Key Points
Analysis of the Japanese genome database showed no firm association between VWF pathogenic variants and phenotypic expression.
Estimating VWD prevalence directly based on the variant allele frequencies risks overestimation.
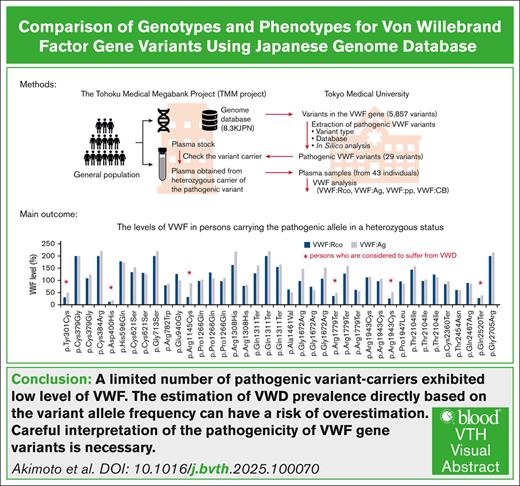
Visual Abstract
von Willebrand disease (VWD) is a common inherited bleeding disorder. The aim of this study was to determine the predicted disease states associated with various pathogenic von Willebrand factor (VWF) variants and their phenotypes using the largest Japanese whole-genome database. Of the 5857 VWF gene variants registered in the Japanese Multi-Omics Reference Panel (jMorp), variants with the following criteria were extracted: (1) caused protein abnormalities due to genetic alterations; (2) have already been detected and included in a database, including known association with VWD; and (3) highly likely pathogenic by in silico analysis. We measured VWF activity, antigen, propeptide, and collagen binding activity in stored plasma samples obtained from heterozygous carriers of the selected variants. A total of 29 VWF variants (26 single nucleotide and 3 small insertions/deletions) were detected, and 6 of these were found in Leiden Open Mutation Database. We obtained 43 plasma samples from individuals carrying these 29 variants as heterozygous. For the 43 variant carriers, their mean age was 43.0 years, and blood group was type O in 17 (39.5%). Analysis of these plasma samples showed low VWF levels (<50%) in 6 (14.0%). Low VWF levels were found in 2 of 8 of the nonsense (25%) and 4 of 31 of the missense variants (12.9%). Taking into consideration the limitation of using stored plasma samples, analysis of the jMorp indicated that most VWF gene variants with predicted pathogenic potential did not correlate with phenotypic expression. Our results supported incomplete penetrance and variable expressivity of the VWF gene variants.
Introduction
von Willebrand factor (VWF) plays a crucial role in primary and secondary hemostasis by facilitating adhesion of platelets to the wound site and acting as a carrier protein for coagulation factor VIII (FVIII).1,2 Quantitative and qualitative congenital abnormalities of VWF cause von Willebrand disease (VWD). VWD is classified into 3 subtypes according to phenotype.3-5 Types 1 and 3 represent quantitative abnormalities, with partial and complete deficiency of VWF, respectively, whereas type 2 represents qualitative abnormality with abnormal interactions between VWF and other molecules, such as platelet glycoprotein Ib, FVIII, ADAMTS13, and collagen. The diagnosis of VWD is confirmed when VWF ristocetin cofactor activity (VWF:Rco) or VWF antigen (VWF:Ag) is <30% or at 30% to 50% coupled with bleeding tendency.6
Identical genetic variation in different individuals, even between consanguineous individuals, is known to be associated with the manifestation of variety of phenotypes, ranging from subclinical to severe disease. This phenomenon is referred to as "incomplete penetrance," which describes a genotype that can either cause or not cause the expected phenotype. In contrast, "variable expressivity" refers to the same genetic variant that is associated with a spectrum of clinical manifestations of varying intensities. Several factors are thought to be responsible for incomplete penetrance and variable expressivity, including general genetic variants, variants in regulatory regions, epigenetic factors, environmental factors, and lifestyle.7 In type 1 VWD, the causative variant is usually fully penetrated in patients with markedly low VWF levels.8 The frequency of sequence variations in the VWF gene increases with decreases in VWF levels, whereas the bleeding score is independent of VWF levels.9 Consequently, the elucidation and diagnosis of VWD, particularly type 1, is challenging due to its heterogeneity.10-12
The estimated prevalence of VWD is ∼1%.3,13 Although the reported prevalence of symptomatic patients is 1 in 1000 in Western countries,14 only 1500 patients with VWD have been diagnosed in Japan by 2021,15 suggesting a prevalence rate of 0.012 per 1000 persons. The low prevalence in Japan may be due to a large number of undiagnosed cases, or it simply reflects a true low prevalence of VWD in Japanese.
Previous research on the prevalence of VWD was conducted in symptomatic patients and their families,16 limiting the value of these studies due to the narrow geographic scope and/or small sample size. Therefore, there is a need for large studies that include the general population.
