.
Allogeneic stem cell transplant has no role in APL in first remission. However, impressive results have been shown using autologous transplant for relapsed APL using cells that are molecularly negative.15 This has never been prospectively studied in patients in first remission who have been successfully treated with ATRA-anthracyclines. It is speculative whether such therapy, using peripheral blood with its attendant low mortality, can further improve the long-term cure rate.
Therapy for Acute Myeloid Leukemia Other Than Acute Promyelocytic Leukemia
Induction therapy
Classic studies by the CALGB two decades ago resulted in the development of the standard induction regimen, which consists of daunorubicin, 45mg i.v. for three days, and cytarabine, 100mg i.v. by continuous infusion for seven days.16 For patients less than 55 to 60 years old, an initial CR of 60-75% can be expected. However, multiple randomized studies compared daunorubicin at a dose of 45mg/m2—while keeping the dose of cytarabine constant—with idarubicin,17 amsacrine,18 aclacinomycin A19 and mitoxantrone.20 Virtually all of these agents have been shown to be either unequivocally superior, or at least with a strong trend towards an improvement, when compared with 45mg/m2 of daunorubicin. Thus, for patients not treated on a clinical trial it is no longer appropriate to use 45mg/m2 of daunorubicin; rather, a higher dose of daunorubicin should be used or an alternative anthracycline or anthraquinone, such as idarubicin or mitoxantrone.
Over the years many variations of the standard 3+7 regimen have been developed and all yield approximately similar results. Intensifying induction therapy through the use of higher doses of cytarabine or the addition of etoposide, while not affecting the initial CR, clearly may have an effect on the disease-free survival.21 However, although intensifying induction therapy may affect the duration of remission in AML, it is not clear that the increased toxicity through more profound myelosuppression is advantageous given the possibility that a similar intensification might be safely added during postremission therapy.22
Currently, the 3+7 induction regimen is recommended for all newly diagnosed patients with AML, including those who present with unfavorable cytogenetics. The latter group also includes most patients with therapy-related or other secondary leukemias. Although multiple publications have advocated alternative regimens,23 there are no data that any form of therapy provides a better outcome than standard induction therapy consisting of an anthracycline and cytarabine. Three large cooperative groups that evaluated this reported CR rates of 55-58% in adult patients presenting with unfavorable cytogenetics (Table 3 ).2,24– 27
Postremission Therapy
While induction therapy may be identical, the choice of postremission therapy must be determined by the prognostic group, most importantly, the cytogenetics at presentation (Table 4 ).
AML with favorable cytogenetics
Although over the past decade there have been several large prospective studies24–,26,28 of postremission therapy in AML, only two of the large studies have rigorously analyzed post-remission data by cytogenetic prognostic groups.2,27 The initial response rate to induction in patients with favorable cytogenetics was approximately 85%. With intensive postremission therapy the overall survival at 5 years exceeds 50%.2,27 There are many chemotherapeutic strategies for postremission therapy, although it is thought by many that high-dose cytarabine is a critical element for the success of postremission therapy.29 However, while the data confirm its effectiveness as postremission therapy, it is doubtful that we need to subscribe to a dogma that one cannot do the same with other regimens. A recent publication, based on CALGB data, suggested that it may be inappropriate not to administer 3-4 cycles of high-dose cytarabine to patients with the t(8;21) abnormality, in which the disease-free survival was about 60%.29 However, the MRC reported an identical disease-free survival in a much larger cohort of patients without using high-dose cytarabine.2,25
Whether autologous stem cell transplantation should be offered as a part of postremission strategy to patients with favorable cytogenetics remains controversial. Data from the CALGB have suggested that intensive chemotherapy yields results that are unlikely to be improved by the substitution of autologous transplantation.29 In contrast, the US Intergroup Study26,27 suggested that autologous transplantation may be particularly useful in this group of patients. In the MRC AML 10 study, patients were randomized to receive autologous stem cell transplant after four cycles of therapy and this was compared with an observation arm.25 The patients with favorable cytogenetics had a markedly lower relapse rate than patients who did not receive an autologous transplant, although a high procedural mortality rate in adults (18%) resulted in being ultimately no difference in the overall survival. The reported data need to be cautiously interpreted and may be influenced by small cohorts of patients, as in the CALGB data29 or the US Intergroup Study.26 Except for young patients in whom fertility remains a consideration, it is probably reasonable to use peripheral autologous stem cell transplantation in experienced centers that have demonstrated a consistently low morbidity and a therapy-related mortality of less than 3-5%.
None of the randomized studies demonstrated an advantage for allogeneic transplant for this group of patients, and given the relatively high transplant-related mortality, this procedure cannot be recommended as standard therapy for such patients. Whether newer methods using less severely myeloablative regimens and relying on the immunological effect of GVL may yield improved results remains to be determined in prospective studies.30
AML with intermediate risk cytogenetics
If an HLA-matched family donor is available, it seems likely that this should be the recommended therapy for patients up to age 55-65 years. Data have consistently shown that this form of therapy provides the best antileukemic effect as judged by the relapse rate.31,32 The study with the largest cohort of prospectively evaluated patients with this subgroup of AML have reported a 3-year survival rate of 65% with a relapse risk at 3 years of 18%.2,25 It should be noted, however, that this advantage for allogeneic transplant was not demonstrated in the US Intergroup Study,26,27 albeit in a smaller cohort of patients.
Timing of allogeneic transplantation in first remission has never been prospectively established. For logistic reasons it may often be necessary to administer chemotherapy after achievement of CR until the availability of a donor and transplant center is established. However, retrospective analysis from the IBMTR suggests that for patients proceeding to allogeneic transplantation in AML there is no added benefit in receiving additional postremission therapy, and if an HLA-matched donor is available, based on the current available data, patients should be referred for this procedure as soon as possible.33
Patients who do not have an HLA-matched sibling should receive intensive postremission chemotherapy using high-dose cytarabine or similar regimen. The optimal dose of high-dose cytarabine—anywhere from 1.5 g/m2 to 3 g/m2—the optimal duration and the total number of doses have never been prospectively established. Only one retrospective analysis, combining several historic studies, suggested that three cycles of high-dose cytarabine are better than a single course.29 While these are important practical questions affecting the management of many patients with AML, there is little current enthusiasm among cooperative groups to study this prospectively in clinical trials.
There are multiple reports of autologous transplants for AML including many patients with intermediate cytogenetics. However, it is difficult to identify and analyze large cohorts of patients who have received this therapy. The MRC study reported a relapse rate of 35% for patients who have also received an autotransplant compared with 55% among patients receiving intensive chemotherapy only. The 5-year survival was 56% versus 48%.2 It is generally assumed that those patients going on to an autologous transplant should receive prior intensive chemotherapy as the best method of in vivo purging. For such patients the intensity of postremission therapy as well as the number of cycles required are unknown and have also never been prospectively studied. Although some of the best results from phase II data of autologous transplants in AML have been reported when patients received no postremission therapy prior to the transplant,34 the preponderance of the data suggest that using several cycles of postremission therapy should be given prior to transplant. Preliminary data have also reported that peripheral blood stem cells can be reliably collected after two cycles of intensive chemotherapy such as high-dose cytarabine with a subsequent very low transplant-related mortality rate.35 Several cooperative groups are currently evaluating the role of gemtuzumab ozogamicin, a humanized monoclonal antibody against CD33 linked to calicheamicin, given in addition to high-dose cytarabine or prior to autologous transplant.
AML with unfavorable cytogenetics
This group has long been recognized to have the poorest outcome among patients with AML. While the initial response rate may exceed 50% (Table 3), the overall long-term survival remains poor, whatever mode of postremission therapy is employed. If a family-matched HLA donor is available, patients should be referred for this procedure as soon as possible after induction therapy. Although this probably represents current practice there aren't abundant prospective data that support this. The MRC AML 10 study reported that among the group with unfavorable cytogenetics allogeneic transplant did not offer any advantage.2 However, there were only 13 patients in this group. In contrast, the US Intergroup study reported a clear advantage for patients undergoing an allogeneic transplant27 with a 5-year survival of 44% compared to 15% for patients undergoing chemotherapy. However, once again, this was based on a very small subgroup of 18 patients using an intent-to-treat analysis and only 11 patients actually received this form of therapy. Nevertheless, this appears to represent a therapy with the greatest potential for prevention of relapse.
Because of the extremely high rate of relapse in this group of patients and the poor long-term outcome, it is important to note that select reports of alternative donors in this group of patients, which used either matched unrelated donors or haploidentically matched family donors, have shown long-term survival rates of 40-50% in patients undergoing such procedures in first remission.36– 39 Whether such information on select patients can be applied to the group as a whole remains to be determined in clinical trials.
Bone Marrow Transplantation
Over the past decade a major effort was made to determine the role of bone marrow transplantation (BMT) in adult AML, especially autologous transplantation, compared to intensive chemotherapy. Several large prospective studies were designed and much effort was expended on these trials.9,24,26,29 The results have been confusing and the data difficult to interpret. It seems that a decade later this issue has still not been resolved. However, certain points need to be emphasized.
The preponderance of the data demonstrate that autologous transplants provide better antileukemic activity than intensive chemotherapy, as judged by the relapse rate (Table 5 ).
Historically, the superior antileukemic benefit of autotransplants was negated by the high procedural mortality of autologous transplants (14% in the US Intergroup study and 18% in the MRC AML 10 study). Were this mortality to be reduced by about 10% then the conclusions from these studies may be quite different. Current use of peripheral blood as a source of stem cells for autotransplants is associated with an extremely low procedural mortality.35
All the major reported trials used bone marrow as the source of the stem cells and may not be relevant to current clinical practice in many centers. Aside from the higher procedural mortality, the morbidity when bone marrow is used is considerable with a median time to neutrophil recovery of approximately 25 days and over 7 weeks for platelet recovery. With such morbidity and mortality, using bone marrow, there had to be a vastly superior advantage to transplants for this to be considered a better option than chemotherapy.
However, most importantly, data from these studies are impossible to interpret due to the small number of patients analyzed per subgroup. The first disappointment was the very high dropout rate of patients, typical in all transplant studies. This dropout occurs at several stages. Only a proportion of patients eligible for randomization after induction actually go on to randomization. Also, a significant number of randomized patients never go on to receive the transplant. In the US Intergroup study approximately half of the patients who were randomized to an autotransplant never received one.26 The validity of intent-to-treat analyses is uncertain when half of the patients do not receive their intended therapy. Furthermore, when these studies were designed over a decade ago AML was considered as a single entity and the number of patients for these studies was determined based on this consideration. With identification of the importance of prognostic groups, it became crucial to consider each of the prognostic subgroups. Once the data for these subgroups are broken down, the number in each patient group is extraordinarily small making any analysis difficult and potentially misleading. As an example, the US Intergroup study26 accrued over 800 patients, and 116 were assigned to autologous transplant. However, only 63 (54%) of these 116 patients actually completed this therapy. These 63 patients were then divided into 3 subgroups: favorable, intermediate and unfavorable cytogenetics, leading to an analysis based on numbers that statisticians would never have agreed to had this been defined as the primary endpoint at the time of design of the study.27 In fact, it has been estimated that in order to conduct such a study in AML patients and obtain meaningful results with data that could be reliably analyzed, an excess of 7,000 patients would be required. Thus, it is unlikely that such a study will ever be carried out, which is the main reason why conflicting results from these transplant studies have been reported, especially when analyzed by subgroups. The most telling example of this may be gleaned from the analysis of the value of high-dose cytarabine in AML patients with favorable cytogenetics. The results from the CALGB studies report a disease-free survival in excess of 60%.29 In contrast, the US Intergroup study reported that for this group of patients the 5-year survival was only 35%. This same study reported that when the identical therapy was applied to a group with less favorable cytogenetics —the intermediate group—the 5-year survival was 55%. These data, while providing some biologic information, emphasize the pitfalls of drawing conclusions when small cohorts are involved and the inability to compare data between different studies.
Acute Myeloid Leukemia in Older Adults
Older adults have a dismal long-term prognosis that has not improved much over the past two decades (Figure 3). They have more unfavorable prognostic factors at presentation and their treatment is made more difficult by their inability to withstand intensive chemotherapy.40 Further, the type of postremission therapy that they receive is generally considered to be sub-optimal even for the more favorable prognostic groups seen in younger adults. The critical factor is that a biologically unfavorable disease is treated sub-optimally.
Older adults who do not have significant co-morbidities should be treated with standard induction therapy. With such therapy 50% of such patients can achieve a CR.41,42 The major difficulty relates to the selection of postremission therapy. Though patients can generally tolerate one cycle of cytarabine given at somewhat lower doses than is usually given for younger adults,41 it has never been shown that this improves the long-term outcome. This population represents the majority of individuals with AML, and major efforts are needed to determine the best therapy in this group of patients with the aim of achieving a possible cure in some patients and a prolongation of the disease-free survival in many others. Maintenance therapy has been studied in the past with some evidence that this can prolong the disease-free survival.43 Current strategies aim to emphasize non-myeloablative immune modulation following induction and limited intensive post-remission therapy and include phase III studies evaluating the role of IL-2/histamine, IL-2 (CALGB) and Flt 3 ligand (ECOG/SWOG/CALGB). Future strategies to prolong the disease-free survival in older adults include proposed studies of gemtuzumab ozogamicin, farnesyltransferase inhibitors and bcl-2 antisense oligonucleotides. Clearly, this is the area with the greatest challenge in AML: applying a less severely myeloablative form of postremission therapy that may, nevertheless, cure the most unfavorable prognostic type of the AML. Achieving this aim has so far been elusive. Such breakthroughs will, likely, also benefit younger adults.
II. Molecular Targets in Acute Myeloid Leukemia
Jerald Radich, MD*
Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, D4-100, P.O. Box 19024, Seattle WA 98109-1024
Acknowledgments: I thank Drs. Derek Stirewalt and Soheil Meshinchi for their helpful comments. In addition, because of size constraints of this review, important work from many colleagues had to be omitted. I apologize that I could not include all the contributions they have made to this field.
Acute myeloid leukemia (AML) is a heterogeneous disease characterized by a myriad of genetic defects. These include translocations involving oncogenes and transcription factors, activation of signal transduction pathways, and alterations of growth factor receptors. If each type of genetic lesion found in AML involved a distinct process to cause leukemia, then “targeted” therapy directed at specific genetic lesions would be futile—quite simply, there would be too many potential targets. However, it appears that many of the pathways perturbed in leukemogenesis interact, so that a limited number of specific targets may be useful in a wide variety of AML cases. Indeed, if multiple genetic abnormalities are needed to cause and sustain AML, then the blunting of a single aberrant pathway may be enough to eliminate the proliferative advantage and curb the disease.
In this short review we will examine the signaling pathways involved in leukemogenesis, with specific emphasis as to how these pathways can be utilized as targets for novel therapy.
Normal Signal Transduction
Signal transduction pathways are designed to translate extracelluar signals (e.g., stimulation to respond to cytokine ligands, interferons) into intracellular action (proliferation, differentiation, survival). Perhaps the best understood pathway involves signaling utilizing the ras family of guanosine nucleotidases (GTPases). A highly simplified cartoon of the ras signal transduction pathway is shown in Figure 4 (see color page 541) . The most important components include the following:
Receptor tyrosine kinase (RTK):
These ligand binding receptors include PDGF, Fms, c-kit, and Flt 3.1– 4 In general their structure includes an extracellular ligand binding region consisting of 5 immunoglobulin-like domains, transmembrane and juxta-membrane domains, and an intracellular domain with kinase activity (Figure 5; see color 541).
Grb-2 and SOS: Grb-2 is an adaptor protein that functionally bridges the association of the RTK with ras. SOS is a guaninine nucleotide exchange protein that facilitates ras-GDP→ras-GTP exchange.
Ras. Harvey (H), Kirsten (K), and N-ras are 21 kd GDP/GTP-binding proteins that serve as the hub of signal transduction.5,6 All three ras proteins are expressed in most tissues, but the constellation of mutations (i.e., N-ras vs. K-ras) tend to be disease specific.
GAP, NF-1: These are GTPase activating proteins, that catalyze the inherently slow GTPase activity of ras, converting active ras-GTP to an inactive ras-GDP form.7
Briefly, extracellular ligand (L) interacts with the RTK that causes receptor dimerization (Figure 4). This prompts activation of the RTK and subsequent receptor autophosphorylation. This phosphorylated RTK can in turn phosphylate and activate Grb-2, the adaptor protein, which when coupled to SOS, causes activation of the ras. Inactive ras remains bound with GDP, and the interaction with Grb2-SOS causes ras to become activated by GTP binding. This produces a conformational change in ras, and promotes interaction with downstream effector proteins. Since ras has an intrinsically slow GTPase activity, the switch back to the inactive ras-GDP state necessitates the activity of the GTPase-activating proteins (p210 GAP and NF-1).
Activation of ras causes activation of several downstream pathways, which may effect cell proliferation, differentiation, and apoptosis.8–,12 The serine/threonine kinase Raf is activated by direct association with ras, and in turn activates the MAP/ERK kinase (MEK), which activates downstream mitogen-activating protein kinases (MAPK), such as extracellular signal-regulated kinases (ERK 1 and 2).13 These in turn phosphylate cytoplasmic targets (Rsk, Mnk) that translocate to the nucleus, causing activation of transcription involved in proliferation. Ras activation also may influence cytoskeleton organization through activation of Rac and Rho. In addition, ras may promote cell cycling through the activation of Cyclin D dependent kinases (CDKs), by interacting alone, with Raf, or with Myc. Lastly, ras may play a part in inhibiting apoptosis. The activation of PI-3 kinase by ras activates c-Akt, which has been demonstrated to protect against apoptosis. Thus, ras pathways may take part in an extraordinary range of pathways regulating cell proliferation and death.