Large-scale genetic studies have been conducted in the last 10 years in several Western countries,17-20 and phenotypic and genomic online databases are currently available, such as the Genome Aggregation Database (gnomAD).21 A similar Japanese genome study is the Tohoku Medical Megabank (TMM) project,22 which includes stored biological samples in addition to genomic data on >150 000 individuals with the general population. The results of various analyses of the TMM data sets are also available online.23,24 These databases provide useful information on a variety of inherited diseases, including VWD. Seidizadeh et al25 reported the results of their analysis of the prevalence and mutational landscape of VWD using gnomAD.
Using data available on the Japanese Multi-Omics Reference Panel (jMorp) and TMM project, this study was conducted to determine the true genotypic and phenotypic status of VWD in Japan, including prevalence. The results showed a relatively lower proportion of phenotypic defects than pathogenic variants, and this finding can be explained by incomplete penetrance and variable expressivity.
Materials and methods
Ethics
This study is a collaboration between Tokyo Medical University and Tohoku University, and it was approved by the ethics committees of both institutions in accordance with the Declaration of Helsinki. In this study, plasma samples, cohort information, and genome data were provided by the TMM Project (research number 2020-51).
Biobank
To determine the genetic variants of VWF gene, we used a database derived from the short-read whole genome sequencing of 8380 Japanese individuals (8.3KJPN), available on jMorp/TMM. Plasma samples from individuals heterozygous for the extracted pathogenic variants were obtained from the TMM, together with basic information about their age, sex, and blood group (no other clinical data are available in the database). For variants with high allele frequencies, we obtained 3 plasma samples from 3 different individuals (no plasma samples were available for the c.1312G>A variant). In addition, individuals with c.2771_2775delGGGTC also had c.2777_2778insTT, probably in a compound heterozygous (cis) status, and thus plasma was provided as c.2771_2775delGGGGTC. In total, 43 samples were provided from 27 different variants. All plasma samples from the TMM were fully anonymized.
Analysis of pathogenicity of variants
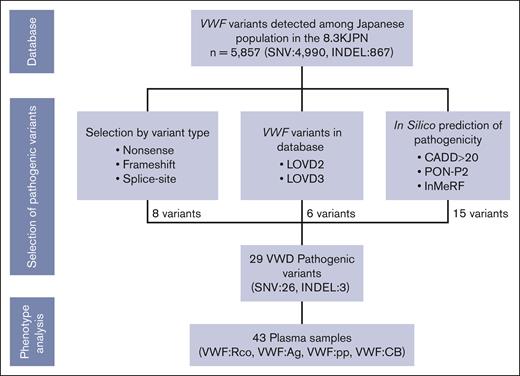
We investigated the pathogenicity of the 5857 variants that have been detected so far in the Japanese population and listed in the 8.3KJPN as of 2021 (Figure 1). Variants were considered pathogenic because they (1) caused protein abnormality due to genetic alterations, (2) were already detected and included in the database as associated with VWD, and (3) strongly suggested to be pathogenic by in silico analysis. The following are the extraction criteria for pathogenicity: (1) nonsense mutations and insertions or deletions resulting in frameshifts; (2) variants within the consensus sequence of the splice site; (3) variants registered in the Liden Open Variant Database (LOVD) version 2 (not available at present) and/or version 3 (accessed September 2020) and associated with VWD; (4) variants with a Combined Annotation Dependent Depletion score of >2026; and (5) missense and small-size insertion-deletion variants (indels) predicted to be pathogenic by both programs, based on results in silico analysis using Individual Meta Random Forest27 and Pathogenic-or-Not-Pipeline2.28 Ensembl Variant Effect Predictor (https://asia.ensembl.org/info/docs/tools/vep/index.html)29 was also referenced to assess the pathogenicity of the variants. In these analyses, GRCh37/hg19 was used as a reference. Registration status with ClinVar was also referenced.
Selection process of pathogenic VWF variants and related plasma samples from jMorp. The pathogenicity of the 5857 VWF variants registered in 8.3KJPN was assessed by registration in the database LOVD, in silico analysis of pathogenicity, and genetic aberrations that do not escape pathogenicity. Because some variants matched >1 criterion, the number of variants selected by each criterion was counted in order of “variant type” first, followed by “database,” and then “in silico prediction.” In total, 29 variants were considered pathogenic; and VWF:Rco, VWF:Ag, VWF:pp, and VWF:CB in 43 plasma samples obtained from the carriers of these variants were analyzed, and their relation to phenotype was investigated. CADD, combined annotation dependent depletion; InMeRF, individual meta random forest; PON-P2, Pathogenic-or-Not-Pipeline2.
Selection process of pathogenic VWF variants and related plasma samples from jMorp. The pathogenicity of the 5857 VWF variants registered in 8.3KJPN was assessed by registration in the database LOVD, in silico analysis of pathogenicity, and genetic aberrations that do not escape pathogenicity. Because some variants matched >1 criterion, the number of variants selected by each criterion was counted in order of “variant type” first, followed by “database,” and then “in silico prediction.” In total, 29 variants were considered pathogenic; and VWF:Rco, VWF:Ag, VWF:pp, and VWF:CB in 43 plasma samples obtained from the carriers of these variants were analyzed, and their relation to phenotype was investigated. CADD, combined annotation dependent depletion; InMeRF, individual meta random forest; PON-P2, Pathogenic-or-Not-Pipeline2.