The initiation of the signal cascade takes place at the intracellular membrane, and thus ras must move from the cytoplastic space to that site of action. For membrane association, ras proteins must undergo a post-translation modification called prenylation, which adds an isoprenoid moiety to the cytoplasmic ras.14 This prenylation is accomplished by the enzymes farnesyl and geranylgeranyl transferases, which add 15 and 17-mer isoprenoids, respectively, to the ras protein. Prevention of prenylation keeps ras in the cytoplasmic space and is the underlying rationale for therapy directed at the inhibition of farnesyl transferase.
The Janus kinase-signal transducer and activator of transcription (Jak/Stat) pathway is utilized by many members of the cytokine receptor superfamily (erythropoietin, interferons, granulocyte colony stimulating factor [G-CSF]).15,16 These receptors, unlike the RTK noted above, lack their own intrinsic tyrosine kinase activity. Binding of ligand to receptor causes autophosphorylation of Jaks, and the activated Jaks in turn phosphorylate the receptor. These phosphorlyated receptors are docking sites for signaling proteins, including Stats, which are in turn activated by phosphorylation. The activated Stats form dimers (either homodimers, or heterodimers with other Stats), translocate into the nucleus, and bind to specific DNA sequences, regulating gene transcription (Figure 4). There are at least 4 Jaks and 7 Stats, and the complex interaction between these regulate gene transcription in an elaborate fashion that is both gene and tissue specific. Further complexity arises in that Jaks and Stats may play a role in pathways not strictly in the Jaks/Stat pathway (e.g., Jak/STAT activation may occur through activation from the RTK/ras/MAPK pathway).
Abnormal Signal Transduction in AML
Perturbations in the signal transduction pathway are common in AML and occur through a variety of mechanisms. The precise cellular consequences of such inappropriate activation are unknown, but functionally it can be thought of as an uncontrolled activation of downstream targets causing inappropriate signals for proliferation and survival.
Tyrosine kinase receptor mutations
Mutations in the Fms, Kit, and Flt3 RTKs have been described frequently in AML (Table 6). Activating point mutations in the kinase domains of Fms have been described in 5-10% of selected AML cases.17–,19 Deletions, insertions, and point mutations have been found occasionally in the Kit receptor, often in cases with a mast-cell phenotype.20,21 However, mutations in the Flt3 RTK appear quite common and may be the most common mutation so far discovered in AML.22– 29
The Flt3 receptor is preferentially expressed on hematopoietic stem cells and mediates stem cell differentiation and proliferation. Flt3 receptor activation causes proliferation of AML cells in vitro, as it appears to both stimulate proliferation and inhibit apoptosis of the AML cells. Recently a unique mutation has been described in the Flt3 gene, whereby a fragment of the JM domain-coding sequence (exons 11 and 12) is duplicated in direct head-to-tail orientation. This creates a so-called internal tandem duplication (ITD) mutation (Figure 5). The length of the ITD varies from approximately 20-200 base pairs and the duplicated sequence is always in-frame. In vitro studies have shown that mutant Flt3/ITD receptors are dimerized in a ligand-independent manner, leading to autophosphorylation of the receptor through constitutive activation of the tyrosine kinase moieties, and leads to autonomous, cytokine independent growth in the mutant cells. Activation of signaling proceeds through the ras/MAPK and Stat 5 pathways.30,31
Several studies have explored the prevalence and significance of the Flt3/ITD mutation. Common themes in these studies are that the mutation is associated with a pronounced leukocytosis, that the prevalence appears to increase with age, and that the presence of the Flt3/ITD may be associated with a poor prognosis, particularly in the pediatric population. The prevalence of the Flt3/ITD in two Japanese pediatric studies ranged from 5-11%, and was associated with a poor clinical outcome.23,32 More recently, analysis of 91 pediatric AML patients treated on a single Children's Cancer Group (CCG) study was performed and showed that 15 of 91 samples (16.5%) were positive for the Flt3/ITD.29 None of the patients with the Flt3/ITD had unfavorable cytogenetic markers. Despite this, the remission induction rate was 40% in patients with the Flt3/ITD compared to 74% in patients without the Flt3/ITD, and event-free survival at 8 years was only 7% for those with the Flt3/ITD compared to 44% for patients without a mutation. The results in adult AML are not as conclusive in regards to Flt3 and outcome. Two retrospective adult studies demonstrated that the presence of the Flt3/ITD was associated with poor outcome. Rombouts et al found Flt3/ITDs in 18/81 patients (22%), and found that the complete response rate (47% vs. 80%), relapse rates (75% vs. 26%), and leukemia-free survival rates (< 10% vs. ~40%) were significantly poorer in patients with Flt3 mutations compared to patients without mutations.33 Kiyoi found the Flt3 mutation in 43/201 (22%) newly diagnosed AML cases but found no effect of the mutation on CR rates. However, survival among those with the Flt3 mutation was inferior to those without the mutation (~20% vs. 50%).24 On the other hand, a large study (N = 143) of “older” (> 55 years) AML cases from SWOG revealed that the presence of Flt3 /ITD did not have an adverse effect on outcomes.27 However, in this elderly AML group, response and outcome was universally (and predictably) poor in all genetic subgroups. This study found a very high rate of Flt3/ITD in 34% of cases, thus fortifying the association of increasing Flt3 with increasing age.
Point mutations in the Flt3 activation loop have recently been described in 7% of adult AML patients.34 Curiously these point mutations were not associated with leukocytosis, unlike Flt3/ITDs, although those having a point mutation shared an equally poor event-free survival as those with the Flt3/ITD compared to patients without the mutation. While Flt3/ITD activation seems to work through ERK and Stat5 pathways, it is unknown if the point mutations behave in the same fashion.
ras mutations
Mutations in N-, K-, or H- ras occur in approximately 10-30% of AML cases (Table 7 ).35–,40 They also occur in myelodysplastic syndrome (MDS) (~5-20%),17,41 juvenile CML (20-30%),42,43 and CMML (30-50%).17,44Ras mutations occur rarely in blast phase CML.44,45 In leukemia (as opposed to solid tumors), mutations are predominately in N-ras, less commonly in K-ras, and quite infrequently in H-ras. These mutations are point mutations in codons 12, 13, and 61, with rare exceptions,46 and act to prevent the hydrolysis of ras-GTP; effectively, this causes the activated ras to be constitutively stuck in the “on” position. Intuitively this activation would likely lead to constitutive activation of downstream pathways normally controlled by ras, but this may not entirely be the case. For example, in a study of primary AML cases, fully one-half had constitutive activation of ERK, but none of these cases had ras mutations. In addition, mutated ras has been found in vitro to bypass some of the normal signal transduction intermediaries and bind directly with the transcription activator, JunB.
NF-1 mutations
Children with neurofibromatosis have an increased incidence of juvenile CML (JCML), often associated with a mutation in the NF-1 gene. The structure and function of NF-1 is similar to GAP, so that a decrease in its activity promotes the maintenance of the ras-GTP state, presumably inappropriately activating the transduction pathway.47,48 N-ras mutations also occur often in patients with JCML, but only in those with normal NF-1.49,50 GAP mutations in other myeloid leukemias are rare.51
Aberrant activation of the Jak/Stat pathway.
The most direct example of aberrant activation of Jak/Stat are from translocations that either directly involve genes or directly activate the pathway. For example, the t(9;12) translocation involving Tel-Jak2 has been found in both lymphocytic and myeloid leukemia.52,53 This translocation contains the helix-loop-helix oligomerization domain of Tel and the catalytic domain of Jak2. Dimerization promotes the activation of Jak, which in turn leads to constitutive activation of the downstream Stat 5. Similar activation of Stat 5 occurs from the inappropriate kinase activity of Bcr-Abl. In addition, inappropriate activation of Stats 1 and 3 have been found in primary acute leukemia cells. For example, mutations in the RTK Flt3 appear to activate Stat 5, whereas activating mutations in c-kit appear to activate Stat 3 and (to a lesser degree) Stat 1.54
Translocations
Translocations have been described that activate the ras pathway directly. For example, in CML specific domains on the BCR moiety of the chimeric BCR-ABL protein interact with the docking protein Grb-2, which then couples with SOS to activate ras. In CMML, the t(5;12) translocation involves the Tel gene and the β chain of PDGFR.55 The Tel dimerization causes autophosphorylation of PDGFR, which activates ras via an interaction with Grb-2/SOS. In addition, the central role of ras activation in CMML is underlined by the frequent mutation in N-ras in those patients without the t(5;12) translocation.
Summary
Taken together, aberrations in signal transduction pathways appear to be quite common in AML. In fact, two studies have measured the frequency of ras and Flt3 mutations in a single population and found that just these two genes account for 30-50% of patients with mutations involving the RTK/ras pathway. Analysis of Kit and Fms receptors might increase the prevalence even further. Thus, drugs designed to target this pathway, particularly at downstream “choke points,” might be effective in a surprisingly large number of AML cases.
New Drugs Aimed at Molecular Targets
To reiterate, mutations in the ras-mediated signal transduction pathway are present in 30-50% of AML by direct mutational analysis. Indeed, approximately 50% of AML cases at diagnosis have abnormal phosphorylation of ERK, indicative of inappropriate pathway activation. Targeted therapy could be leveraged at many fronts.
Rationale for RTK inhibition
As noted above, mutations in RTKs are a common abnormality in AML. The exciting success of the tyrosine kinase inhibitor (TKI) STI571 in CML has caused a flurry of activity toward developing TKIs directed at aberrant RTK function.56,57 Unfortunately, while STI571 inhibits Bcr-Abl, Abl, and PDGF, it has limited activity on Flt3 or Fms;58 however, it has substantial activity against c-kit and may be effective in the small subset of leukemia patients harboring that mutation. In c-kit mutant cell lines, the addition of STI571 inhibits Kit autophosphorylation and effectively blocks activation of ERK and Akt.59 Inhibition of Flt3 in mouse and human leukemia cells can be accomplished with the TKIs herbimycin A and AG1296, which inhibit mutant Flt3 autophosphorylation as well as abrogate in vitro growth independence of Flt3/ITD cell lines.59
Treatment with the novel tyrosine kinase inhibitor SU5416 has recently been described in an AML patient in refractory second relapse.60 SU5416 blocks the activity both vascular endothelial growth factor receptor 2 (VEGFR-2) and the stem cell factor (SCF) receptor c-kit. The patient treated had evidence by flow cytometry of blasts that expressed both VEGFR-2 and c-kit. SU5416 monotherapy was instituted and by 12 weeks a CR was achieved. This CR was durable for an additional 6 months on maintenance SU5416 therapy alone. Analysis of the bone marrow microenvironment revealed a decrease in microvessel density suggesting a decline in angiogenesis caused by VEGFR-2 activity. The report is the first documented durable remission induced with specific RTK inhibition in AML.
Ras inhibition
Farnesyltransferase inhibitors (FI) target the post-translational modification of ras to prevent subcellular localization necessary for participation in signal transduction. The first phase I trial of a FT inhibitor in hematological malignancies has recently been completed in 35 adults with refractory and relapsed acute leukemias.61 The non-peptidomimetic FT inhibitor R115777 was given at doses from 100 mg b.i.d. to 1200 mg b.i.d. for up to 21 days. Dose-limiting neurotoxicity was encountered at 1200 mg b.i.d. The overall response rate in 25 AML patients was 32% (8/25, with 6 partial and 2 complete responses). Biochemical assays showed that the FT activity was inhibited by a dose of 600 mg b.i.d., and at this level clinical responses were seen in 2/7 (29%) AML patients, suggesting that this may be a reasonable drug level for future phase 2 trials. Activation of the MAPK pathway was found in 8 patients, and in 4 this activity was curbed after R115777 treatment. It is unclear if the patients with ERK response were the same that revealed clinical responses. Curiously, none of the 25 AML patients had evidence of N-ras mutations; RTK mutations were not evaluated. However, 3/5 patients with monosomy 7, a defect that may be associated with aberrant ras expression, had a clinical response, implicating some activity against ras activity. How, then, is R115777 working in the bulk of these patients? Other effectors of ras activation, such as RhoB, and members of the PI3/AKT-2 pathway, need farnesylation for activity, and perhaps these downstream effectors represent more crucial targets for FT inhibition.62,63
There may be other targets for ras inhibition, based on its necessary physical interactions with downstream effectors. X-ray crystal structures of normal and oncogenic ras have been determined, and crucial binding sites of SOS, GAP, and Raf have been defined.56,57 These are rational targets for small molecules designed to block these areas of protein-protein interaction. Moreover, elucidation of the conformational changes that occur in the mutant ras protein structure may provide structural targets for therapy. Lastly, the finding that mutated ras binds Jun, perhaps bypassing normal ras signaling pathways, offers a potential target that would maintain normal ras function.
Rationale for Jak/Stat inhibitors
Activation of the Jak/Stat pathway appears common in AML, especially involving Stats 3 and 5. Inhibiting activation of Stat could be accomplished at the receptor level (by blocking ligand binding or inactivating RTK activity), by blocking Stat phosphorylation and subsequent dimerization, by small molecule interactions with the SH2 domain, or by small molecules targeting Stat consensus DNA binding domains.64 In addition, oligonucleotide therapy has been directed at inhibiting Stat expression.65 These novel approaches are currently in the pre-clinical phase of development.
The Discovery of New Molecular Targets
The study of the molecular biology of leukemia has been limited by the painstaking process of gene identification and the difficulty of unraveling the complicated networks that drive normal (and abnormal) cellular function. However, the payoff of the Human Genome Project and the advances in micro-engineering and informatics has ushered in an area of genetic research where it is possible to study > 10,000 genes simultaneously by use of mRNA expression arrays. While this technology is in its infancy, it has already been demonstrated that it may be a powerful tool for finding new biological classification methods in leukemia and lymphoma. For example, the work of Golub et al suggests that gene arrays can be used to determine a set of genes that distinguish ALL from AML, and holds considerable promise towards a molecular classification of cancer.66 As a model approach, the study demonstrated the feasibility of molecular classification and described a general strategy for discovering new classification schemes independent of previous knowledge or biases. Such technology may also be used to study pathways as well. Experimental studies have been successfully performed in yeast, where pathways have been mapped by using controlled manipulations of variables followed by mRNA expression analysis.67 This has elucidated considerable, unanticipated “cross-talk” between several MAPK mediated pathways, such as those regulating filamentous growth and mating responses. Similar approaches can be imagined in human cancers. Unanticipated and unique pathways might be uncovered in leukemia cells that are quite different than normal pathways. The effects of potential drugs on various pathways can be assessed, and interactions discovered that would likely never be apparent with conventional methodology.
Conclusion
Leukemia cells bypass normal control of growth, differentiation, and apoptosis. However, in skirting these normal checks and balances, they place themselves in the tenuous position of relying on aberrant cellular mechanisms for survival. For example, if the activation of the ras pathway causes both inappropriate proliferation and a block in apoptosis, the inactivation of the pathway may both decrease the proliferation drive, as well as relieve the check of programmed death. The results with STI571 have shown how ungoverned signal transduction can be used as “pharmaceutical judo” to control disease when the aberrant signal is suddenly blocked. Further characterization of signaling pathways in AML, partnered with novel drug development, promises a new approach to the treatment of leukemia.
III. Immunologic Approaches to the Treatment of Acute Myeloid Leukemia
Frederick R. Appelbaum, MD*
Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, D5-310, P.O. Box 19024, Seattle WA 98109-1024
The creation of an effective immunologic approach to the treatment of acute myeloid leukemia (AML) has been a goal of many researchers over the past two decades. Finally, with the development of gemtuzumab ozogamicin (Mylotarg), there is one example of a therapy based at least in part on an immunologic approach that has won FDA approval for the treatment of AML. Recent advances in immunology give us hope that this example will be neither the last nor the best. This brief article offers a review of preclinical and clinical work currently underway in the field.
Antibody-Based Approaches to AML
Unconjugated monoclonal antibodies
Unconjugated monoclonal antibodies can kill tumor cells in one of three general ways. They can induce fatal immunologic injury via complement-dependent cytotoxicity (CDC) or antibody-dependent cell-mediated cytotoxicity (ADCC). They can react with cell receptors resulting in signal transduction events that directly lead to apoptosis without a significant contribution from CDC or ADCC. And finally, they can, at least theoretically, block the binding of other factors necessary for cell survival.
The only unconjugated antibodies systematically studied in AML target the CD33 antigen. CD33 is a glycoprotein found on blasts from more than 90% of cases of AML. It is expressed on almost all normal early myeloid and erythroid progenitors but is not on normal hematopoietic stem cells or nonhematopoietic tissue. Although the normal function of CD33 is not known, it is thought to be a member of the Siglec family with immunoreceptor tyrosine-based inhibitory motifs in its cytoplasmic domain. There is no evidence that ligation of CD33 induces apoptosis; thus, use of unconjugated antibody therapy in AML directed at CD33 is thought to depend on the antibody's ability to inflict immunologic injury on the tumor cell.
Initial studies of murine anti-CD33 antibodies showed that the antibody could be administered with relatively little toxicity other than fever and chills, that there was rapid uptake of antibody in marrow and spleen, and that antigenic sites on leukemic and normal cells were saturated with antibody doses of 5-10 mg/m2.1,2 While transient drops in circulating blast counts occurred in some patients, no sustained responses were seen, demonstrating that the murine antibody was incapable of initiating an effective immunologic response.
In an effort to increase immunologic potency, the Sloan-Kettering group developed a chimeric humanized form of one anti-CD33 antibody, termed HuM195. In a phase II trial of this agent in 35 patients, transient drops in peripheral blast counts were seen, and one patient with less than 30% blasts at the start of treatment achieved a complete remission.3 Attempts to augment the immunologic reactivity of HuM195 by combining it with IL-2 did not lead to a marked increase in clinical activity.4
Given the relative lack of activity in overt AML, subsequent trials of unconjugated HuM195 have been conducted in patients in clinical remission. In one study, patients with acute promyelocytic leukemia (APL) in remission following treatment with chemotherapy and retinoic acid were given 3 mg/m2 HuM195 twice weekly for six doses. Of 27 patients in first remission, 22 had evidence of minimal residual disease by reverse transcription PCR assay for PML/RARα rearrangements. Of these 22, 11 became PCR negative after antibody therapy.5 Current trials are examining HuM195 in combination with retinoic acid and arsenic trioxide in APL and in combination with conventional chemotherapy for patients with AML in first relapse.