Analysis of phenotypes
VWF:Rco and VWF:Ag were measured by BC von Willebrand reagent and VWF Ag reagent (Siemens Healthcare Diagnostics Inc, Marburg, Germany), respectively, using the automated coagulation analyzer, CS-2400 (Sysmex, Kobe, Japan), according to the instructions provided by the manufacturer. When the measured values of VWF:Rco and VWF:Ag were in excess of the upper limit of the measurement range (200% and 220%, respectively), the upper limit was used as the measurement. VWF propeptide (VWF:pp) was measured with a Senova von Willebrand Factor Propeptide ELISA Test Kit (Senova, Weimar, Germany), and VWF collagen binding activity (VWF:CB) was measured by Zymutest vWF:CBA (Hyphen, Neuville-sur-Oise, France), each using the instructions provided by the respective manufacturer. Based on previous studies, the threshold for VWF:pp/VWF:Ag and VWF:CB/VWF:Ag were set at 1.6 and 0.7, respectively.30,31
Validation of VWF:Rco and VWF:Ag measurements of EDTA-anticoagulated plasma
Sodium citrate–plasma samples are routinely used in the aforementioned VWF measurements. However, the plasma samples obtained from the biobank were prepared with disodium EDTA (EDTA-2Na). For this reason, we conducted a validation study to examine the effects of these 2 anticoagulants on the results of the aforementioned VWF assays. Specifically, we measured VWF levels in EDTA-2Na–plasma samples and sodium citrate–plasma samples obtained from 6 healthy donors and 9 patients with VWD (supplemental Figure 1A). The results showed that VWF:Rco and VWF:Ag in EDTA plasma were 1.23- and 1.22-fold higher than those in citrated plasma, respectively. The main reason for this difference can be explained by the fact that EDTA-2Na is used as a dry powder, whereas citrate is used as a solution and is mixed 1:9 with blood, resulting in a 1.1-fold dilution of the blood.
We also considered that the VWF levels obtained in our laboratory might be different from those obtained by commercial laboratory. To test for this, another preliminary study was conducted using 11 plasma samples provided by the biobank and 9 plasma samples from patients with VWD receiving care at our hospital (supplemental Figure 1B). The results indicated that the measurements of VWF:Rco and VWF:Ag measured by our laboratory personnel were 1.04- and 1.1-fold higher, respectively, than those of the commercial laboratory.
The abovementioned 2 validation studies confirmed the validity of the use of EDTA plasma and our laboratory measurements.
With regard to the measurements of VWF:pp and VWF:CB, preliminary experiments in 6 healthy donors showed that the values in EDTA plasma tended to be higher than those of citrate plasma, similar to our findings for VWF:Rco and VWF:Ag, although the extent of these differences varied considerably on an individual basis (supplemental Figure 1C). We concluded that the procedure used in our laboratory seemed to provide an approximate estimate of VWF:pp and VWF:CB plasma levels, although the measured values were not suitable for accurate disease typing applications.
Statistical analysis
Linear regression analysis was used to determine the correlation between 2 parameters. A P value of <.05 denoted statistical significance.
Results
Extraction of pathogenic variants from jMorp
The 8.3KJPN contained 5857 VWF variants, including 4990 single—nucleotide variants (SNVs) and 867 indels, in the VWF gene from whole-genome sequencing of 8380 individuals. In this analysis, considering the limitations due to the next-generation sequencing (NGS) principles, large-size variants were excluded from the analysis, and only SNVs and small indels were subjected to detailed analysis. Using the aforementioned criteria, 29 different variants were considered pathogenic, including 26 SNVs and 3 indels (Figure 1; Table 1). Further analysis showed 21 missense (72.4%), 4 nonsense (13.8%), 3 frameshift (10.3%), and 1 splice site (3.4%). Using the American College of Medical Genetics and Genomics guidelines, 17, including 14 SNVs and 3 indels, were classified as "pathogenic" and "likely pathogenic" variants (Table 2). Nine of these (31%) are already listed in the LOVD database. The number of variants associated with types 1, 2A, 2B, 2N, and 3 VWD were 2, 2, 2, 1, and 2, respectively (Table 1; supplemental Table 1).
Characteristics of pathogenic variants extracted from jMorp
| No. . | HGVS.c . | HGVS.p . | rs No. . | Allele frequency . | Allele count . | SIFT . | PolyPhen . | CADD . | PON-P2 . | InMeRF . | LOVD . | Selection criteria . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | c.902A>G | p.Tyr301Cys | — | 0.0001 | 1 | deleterious(0) | probably_damaging(0.999) | 25.3 | Pathogenic | Pathogenic | — | PON+InM |
| 2 | c.1135T>G | p.Cys379Gly | rs763461692 | 0.0001 | 1 | deleterious(0) | probably_damaging(0.972) | 26.7 | Pathogenic | Pathogenic | — | PON+InM |
| 3 | c.1150T>C | p.Cys384Arg | rs762146804 | 0.0001 | 1 | deleterious(0) | benign(0.303) | 23.7 | Pathogenic | Pathogenic | — | PON+InM |
| 4 | c.1198G>C | p.Asp400His | — | 0.0001 | 1 | deleterious(0) | probably_damaging(0.999) | 26.9 | Pathogenic | Pathogenic | — | PON+InM |
| 5 | c.1312G>A | p.Ala438Thr | rs756620872 | 0.0001 | 1 | deleterious(0) | probably_damaging(0.995) | 25.1 | Pathogenic | Pathogenic | — | PON+InM |
| 6 | c.1788T>A | p.His596Gln | 0.0001 | 1 | deleterious(0) | probably_damaging(0.985) | 23.3 | Pathogenic | Pathogenic | — | PON+InM | |
| 7 | c.1862G>C | p.Cys621Ser | rs886049744 | 0.0001 | 1 | deleterious(0.02) | probably_damaging(0.985) | 28 | Pathogenic | Pathogenic | — | PON+InM |
| 8 | c.2137G>A | p.Gly713Ser | rs1418751948 | 0.0001 | 1 | deleterious(0) | probably_damaging(0.999) | 27.5 | Pathogenic | Pathogenic | — | PON+InM |
| 9 | c.2344C>T | p.Arg782Trp | rs61748471 | 0.0001 | 1 | deleterious(0) | possibly_damaging(0.828) | 22.8 | — | Pathogenic | Type 2N | database |
| 10 | c.2771_2775delGGGTC | p.Arg924HisfsTer10 | — | 0.0001 | 1 | — | — | — | — | — | — | variant type |
| 11 | c.2777_2778insTT | p.Ile927SerfsTer16 | — | 0.0001 | 1 | — | — | — | — | — | — | variant type |
| 12 | c.2819A>G | p.Glu940Gly | rs1268610851 | 0.0001 | 1 | deleterious(0.01) | probably_damaging(0.985) | 33 | — | — | — | CADD>20 |
| 13 | c.3433C>T | p.Arg1145Cys | — | 0.0001 | 1 | deleterious(0) | probably_damaging(1) | 27.8 | Pathogenic | Pathogenic | — | PON+InM |
| 14 | c.3797C>A | p.Pro1266Gln | rs61749370 | 0.001 | 12 | deleterious(0.01) | probably_damaging(0.994) | 23.5 | — | Pathogenic | Type 2B | database |
| 15 | c.3923G>A | p.Arg1308His | rs61749388 | 0.0001 | 1 | tolerated(1) | benign(0) | 17.38 | — | Pathogenic | Type 2A | database |
| 16 | c.3931C>T | p.Gln1311Ter | rs267607337 | 0.0005 | 8 | — | — | 42 | — | — | Type 3 | variant type, database, CADD>20 |
| 17 | c.4382C>T | p.Ala1461Val | rs61750089 | 0.0001 | 1 | tolerated(0.32) | possibly_damaging(0.596) | 21.7 | — | Pathogenic | Type 2B | database |
| 18 | c.5014G>A | p.Gly1672Arg | rs61750598 | 0.0023 | 38 | tolerated(0.55) | benign(0) | 10.01 | — | Pathogenic | Type 2A | database |
| 19 | c.5093delG | p.Gly1698AlafsTer13 | — | 0.0001 | 1 | — | — | — | — | — | — | variant type |
| 20 | c.5335C>T | p.Arg1779Ter | rs61750606 | 0.0002 | 3 | — | — | 41 | — | — | Type 1 | variant type, database, CADD>20 |
| 21 | c.5620+2T>G | — | rs758879278 | 0.0001 | 1 | — | — | 34 | — | — | — | variant type, CADD>20 |
| 22 | c.5827C>T | p.Arg1943Cys | — | 0.0002 | 3 | deleterious(0) | probably_damaging(0.926) | 32 | Pathogenic | — | — | CADD>20 |
| 23 | c.5840C>T | p.Pro1947Leu | rs1312789020 | 0.0001 | 1 | deleterious(0) | probably_damaging(0.997) | 32 | Pathogenic | — | — | CADD>20 |
| 24 | c.6311C>T | p.Thr2104Ile | rs61750616 | 0.0033 | 56 | deleterious(0) | probably_damaging(0.997) | 25.7 | Pathogenic | — | Type 1 | database |
| 25 | c.7080C>A | p.Cys2360Ter | — | 0.0001 | 1 | — | — | 35 | — | — | — | variant type, CADD>20 |
| 26 | c.7361C>A | p.Thr2454Asn | rs200486416 | 0.0001 | 1 | deleterious(0) | probably_damaging(0.979) | 25.6 | Pathogenic | Pathogenic | — | PON+InM |
| 27 | c.7400A>G | p.Gln2467Arg | — | 0.0001 | 1 | deleterious(0) | possibly_damaging(0.739) | 26.2 | Pathogenic | Pathogenic | — | PON+InM |
| 28 | c.7558C>T | p.Gln2520Ter | rs1448479214 | 0.0001 | 1 | — | — | 40 | — | — | Type 3 | variant type, database, CADD>20 |
| 29 | c.8113G>A | p.Gly2705Arg | rs7962217 | 0.0001 | 1 | deleterious(0) | probably_damaging(0.999) | 32 | Pathogenic | — | — | CADD>20 |