Drug conjugates and immunotoxins
Although unconjugated antibodies to CD33 do not have major clinical activity in overt AML, the antibodies are capable of reaching sites of leukemia, saturating the antigenic sites on leukemic cells and internalizing after cell surface binding. These observations together with the fact that CD33 is absent from the surface of the normal hematopoietic stem cell and all non-hematopoietic sites suggested that antibodies to CD33 might serve as an effective vehicle to target potent drug conjugates to leukemic cells while sparing the normal hematopoietic stem cell and normal organs.
Working with investigators from Lederle Laboratories, the Seattle group developed the drug immunoconjugate Mylotarg, which joins a humanized anti-CD33 IgG4 antibody to the potent antitumor antibiotic calicheamicin. Mylotarg was initially evaluated in a phase I dose escalation trial involving 40 patients with refractory or relapsed AML.6 Patients received 0.25-9 mg/m2/dose q14 days for up to 3 doses. Dose escalation was stopped at 9 mg/m2 because at that dose more than 90% of antigenic sites on leukemic cells were saturated. The most common side effects were fever, chills, transient transaminasemia and the expected myelosuppression. Peripheral blast counts dropped in virtually all patients receiving doses of 6 mg/m2 or more of drug. When all dose levels were considered, 8 of 40 patients (20%) had complete clearance of bone marrow blasts, 3 of whom had full hematopoietic recovery.
The combined results of three multicenter phase II trials of Mylotarg have recently been reported.7 In these studies, 142 patients with AML in first relapse were treated with 9 mg/m2 of Mylotarg on days 1 and 14. Most patients experienced the post-infusion syndrome of fever, chills and hypotension, but this usually cleared by 8 hours. Grade 3-4 mucositis (4%), nausea and vomiting (11%) and serious documented infections (28%) were less frequent than seen with most aggressive reinduction regimens. Grade 3-4 elevation in transaminases was seen in 17% of patients and was usually transient, although one of the 142 patients died of apparent veno-occlusive disease of the liver. Overall, 30% of patients achieved remission, defined as less than 5% blasts in marrow and recovery of red cell and neutrophil counts to normal and platelet transfusion independence. Of these 30, 11 had not recovered to 100,000 platelets prior to receiving subsequent post-reinduction therapy. Based on its activity and favorable safety profile, Mylotarg was approved by the FDA for the treatment of CD33 positive AML in first relapse in patients 60 years and older who are not good candidates for aggressive reinduction regimens.
An analysis of leukemic blasts from 126 of the 142 patients treated on the phase II Mylotarg studies described above found that increased surface expression of P-glycoprotein (Pgp) and increased Pgp function, as demonstrated by cyclosporine (CSA) inhibitable dye efflux, correlated with treatment failure.8 Specifically, 52% of samples from patients who failed to achieve remission exhibited dye efflux compared to only 24% of those from patients who achieved remission. Similarly, apoptosis induced by in vitro exposure to Mylotarg was reduced in blasts with high levels of Pgp expression and in those from patients who failed to achieve remission. Addition of cyclosporine to cultures containing Mylotarg significantly increased apoptosis in approximately 1/3 of such cases.
Current trials of Mylotarg include the use of the agent as de novo treatment in older individuals, in combination with cytarabine or cytarabine plus an anthracycline, as a debulking agent prior to nonablative transplantation, as maintenance therapy in younger patients in remission, and in combination with cyclosporine.
As an alternative to targeting CD33, Frankel et al have developed a fusion protein consisting of granulocyte-macrophage colony-stimulating factor (GM-CSF) combined with diphtheria toxin as a means to specifically target myeloid cells. An early report of a phase I dose escalation trial reported on 26 patients with relapsed or refractory AML.9 Frequently seen toxicities were generally limited to fever and chills, and in 3 of the 26 patients greater than 90% reduction in marrow blasts was seen.
Radioimmunoconjugates
Radioimmunoconjugates have been investigated as therapy for AML both as a stand-alone treatment and in the context of hematopoietic cell transplantation. Because leukemia cells are adjacent to normal hematopoietic stem cells, the only real hope of delivering adequate doses of radiotherapy to the tumor cell without also irradiating normal stem cells would be with a radionuclide possessing an extremely short path length, such as an alpha emitter like 213Bi. The Sloan-Kettering group has explored a 213Bi-HuM195 conjugate in a dose escalation trial.10 Seventeen patients with recurrent AML were treated with HuM195 conjugated to 0.28-1 mCi/kg 213Bi and, although blasts decreased in numbers in 12 of 17, no complete responses occurred and pancytopenia was significant at the higher dose levels.
Given the obvious concern that radionuclides targeted to leukemic cells would irradiate adjacent normal stem cells, most trials of antibody targeted radiotherapy for AML have now been conducted in the context of hematopoietic cell transplantation. To date, three different targets have been explored: CD33, CD45 and CD66. The rationale for these studies comes, in part, from prior randomized trials attempting to identify an optimal dose of total body radiation (TBI) included in transplant preparative regimens. In these studies, it was found that increasing the dose of TBI from 12 to 15.75 Gy significantly reduced the risk of post-transplant relapse but was associated with an increase in non-relapse mortality.11,12 If, by using radioimmunoconjugates, the dose of radiation to sites of leukemia could be similarly increased without affecting normal tissues such as the liver, lung and gastrointestinal mucosa, then cure rates might be significantly improved.
An initial trial in Seattle involved the use of 131I-labeled anti-CD33 in 9 patients with recurrent AML.2 The trial design involved first administering a tracelabeled dose of the antibody to determine biodistribution and, if more radiation was predicted to be delivered to the marrow, spleen and other known sites of leukemia than to normal organs (a finding termed “favorable biodistribution”), treating patients on a dose escalation trial by adding increasing doses of 131I-labeled to anti-CD33 to a standard 120 mg/kg cyclophosphamide (CY) plus 12 Gy TBI regimen. Although rapid uptake of the trace-labeled antibody in the marrow and spleen was seen, the residence time of the radionuclide was very short, due to antigen-antibody internalization and dehalogenation, and in only 4 of 9 patients was “favorable” biodistribution found.
Jurcic et al from Sloan-Kettering have explored a similar approach, adding 131I-M195 (120-230 mCi/m2) in 2-4 divided doses to a standard busulfan (BU) 16 mg/kg plus CY 120 mg/kg preparative regimen.13 They report that among 19 patients all engrafted, and no unexpected toxicities were seen. However, because of the short retention of the radionuclide in the marrow, this approach required multiple infusions of the radionuclide to achieve the desired marrow dose, which led to prolongation of the preparative regimen and the peri-transplant neutropenic phase. Given this experience, the Sloan-Kettering group is now exploring the use of 90Y-HuM195, a radioimmunoconjugate that they hypothesize should reside longer in marrow and leukemic sites. An initial phase I trial appears to support this hypothesis.14 Their group is now exploring 90Y-HuM195 plus etoposide as a stem cell transplant preparative regimen.
CD45 has been explored as an alternative target for radioimmunotherapy in AML. It is expressed by most hematopoietic cells, save mature red cells and platelets, and is not expressed by non-hematopoietic cells. Compared to CD33, it is found in far higher copy numbers per cell and does not internalize upon antibody binding. Preclinical studies in mice and macaques showed that 131I-labeled anti-CD45 antibodies could deliver higher doses of radiation to spleen (13-fold) and marrow (3- to 4-fold) than to any normal organ.15,16
Given these data, Matthews and the Seattle group conducted a phase I trial in which patients with recurrent acute leukemia were given a trace-labeled dose of an 131I-labeled murine anti-CD45 antibody (BC8) following which biodistribution studies were conducted.17 If favorable biodistribution was found, patients went on to receive increasing doses of 131I conjugated to BC8 combined with CY (120 mg/kg) plus 12 Gy TBI followed by marrow transplantation. Among 44 patients, favorable biodistribution was found in 84%, with marrow and spleen receiving 6-13 cGy/mCi as opposed to 2.8, 1.8 and 0.6 delivered to liver, lungs and total body. Mucositis became dose limiting at doses above 10.5 Gy delivered to normal organs. This, in turn, meant that it was possible to administer approximately 20 Gy of marrow via the radioimmunoconjugate in addition to the standard CY plus TBI regimen. Approximately 30% of patients treated on this study became long-term survivors, but given patient heterogeneity, no conclusions about possible impact of the added radioimmunotherapy on outcome are possible.
A phase II trial of 131I-BC8 combined with standard dose BU (16 mg/kg) plus CY (120 mg/kg) as a preparative regimen for patients with AML with first remission is ongoing.18 The dose of 131I-BC8 is calculated to deliver 5.25 Gy to liver, or approximately 12 Gy and 29 Gy to marrow and spleen. Among 40 patients so far treated, relapse rates have been low (15%), grade 3-4 toxicity acceptable, and 70% of patients are calculated to be alive, disease-free, at 5 years.