| No. . | HGVS.c . | HGVS.p . | rs No. . | Allele frequency . | Allele count . | SIFT . | PolyPhen . | CADD . | PON-P2 . | InMeRF . | LOVD . | Selection criteria . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | c.902A>G | p.Tyr301Cys | — | 0.0001 | 1 | deleterious(0) | probably_damaging(0.999) | 25.3 | Pathogenic | Pathogenic | — | PON+InM |
| 2 | c.1135T>G | p.Cys379Gly | rs763461692 | 0.0001 | 1 | deleterious(0) | probably_damaging(0.972) | 26.7 | Pathogenic | Pathogenic | — | PON+InM |
| 3 | c.1150T>C | p.Cys384Arg | rs762146804 | 0.0001 | 1 | deleterious(0) | benign(0.303) | 23.7 | Pathogenic | Pathogenic | — | PON+InM |
| 4 | c.1198G>C | p.Asp400His | — | 0.0001 | 1 | deleterious(0) | probably_damaging(0.999) | 26.9 | Pathogenic | Pathogenic | — | PON+InM |
| 5 | c.1312G>A | p.Ala438Thr | rs756620872 | 0.0001 | 1 | deleterious(0) | probably_damaging(0.995) | 25.1 | Pathogenic | Pathogenic | — | PON+InM |
| 6 | c.1788T>A | p.His596Gln | 0.0001 | 1 | deleterious(0) | probably_damaging(0.985) | 23.3 | Pathogenic | Pathogenic | — | PON+InM | |
| 7 | c.1862G>C | p.Cys621Ser | rs886049744 | 0.0001 | 1 | deleterious(0.02) | probably_damaging(0.985) | 28 | Pathogenic | Pathogenic | — | PON+InM |
| 8 | c.2137G>A | p.Gly713Ser | rs1418751948 | 0.0001 | 1 | deleterious(0) | probably_damaging(0.999) | 27.5 | Pathogenic | Pathogenic | — | PON+InM |
| 9 | c.2344C>T | p.Arg782Trp | rs61748471 | 0.0001 | 1 | deleterious(0) | possibly_damaging(0.828) | 22.8 | — | Pathogenic | Type 2N | database |
| 10 | c.2771_2775delGGGTC | p.Arg924HisfsTer10 | — | 0.0001 | 1 | — | — | — | — | — | — | variant type |
| 11 | c.2777_2778insTT | p.Ile927SerfsTer16 | — | 0.0001 | 1 | — | — | — | — | — | — | variant type |
| 12 | c.2819A>G | p.Glu940Gly | rs1268610851 | 0.0001 | 1 | deleterious(0.01) | probably_damaging(0.985) | 33 | — | — | — | CADD>20 |
| 13 | c.3433C>T | p.Arg1145Cys | — | 0.0001 | 1 | deleterious(0) | probably_damaging(1) | 27.8 | Pathogenic | Pathogenic | — | PON+InM |
| 14 | c.3797C>A | p.Pro1266Gln | rs61749370 | 0.001 | 12 | deleterious(0.01) | probably_damaging(0.994) | 23.5 | — | Pathogenic | Type 2B | database |
| 15 | c.3923G>A | p.Arg1308His | rs61749388 | 0.0001 | 1 | tolerated(1) | benign(0) | 17.38 | — | Pathogenic | Type 2A | database |
| 16 | c.3931C>T | p.Gln1311Ter | rs267607337 | 0.0005 | 8 | — | — | 42 | — | — | Type 3 | variant type, database, CADD>20 |
| 17 | c.4382C>T | p.Ala1461Val | rs61750089 | 0.0001 | 1 | tolerated(0.32) | possibly_damaging(0.596) | 21.7 | — | Pathogenic | Type 2B | database |
| 18 | c.5014G>A | p.Gly1672Arg | rs61750598 | 0.0023 | 38 | tolerated(0.55) | benign(0) | 10.01 | — | Pathogenic | Type 2A | database |
| 19 | c.5093delG | p.Gly1698AlafsTer13 | — | 0.0001 | 1 | — | — | — | — | — | — | variant type |
| 20 | c.5335C>T | p.Arg1779Ter | rs61750606 | 0.0002 | 3 | — | — | 41 | — | — | Type 1 | variant type, database, CADD>20 |
| 21 | c.5620+2T>G | — | rs758879278 | 0.0001 | 1 | — | — | 34 | — | — | — | variant type, CADD>20 |
| 22 | c.5827C>T | p.Arg1943Cys | — | 0.0002 | 3 | deleterious(0) | probably_damaging(0.926) | 32 | Pathogenic | — | — | CADD>20 |
| 23 | c.5840C>T | p.Pro1947Leu | rs1312789020 | 0.0001 | 1 | deleterious(0) | probably_damaging(0.997) | 32 | Pathogenic | — | — | CADD>20 |
| 24 | c.6311C>T | p.Thr2104Ile | rs61750616 | 0.0033 | 56 | deleterious(0) | probably_damaging(0.997) | 25.7 | Pathogenic | — | Type 1 | database |
| 25 | c.7080C>A | p.Cys2360Ter | — | 0.0001 | 1 | — | — | 35 | — | — | — | variant type, CADD>20 |
| 26 | c.7361C>A | p.Thr2454Asn | rs200486416 | 0.0001 | 1 | deleterious(0) | probably_damaging(0.979) | 25.6 | Pathogenic | Pathogenic | — | PON+InM |
| 27 | c.7400A>G | p.Gln2467Arg | — | 0.0001 | 1 | deleterious(0) | possibly_damaging(0.739) | 26.2 | Pathogenic | Pathogenic | — | PON+InM |
| 28 | c.7558C>T | p.Gln2520Ter | rs1448479214 | 0.0001 | 1 | — | — | 40 | — | — | Type 3 | variant type, database, CADD>20 |
| 29 | c.8113G>A | p.Gly2705Arg | rs7962217 | 0.0001 | 1 | deleterious(0) | probably_damaging(0.999) | 32 | Pathogenic | — | — | CADD>20 |
The allele frequency expressed as "0.0001" is a rounded value to the fifth decimal place in the biobank; the actual value is "0.00006.”
CADD, Combined Annotation Dependent Depletion; HGVS, Human Genome Variation Society; HGVS.c, variant designations according to the HGVS nomenclature, based on coding DNA reference sequences; HGVS.p, variant designations according to HGVS nomenclature, based on protein reference sequence; InMeRF, Individual Meta Random Forest; PolyPhen, polymorphism phenotyping; PON-P2, Pathogenic-or-Not-Pipeline2; rs, reference SNP; SIFT, Sorting Intolerant from Tolerant.
Location and evaluation of pathogenicity of selected variants within the VWF gene
| No. . | HGVS.c . | HGVS.p . | VWF . | Novel . | ClinVar . | ACMG . | Seidizadeh et al25 . | ||
|---|---|---|---|---|---|---|---|---|---|
| Exon . | Pseudogene . | Domain . | |||||||
| 1 | c.902A>G | p.Tyr301Cys | 8 | — | D1 | — | — | Likely pathogenic | — |
| 2 | c.1135T>G | p.Cys379Gly | 10 | — | D1 | — | — | Uncertain significance | Yes |
| 3 | c.1150T>C | p.Cys384Arg | 10 | — | D1 | — | — | Likely pathogenic | Yes |
| 4 | c.1198G>C | p.Asp400His | 11 | — | D2 | Yes | — | Likely pathogenic | — |
| 5 | c.1312G>A | p.Ala438Thr | 12 | — | D2 | — | — | Uncertain significance | Yes |
| 6 | c.1788T>A | p.His596Gln | 15 | — | D2 | — | — | Likely pathogenic | — |
| 7 | c.1862G>C | p.Cys621Ser | 15 | — | D2 | — | Uncertain significance | Likely pathogenic | — |
| 8 | c.2137G>A | p.Gly713Ser | 16 | — | D2 | — | — | Uncertain significance | — |
| 9 | c.2344C>T | p.Arg782Trp | 18 | — | D' | — | Uncertain significance | Uncertain significance | Yes |
| 10 | c.2771_2775delGGGTC | p.Arg924HisfsTer10 | 21 | — | — | — | — | Likely pathogenic | — |
| 11 | c.2777_2778insTT | p.Ile927SerfsTer16 | 21 | — | — | — | — | Likely pathogenic | — |
| 12 | c.2819A>G | p.Glu940Gly | 21 | — | D3 | — | — | Likely pathogenic | — |
| 13 | c.3433C>T | p.Arg1145Cys | 26 | No | D3 | — | — | Uncertain significance | — |
| 14 | c.3797C>A | p.Pro1266Gln | 28 | No | D3 | — | — | Likely benign | Yes |
| 15 | c.3923G>A | p.Arg1308His | 28 | No | A1 | — | — | Likely pathogenic | Yes |
| 16 | c.3931C>T | p.Gln1311Ter | 28 | Yes | — | — | Pathogenic | Pathogenic | Yes |
| 17 | c.4382C>T | p.Ala1461Val | 28 | No | A1 | — | — | Likely pathogenic | — |
| 18 | c.5014G>A | p.Gly1672Arg | 28 | No | A2 | — | Uncertain significance | Likely benign | Yes |
| 19 | c.5093delG | p.Gly1698AlafsTer13 | 29 | No | — | — | — | Likely pathogenic | — |
| 20 | c.5335C>T | p.Arg1779Ter | 31 | No | — | — | Pathogenic | Pathogenic | Yes |
| 21 | c.5620+2T>G | — | — | No | — | — | — | Likely pathogenic | — |
| 22 | c.5827C>T | p.Arg1943Cys | 34 | No | D4 | Yes | — | Uncertain significance | — |
| 23 | c.5840C>T | p.Pro1947Leu | 34 | No | D4 | — | — | Pathogenic | — |
| 24 | c.6311C>T | p.Thr2104Ile | 37 | — | D4 | — | Uncertain significance | Uncertain significance | Yes |
| 25 | c.7080C>A | p.Cys2360Ter | 41 | — | — | — | Pathogenic | Pathogenic | — |
| 26 | c.7361C>A | p.Thr2454Asn | 43 | — | C3 | — | — | Uncertain significance | Yes |
| 27 | c.7400A>G | p.Gln2467Arg | 43 | — | C3 | — | — | Uncertain significance | — |
| 28 | c.7558C>T | p.Gln2520Ter | 45 | — | — | — | — | Likely pathogenic | Yes |
| 29 | c.8113G>A | p.Gly2705Arg | 49 | — | C6 | — | Benign/Likely benign | Benign | — |
| No. . | HGVS.c . | HGVS.p . | VWF . | Novel . | ClinVar . | ACMG . | Seidizadeh et al25 . | ||
|---|---|---|---|---|---|---|---|---|---|
| Exon . | Pseudogene . | Domain . | |||||||