CD66 is present on maturing hematopoietic cells but not on leukemic blasts. However, by targeting CD66, it should be possible to deliver radiation to leukemic cells as innocent bystanders. Bunjes et al have been exploring the use of 88Re-anti-CD66 as an adjunct to a standard preparative regimen and report that they can deliver approximately 15 Gy to marrow in addition to a standard preparative regimen.19 Among 36 patients with myeloid malignancies, 58% are alive in remission 17 months after treatment with this approach.
Cellular-Based Approaches to AML
Allogeneic hematopoietic cell transplantation
Although the existence of a graft-versus-leukemia (GVL) effect has been recognized since it was first described by Barnes et al in 1956,20 only in the last few years have investigators begun to focus on allogeneic HSCT as a potential immunotherapeutic approach rather than primarily as a vehicle for delivering high dose therapy.21 Three general observations have led to this change in focus. First, studies have consistently found that after allogeneic HSCT, relapse rates are least in patients who develop both acute and chronic graft-versus-host disease (GVHD), higher in those who develop no clinically evident GVHD, and higher still if T cells are depleted from the marrow graft.22 A second observation emphasizing the potential power of GVL comes from the results of treating patients for post transplant relapse by infusing viable donor lymphocytes. Complete sustained responses have been reported in substantial proportions of patients including up to 75% of patients with CML in chronic phase and 30% of patients with AML.23 A final set of observations that have dramatically increased interest in the GVL effect show that allogeneic engraftment can be achieved following administration of preparative regimens that are not truly myeloablative. For example, the M.D. Anderson group has been exploring the use of purine analogs (2-CDA or fludarabine) combined with various combinations of melphalan, idarubicin, or cytarabine to treat older or debilitated patients with myeloid malignancies.24 Slavin et al and the group from Jerusalem have explored the use of fludarabine combined with antithymocyte globulin and busulfan (8 mg/kg) as a preparative regimen with reduced intensity.25 Storb and the Seattle group have shown that if intensive post-transplant immunosuppression is given, including cyclosporine and mycophenolate mofetil, sustained engraftment can be obtained in virtually all recipients of transplants from matched siblings following a preparative regimen consisting only of fludarabine 90 mg/m2 and 200 cGy TBI.26 While each of these approaches has its own unique potential advantages and drawbacks, they all support several consistent conclusions. First, sustained complete engraftment of allogeneic stem cells can be achieved with non-myeloablative preparative regimens. Second, such transplants are associated with considerably less toxicity than traditional transplant approaches. For example, among the first 100 patients treated on the Seattle regimen, with a median age of 53, the 100-day transplant-related mortality was 4.5%. Third, substantial numbers of patients with active disease at the time of transplant have achieved complete remission and, although the follow-up is still short, most patients transplanted while in remission have remained in remission. However, the numbers of patients in any one study are still quite limited and follow-up is still too brief to allow for definitive conclusions about the efficacy of this approach. Nonetheless, the potential power of the GVL effect coupled with the ability to achieve allogeneic engraftment without the toxicities associated with very high dose therapy has generated increased interest in transplantation as an immunotherapeutic approach.
While it is critically important to define the clinical utility of existing non-myeloablative transplant approaches for the treatment of AML, the procedure, as currently applied, is still accompanied by a substantial incidence of GVHD. Further, it is unlikely that the anti-leukemic effects of non-myeloablative approaches will be greater than seen with conventional transplantation and likely will turn out to be less. Thus, methods to augment the GVL effect without causing GVHD are needed.
Polymorphic minor histocompatibility antigens as targets for GVL
One approach to segregating the anti-tumor from anti-host reactions following allogeneic HSCT has been to identify polymorphic minor histocompatability antigens with expression largely limited to hematopoietic tissues. Such antigens might serve as useful targets for post-transplant donor derived T cell therapy that should, in principle, be capable of eradicating host normal and malignant hematopoietic tissue. A number of minor histocompatibility antigens fulfilling this description have been identified by the groups from Leiden and Seattle.27,28 Studies have now been initiated in which donor derived T cells recognizing such antigens are isolated, expanded, and then used post-transplant in those settings where non-specific DLI infusions have previously been used.29
Non-allogeneic targets for cellular therapy
Use of polymorphic minor histocompatibility antigens as a means of segregating GVL from GVHD, while rational, will always require the setting of allogeneic HCT for application. Thus, attempts have been made to identify non-allogeneic peptides associated with the malignant phenotype that then might be used as candidate antigens for both allogeneic and autologous T cell therapy or possibly as peptide vaccines. The two categories of antigens that have been studied so far are mutational, such as BCR-abl and DEK-can, and overexpressed self-antigens, including PR3 and WT1.
BCR-abl encodes a fusion-product protein not expressed by normal cells, which thus could serve as a tumor-specific peptide, at least in CML, some cases of ALL and rare cases of AML. In vitro studies have found that peptides derived from BCR-abl protein can elicit autologous T cell responses that are class I restricted. A phase I clinical trial has evaluated a vaccine consisting of 4 peptides able to bind to HLA class I. The vaccine was studied in 12 patients with CML, 3 at each of 4 dose levels.30 Two of the 12 patients developed a possible delayed type hypersensitivity reaction to the vaccine following therapy and 3 showed proliferative responses when T cells were cultured with the peptides. However, no cytotoxic T cell responses could be detected.
A second fusion protein under study is the DEK-can protein derived from the (6;9) translocation sometimes seen in AML. In one study, a CD4-positive T cell line was shown able to kill an autologous B cell line pulsed with peptides derived from the fusion protein.31
Two non-mutated proteins highly overexpressed in AML cells have been the focus of a number of recent studies. PR3 is a neutral serine proteinase with expression largely restricted to the promyelocytic stage of myeloid differentiation. It is expressed by leukemic progenitors from patient with AML and CML but is minimally expressed by normal marrow progenitors. CD8-positive T cells specific for PR3 have been generated that can selectively lyse leukemic blasts but not normal bone marrow cells. Further, CD8-positive cytotoxic T cells specific for PR1, a peptide derived from PR3, were able to be isolated from the peripheral blood of patients with CML in remission after allogeneic transplantation or interferon.32 Based on these findings, a vaccine based on the PR1 peptide is now being evaluated in a phase I trial.
WT1 is a zinc finger transcription factor, abundantly overexpressed by most human leukemias including AML, CML and ALL. T cells can be generated that recognize WT1 peptides and lyse leukemic CD34-positive cells but not normal CD34-positive cells and inhibit growth of leukemic, but not normal, myeloid colonies.33 Antibodies to WT1 have been detected in the serum of some patients with AML suggesting, as in the case of PR3, that an active immune response to the antigen is not necessarily associated with obvious toxicities, making it a candidate for an adoptive T cell or vaccine strategy.
Whole cell vaccine strategies
An alternative to identifying specific antigens to use as targets for T cell clones or as vaccines is, instead, to vaccinate with genetically altered autologous tumor cells. Previous studies demonstrated that mice vaccinated with syngeneic tumors engineered to secrete GM-CSF develop particularly potent immune responses.34 Accordingly, studies have been conducted in murine models of AML testing the utility of AML cells transduced to secrete GM-CSF and found that such vaccines can improve cure rates in mice with established tumors treated with chemotherapy.35 Similarly, a vaccine composed of AML cells transduced with the gene for IL-12 has been found to have high activity in a similar murine model.36 Other studies have suggested that immune responses can be further enhanced by performing such vaccinations after autologous transplantation, perhaps because the transplant eliminates an inhibitory effect of the intact immune system.37
A related approach to the development of whole cell vaccines has been to culture peripheral blood mononuclear cells of leukemic patients with cytokines, including GM-CSF and IL-4, in an effort to induce the leukemic cells to assume characteristics of dendritic cells. These cells should theoretically become much more immunogenetic and thus could serve as tumor vaccines.38
IV. Modeling Human Leukemia in Vivo
John E. Dick, PhD*
Program in Cancer/Blood Research, The Hospital for Sick Children, 555 University Avenue, Toronto ONT M5G 1X8, Canada
Acknowledgments: Supported by grants from the Canadian Institutes for Health Research (CIHR), the National Cancer Institute of Canada (NCIC) with funds from the Canadian Cancer Society, the Canadian Genetic Disease Network of the National Centers of Excellence, and a CIHR Scientist award.
Our understanding of the leukemogenic disease process has, to a large extent, been formed from many decades of research on human subjects involving characterization of the cellular phenotype of acute leukemia and other aspects of the clinical picture. One of the major difficulties with this approach is the limited ability for experimental intervention in human subjects. Moreover, it is almost impossible to gain insight into the early events of the leukemogenic process before they become clinically apparent. Until the last decade, most experimental approaches have involved the study of naturally occurring animal (mostly murine) leukemia and experimentally induced disease following transgenic or gene knock-out methods. However, while many aspects of these murine leukemias recapitulate the human disease, there can be significant differences with the human disease. For example, some translocations that cause lymphoid diseases in humans (e.g. E2A-PBX, HOX 11) result in myeloid disease when expressed in mice. Moreover, marked differences in genomic stability between humans and inbred mice strains suggest that the leukemogenic process might be subtly different. Ultimately, one would like to complement murine experiments with model systems that utilize human leukemia to ensure that they are relevant to the human situation and that therapies based on this knowledge will have a higher likelihood of efficacy in humans. The transplantation of normal and leukemic human cells into immune-deficient mice provides such a system. This review will examine progress in using this xenograft model to characterize the leukemic clone, with particular emphasis on the identification of the leukemic stem cell in AML, and to develop novel therapeutic strategies.
Heterogeneity of Normal Human Stem Cells
The mammalian hematopoietic system is a hierarchy derived from stem cells that possess extensive self-renewal, proliferative, and differentiative capacity. Hematopoietic stem cells maintain the hematopoietic system throughout life, and stem cell regulation is a critical element in the control of normal hematopoiesis. The stem cell developmental program is tightly regulated by a combination of intrinsic factors as well as external stimuli such as soluble cytokines and contact with stroma. Disregulation of this tightly controlled developmental program as a consequence of aberrant expression of oncogenes results in leukemic proliferation. Thus, understanding the cellular and molecular factors that regulate the developmental program of normal stem cells and those that initiate proliferative diseases such as leukemia remains one of the major challenges in biology.
Hematopoietic stem cells possess extensive proliferation, differentiation, and self-renewal potential; properties that can only be conclusively examined by in vivo repopulation. The composition of the human hematopoietic stem cell compartment is poorly understood because of the historic absence of experimental tools to characterize the developmental program of individual stem cells. We have used repopulation of immune-deficient BNX, SCID and NOD/SCID mice to develop a quantitative assay for human stem cells that have been termed SCID-repopulating cells (SRC).1–,4 The key property that defines SRC is the potential for repopulation of multiple hematopoietic lineages. This system closely models conventional methods of human bone marrow transplantation and murine reconstitution assays of stem cell function. A detailed characterization of SRC is emerging in terms of frequency, cell surface phenotype, and cytokine responsiveness.5–,11 However, it is not known if the SRC assay detects a functionally homogenous population of stem cells or if, like the murine system, there is heterogeneity in the reconstitution potential of individual stem cells. The recent discovery of Lin-negative CD34-negative CD38-negative SRC and sheep-repopulating cells that appear to be precursors of the more numerous SRC from the Lin-negative CD34-positive CD38-negative fraction suggest phenotypic heterogeneity might exist in the human stem cell compartment.12,13
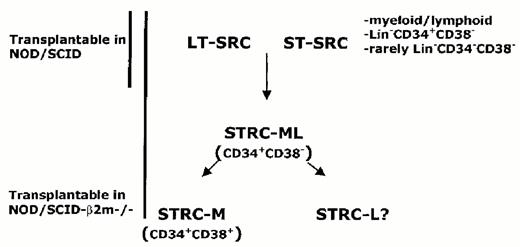
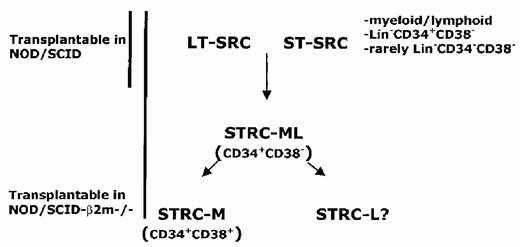
To understand the composition of the human hematopoietic stem cell compartment, we have tracked the in vivo fate of individual SRC during repopulation of NOD/SCID mice by analysis of the unique clonal markers that were introduced with retroviral vectors.14 The vector integration site provides a marker that is stably inherited by all progeny of an active stem cell. Analysis of serial bone marrow aspirations from NOD/SCID mice transplanted with transduced cord blood demonstrated that the repopulation was oligoclonal with extensive variability in self-renewal capacity as well as in the lifespan and proliferative capacity of individual SRC. Some clones only contributed for several weeks after the transplant and disappeared, while others appeared later and persisted. Secondary repopulation experiments demonstrated that there was heterogeneity in the self-renewal capacity of the transduced SRC. These data point to the existence of different classes of human stem cells with short- and long-term-repopulating capacity (ST- and LT-SRC, respectively (Figure 6 ).
Using NK cell-deficient, β2-microglobulin negative/negative/NOD-SCID mice, Glimm et al have found that short-term human repopulation can occur within the first several weeks following transplantation of Lin-negative CD34-positive CD38-positive cells.15 Unlike SRC, many of these short-term repopulating cells (STRC) were restricted to the myeloid lineage. Furthermore, Lin-negative CD34-positive CD38-positive cells cannot engraft NOD/SCID mice, pointing to a fundamental difference between STRC and SRC, which possess lympho-myeloid differentiation capacity. However, lin-negative CD34-positive CD38-positive cells can engraft short-term in NOD-SCID mice treated with anti-NK antibodies, similar to the β2-microglobulin/negative/negative NOD-SCID mice.44 Taken together with the findings of Guenechea et al,14 these findings provide convincing evidence that the stem cell compartment, as assayed by repopulation, is heterogeneous in terms of cell surface phenotype as well as in functional properties (Fig. 6).
Acute Myeloid Leukemia
AML, an impaired differentiation program results in the excess production of leukemic blasts, of which the vast majority have limited proliferative capacity.16 Therefore, the leukemic clone must be maintained by rare sub-populations of leukemic stem cells with extensive proliferative and self-renewal capacity.17,18 Thus, identification of AML stem cells is central to understanding the leukemogenic process, and their elimination is the only real target for effective therapy. The blasts from different patients are heterogeneous in their differentiation stage. The mechanism underlying this heterogeneity is unknown, as is the nature of the leukemic stem cells. Further, the identity of the target cell that undergoes leukemic transformation in AML has been controversial. Two major hypotheses have been put forward to explain heterogeneity in AML (reviewed in 4,19). One model (Figure 7; see color page 542 ) suggests that many different cell types in the stem/progenitor hierarchy are susceptible to transformation.17,20 The level of commitment of the target cell influences the characteristics of the resulting leukemic blast cells. This model predicts that the phenotype of the leukemic stem cells from patients with myelomonocytic blasts, for example, would be different from the leukemic stem cells from patients whose blasts expressed few lineage markers. The second model (Figure 8; see color page 542 ) suggests that critical mutations/translocations responsible for transformation and progression exert a leukemogenic effect only in HSC.18,21 The leukemic clone that arises is organized as a hierarchy with a leukemic stem cell generating clonogenic progenitors and leukemic blasts. According to this model, heterogeneity results from the variable ability of these primitive leukemic stem cells to differentiate or acquire lineage markers, depending upon the repertoire and direct influence of specific transformation or progression-related gene(s) and not on the degree of commitment of the target cell. This model predicts little variability in the phenotype of the leukemic stem cells among different patients. Another consequence of the initial transforming event(s) would be impairment of differentiation to other lineages (e.g. erythroid or lymphoid) emanating from the pluripotential stem cell target.
Xenotransplant Assay for Acute Myeloid Leukemia Stem Cells
Initial attempts to develop animal models for human leukemia involved subcutaneous transplantation of patient samples or leukemic cell lines into nude mice. This method created myelosarcomas or localized solid tumors, neither of which is characteristic of the disease. SCID-leukemia and NOD/SCID-leukemia models have been developed using the same paradigm as described above for normal human hematopoiesis. Leukemic cell transplantation successfully engrafts these mice and faithfully recapitulates the pathology of the original human leukemia. These systems have been applied to the study of most human leukemias, including AML, ALL, CML, and JCML (reviewed in 4).
Identification of the AML stem cell, based upon initiation of AML in immune-deficient mice (termed the SCID-Leukemia initiating cell, SL-IC), provides insight into the organization of the cells that comprise the leukemic clone and a means to rationally develop novel therapies directly targeted to the stem cell.21,22 SL-IC were found in the primitive CD34++CD38- fraction (similar to normal HSC) of AML blood regardless of the differentiation stage or FAB subtype of the leukemic blasts suggesting that the initial transformation events occur in HSC consistent with the model outlined in Figure 3. Moreover, we conclusively showed that the AML clone was organized as a hierarchy that is maintained by stem cells that undergo leukemic differentiation. Stem cells within the highly purified CD34-positive CD38-negative cell fraction from AML samples could differentiate, albeit abnormally, to generate a leukemic cell phenotype identical to the donor. Moreover, the dissemination characteristics of either M4/M5 samples (widespread to non-hematopoietic tissues) or M1 (limited to hematopoietic tissues) was reproduced, suggesting that the SL-IC assay can be used to study this mechanism.21,22 Limiting dilution assays showed that the frequency of SL-IC ranged from 0.2-100/106, while serial transplantation into secondary mice showed that the SL-IC possessed extensive self-renewal capacity, a key determinant of stem cells. It remains to be seen whether the SL-IC frequency correlates with patient response or outcome. Recent studies on a large number of AML samples found that secondary AML or primary AML that fail to respond to therapy engraft more extensively in NOD/SCID mice compared to primary samples.23 Moreover the most significant correlate to high level engraftment was WBC count of the donor sample, suggesting that the overall proliferative capacity of the SL-IC may vary greatly. Since the ability to detect an SL-IC depends on its proliferative capacity within the murine microenvironment, variance in frequency of SL-IC observed between patients may reflect proliferative capacity more than a true measure of actual stem cell frequency.23 Nevertheless, this quantitative assay enables the monitoring of patients undergoing drug therapy, cytokine therapy, purging, and BM transplantation by examining the effect of these treatments on leukemic stem cells.