| 1 | c.902A>G | p.Tyr301Cys | 8 | — | D1 | — | — | Likely pathogenic | — |
| 2 | c.1135T>G | p.Cys379Gly | 10 | — | D1 | — | — | Uncertain significance | Yes |
| 3 | c.1150T>C | p.Cys384Arg | 10 | — | D1 | — | — | Likely pathogenic | Yes |
| 4 | c.1198G>C | p.Asp400His | 11 | — | D2 | Yes | — | Likely pathogenic | — |
| 5 | c.1312G>A | p.Ala438Thr | 12 | — | D2 | — | — | Uncertain significance | Yes |
| 6 | c.1788T>A | p.His596Gln | 15 | — | D2 | — | — | Likely pathogenic | — |
| 7 | c.1862G>C | p.Cys621Ser | 15 | — | D2 | — | Uncertain significance | Likely pathogenic | — |
| 8 | c.2137G>A | p.Gly713Ser | 16 | — | D2 | — | — | Uncertain significance | — |
| 9 | c.2344C>T | p.Arg782Trp | 18 | — | D' | — | Uncertain significance | Uncertain significance | Yes |
| 10 | c.2771_2775delGGGTC | p.Arg924HisfsTer10 | 21 | — | — | — | — | Likely pathogenic | — |
| 11 | c.2777_2778insTT | p.Ile927SerfsTer16 | 21 | — | — | — | — | Likely pathogenic | — |
| 12 | c.2819A>G | p.Glu940Gly | 21 | — | D3 | — | — | Likely pathogenic | — |
| 13 | c.3433C>T | p.Arg1145Cys | 26 | No | D3 | — | — | Uncertain significance | — |
| 14 | c.3797C>A | p.Pro1266Gln | 28 | No | D3 | — | — | Likely benign | Yes |
| 15 | c.3923G>A | p.Arg1308His | 28 | No | A1 | — | — | Likely pathogenic | Yes |
| 16 | c.3931C>T | p.Gln1311Ter | 28 | Yes | — | — | Pathogenic | Pathogenic | Yes |
| 17 | c.4382C>T | p.Ala1461Val | 28 | No | A1 | — | — | Likely pathogenic | — |
| 18 | c.5014G>A | p.Gly1672Arg | 28 | No | A2 | — | Uncertain significance | Likely benign | Yes |
| 19 | c.5093delG | p.Gly1698AlafsTer13 | 29 | No | — | — | — | Likely pathogenic | — |
| 20 | c.5335C>T | p.Arg1779Ter | 31 | No | — | — | Pathogenic | Pathogenic | Yes |
| 21 | c.5620+2T>G | — | — | No | — | — | — | Likely pathogenic | — |
| 22 | c.5827C>T | p.Arg1943Cys | 34 | No | D4 | Yes | — | Uncertain significance | — |
| 23 | c.5840C>T | p.Pro1947Leu | 34 | No | D4 | — | — | Pathogenic | — |
| 24 | c.6311C>T | p.Thr2104Ile | 37 | — | D4 | — | Uncertain significance | Uncertain significance | Yes |
| 25 | c.7080C>A | p.Cys2360Ter | 41 | — | — | — | Pathogenic | Pathogenic | — |
| 26 | c.7361C>A | p.Thr2454Asn | 43 | — | C3 | — | — | Uncertain significance | Yes |
| 27 | c.7400A>G | p.Gln2467Arg | 43 | — | C3 | — | — | Uncertain significance | — |
| 28 | c.7558C>T | p.Gln2520Ter | 45 | — | — | — | — | Likely pathogenic | Yes |
| 29 | c.8113G>A | p.Gly2705Arg | 49 | — | C6 | — | Benign/Likely benign | Benign | — |
“Seidizadeh et al” indicates whether described in the study of Seidizadeh et al,25 which was conducted using similar analysis.
ACMG, American College of Medical Genetics and Genomics variant classification.
Allele frequencies
Table 1 summarizes the allele frequencies of each of the identified 29 VWF variants. The minimum allele frequency was 0.0001. However, this value was rounded to the fifth decimal point at the biobank, indicating that 1 variant allele was present among ∼16 760 alleles analyzed. Consequently, the actual value is 0.00006. Most variants were present at a frequency of 1 allele within the analyzed population. However, the frequencies of the 6 variants (p.Pro1266Gln, p.Gln1311Ter, p.Gly1672Arg, p.Arg1779Ter, p.Arg1943Cys, and p.Thr2104Ile) were 0.001, 0.0005, 0.0023, 0.002, 0.002, and 0.0033, respectively. A review of the biobank data confirmed that all selected variants were present in the heterozygous state.