Phenotype of SL-IC
While initial studies demonstrated phenotypic similarity between normal and leukemic stem cells, subtle differences in the cell surface phenotype that might be exploited clinically have recently been discovered. For example, SL-IC have been found within the CD34-positive Thy-1-negative and CD34-positive CD71-negative HLA-DR-negative sub-population, in contrast to normal HSC.24,25 Strikingly, the SL-IC appear to uniquely express IL-3 receptor α chain (CD123) while normal stem cells do not appear to express this marker.26 The biological significance of this result is not clear since this receptor does not appear to signal through the common pathways associated with this receptor. Expression of c-kit is usually associated with normal stem cells; however, transplantation studies showed that only CD34-positivec-kit-negative cells contained SL-IC.27 Again, the biological significance is not clear since the cells were able to respond to the c-kit ligand, SCF, suggesting that undetectable levels might still be present. Nevertheless, the surface expression of this receptor is clearly different between normal and leukemic stem cells. All of these studies provide new reagents to characterize the leukemic stem cell and suggest purification strategies for future purging experiments.
The significant enrichment of SL-IC makes possible a detailed analysis of the biology of these elusive cells. Progress along these lines is beginning. Gene expression analysis of highly purified normal and AML-derived CD34-positive CD38-negative cells has revealed a number of interesting conserved genes that were not expected to be expressed in neoplastic cells including the tumor-suppressor genes, interferon regulatory factor 1 (IRF-1) and death associated protein kinase (DAPK). These data are surprising in that pro-apoptotic factors are typically absent from malignant cells, indicating that IRF-1 and DAPK may play a role in the biology of early leukemogenic cells.45 Future studies need to focus on identification of the full expression profile of leukemic stem cells as compared with normal stem cells.
The discovery of CD34-negative HSC12,13 in normal hematopoietic tissues raises the possibility that this cell might also be a target of AML transformation and that SL-IC with this phenotype might exist. Conversely, these stem cells might not be the target for leukemic transformation and therefore represent a pool of normal stem cells that might be exploited clinically. A very small number of patients have been found to contain both CD34-negative and CD34-positive SL-IC,28 but it is not known if leukemic progression-related changes disregulated CD34 expression or if this reflects the existence of a primitive target cell. Recent studies using normal mouse hematopoietic cells have identified stem cells based on their efflux of Hoechst 33342 (termed side population or SP-positive).29 In the mouse, they are largely CD34(negative) and enriched for primitive progenitors and stem cells. Characterization of AML samples has revealed the existence of SP-positive CD34-negative cells that are part of the leukemic clone and have SL-IC activity.30,46 However, additional subfractionation has found that the SP-positive CD34-positive CD38-negative cells have SRC activity and are non-leukemic.30 These early studies point the way to interesting areas for future research both for leukemic stem cell biology and for their potential to identify differences between normal and leukemic stem cells that might be exploited clinically.
APL (FAB M3) may be a distinct entity and represent a real exception to the stem cell model. It appears that the leukemic stem cell in APL may not be derived from the primitive hematopoietic compartment because the PML-RARα fusion gene was present only in the CD34-positive CD38-positive population.31 Interestingly, APL cells do not appear to engraft SCID or NOD/SCID mice, supporting this idea.21,22 Thus, transformation at the level of committed myeloid progenitors (or STRC as illustrated Figure 6) may be the exception rather than the rule.
Therapeutic Strategies
The availability of a model that faithfully reproduces the human disease in mice provides a powerful tool to develop and test new approaches to AML treatment. There is little question that effective treatment must eliminate the AML stem cell or relapse will occur. A large literature is developing on the use of SCID and NOD/SCID-leukemia models to test new therapies. I will only focus on several selected examples here. The first key element of these approaches is the use of primary AML samples rather than cell lines that might have acquired alterations during establishment of the cell line that are not reflective the original donor. The second element is the use of the model in such a way as to point to killing of the leukemic stem cell.
The efficacy of allogeneic HSCT as a curative therapy for acute and chronic leukemia depends both on the intensive chemoradiotherapy administered prior to transplant and a GVL effect mediated by donor T cells. However, a significant fraction of patients with advanced leukemia at the time of HSCT will relapse due to persistence of leukemic progenitor cells. Escalation of the doses of chemoradiotherapy has not improved survival due to increased toxicity and efforts to augment the GVL effect after transplant such as by the administration of unselected donor lymphocytes or the administration of interleukin-2 were only partially effective and/or complicated by increased GVHD.32,33 Adoptive T cell immunotherapy targeting human minor histocompatibility (H) antigens expressed in recipient hematopoietic cells but not in nonhematopoietic cells such as skin fibroblasts and keratinocytes has been proposed as one strategy for inducing a GVL effect without causing GVHD34–,36 CD8-positive CTL clones specific for minor H antigens have been demonstrated to lyse a proportion of myeloid and lymphoid leukemic cells in vitro and inhibit the growth of clonogenic myeloid leukemic progenitor cells in methylcellulose culture.37–,39 These CD8-positive CTL clones inhibit the engraftment of human AML cells in NOD/SCID mice following short-term incubation with AML cells prior to transplant into NOD/SCID mice.40 The inhibition was mediated by direct CTL recognition of SL-ICs. These results implicate CD8-positive minor H antigen-specific CTL as mediators of the GVL effect associated with allogeneic HSCT, and provide an experimental model to identify and select T cell clones for immunotherapy to prevent or treat relapse after allogeneic HSCT.
Other approaches have involved the development of immunotoxins that target toxic molecules to the leukemic stem cell. Several groups have focused on the diphtheria toxin/GM-CSF receptor immunotoxin.41,42 These studies have indicated that the SL-IC expresses the GM-CSF molecule since SL-IC were specifically killed by treating the AML cells with these molecules.
Conclusions
Leukemic stem cells hold the key to understanding origin and maintenance of AML and for developing effective therapy. In the future, comparison of the gene expression profiles of highly purified fractions of normal and leukemic stem cell fractions should provide the basis for identification of leukemia-specific patterns of gene expression. It will be possible to determine the biological function of these newly discovered genes in terms of their ability to interfere with the normal developmental program of normal human stem cells, using experimental models where leukemia-associated oncogenes are expressed in primary human hematopoietic cells using retroviral vectors.43 Together, these approaches should identify novel targets for leukemic therapy, which can, in turn, be tested in vivo using the NOD/SCID-leukemia system.
Survival of almost 3,000 consecutive patients treated on ECOG protocols for newly diagnosed acute myeloid leukemia (AML) since 1973. The only exclusion from this curve is acute promyelocytic leukemia (APL) patients treated on all-trans retinoic acid (ATRA).
Survival of almost 3,000 consecutive patients treated on ECOG protocols for newly diagnosed acute myeloid leukemia (AML) since 1973. The only exclusion from this curve is acute promyelocytic leukemia (APL) patients treated on all-trans retinoic acid (ATRA).
Patients≤55 years with newly diagnosed acute myeloid leukemia (AML) treated on Eastern Cooperative Oncology Group (ECOG) protocols since 1973.
Patients≤55 years with newly diagnosed acute myeloid leukemia (AML) treated on Eastern Cooperative Oncology Group (ECOG) protocols since 1973.
Patients > 55 years with newly diagnosed acute myeloid leukemia (AML) treated on Eastern Cooperative Oncology Group (ECOG) protocols since 1973.
Patients > 55 years with newly diagnosed acute myeloid leukemia (AML) treated on Eastern Cooperative Oncology Group (ECOG) protocols since 1973.
Heterogeneity of transplantable stem cells from Glimm et al15 and Guenechea et al.14Long-term and short-term SCID-repopulating cells (LT-SRC and ST-SRC, respectively) are detected in NOD/SCID mice and generate another class of short-term repopulating cell (STRC) that can only be assayed in the NK cell deficient β2-microglobulin knock-out/NOD-SCID mouse or in anti-NK treated NOD/SCID mice. The STRC can generate either myelo-lymphoid cells (STRC-ML) or are restricted to either lineage, STRC-M or STRC-L.
Heterogeneity of transplantable stem cells from Glimm et al15 and Guenechea et al.14Long-term and short-term SCID-repopulating cells (LT-SRC and ST-SRC, respectively) are detected in NOD/SCID mice and generate another class of short-term repopulating cell (STRC) that can only be assayed in the NK cell deficient β2-microglobulin knock-out/NOD-SCID mouse or in anti-NK treated NOD/SCID mice. The STRC can generate either myelo-lymphoid cells (STRC-ML) or are restricted to either lineage, STRC-M or STRC-L.