Phenotype
Supplemental Table 1 shows the VWF-related measurements of plasma samples examined in this study, along with the age, sex, and blood groups of the individuals. The VWF measurements were in general higher than anticipated. We selected the 29 variants that could cause VWD and analyzed 43 plasma samples derived from the carriers of these variants. Contrary to expectations, the VWF:Rco and VWF:Ag levels were <50% in only 6 samples (Figure 2A; supplemental Table 1). The 6 samples with VWF levels <50% were collected from individuals with blood group O. The variants present in the 6 samples with low VWF levels were 4 missense (3 with substitutions to cysteine residues) and 2 nonsense variants. Finally, 2 of the 6 were novel. Although VWF levels were >50%, the VWF:Rco/VWF:Ag ratio in 1 individual with p.Arg1308His and 2 individuals with p.Gly1672Arg was ∼0.7, suggesting type 2 VWD (supplemental Table 1).
VWF levels for each variant. (A) Plasma samples with <50% VWF:Rco were considered diagnostic for VWD. Two of 6 samples were nonsense variants, and the remaining were missense variants. Of the 4 missense variants, 3 showed amino acid substitution to cysteine. Two missense variants, p.Asp400His and p.Arg1943Cys, were novel. (B) VWF levels for missense variants in plasma samples obtained from 3 different individuals. VWF levels were different in different plasma samples, even for those with the same variant. (C) VWF levels for nonsense variants. We identified a number of cases with nonsense variants who did not have low VWF levels. Even for those with the same variant, VWF levels varied widely from sample to sample. For cases with VWF:Rco of >200% and VWF:Ag of >220%, their values were entered as 200% and 220%, respectively.
VWF levels for each variant. (A) Plasma samples with <50% VWF:Rco were considered diagnostic for VWD. Two of 6 samples were nonsense variants, and the remaining were missense variants. Of the 4 missense variants, 3 showed amino acid substitution to cysteine. Two missense variants, p.Asp400His and p.Arg1943Cys, were novel. (B) VWF levels for missense variants in plasma samples obtained from 3 different individuals. VWF levels were different in different plasma samples, even for those with the same variant. (C) VWF levels for nonsense variants. We identified a number of cases with nonsense variants who did not have low VWF levels. Even for those with the same variant, VWF levels varied widely from sample to sample. For cases with VWF:Rco of >200% and VWF:Ag of >220%, their values were entered as 200% and 220%, respectively.
Missense variants
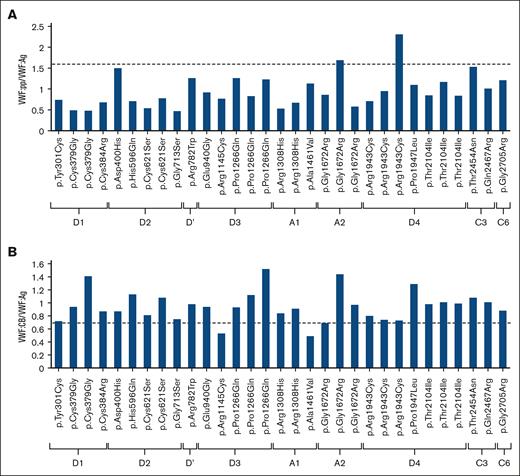
A total of 31 missense variants of 20 different kinds in the VWF gene were examined and characterized. Supplemental Table 1 summarizes the results of VWF-related tests, and Figure 3 provides the VWF:pp/VWF:Ag and VWF:CB/VWF:Ag ratios for individual missense variants. For the VWF:pp/VWF:Ag ratio, a measure of VWF clearance, only 1 of the 3 samples each for p.Gly1672Arg and p.Arg1943Cys exceeded the threshold of 1.6. The VWF:CB/VWF:Ag ratio, which serves as an indicator of the collagen-binding capacity of VWF, was below the threshold value of 0.7 in 4 samples (p.Arg1145Cys, p.Gln1311Ter, p.Ala1461Val, and p.Gly1672Arg). The collagen-binding sites of the VWF molecule are located in the A1 and A3 domains. The value of VWF:CB/VWF:Ag ratio not only confirms impaired binding of VWF to collagen but also the loss of high or medium molecular weight VWF multimers. Thus, this ratio is basically reduced in type 2 VWD cases with abnormal multimers. In this study, however, the ratio was below the threshold in only a minority of the variants previously reported to cause type 2 VWD.
Location of each variant analyzed and its respective VWF:pp/VWF:Ag and VWF:CB/VWF:Ag ratios. (A) The VWF:pp/VWF:Ag ratio of the Gly1672Arg and Arg1943Cys variants was >1.6, suggesting abnormality with enhanced clearance. (B) The VWF:CB/VWF:Ag ratios of Arg1145Cys and Ala1461Val were below the threshold value of 0.7, suggesting molecular abnormalities.
Location of each variant analyzed and its respective VWF:pp/VWF:Ag and VWF:CB/VWF:Ag ratios. (A) The VWF:pp/VWF:Ag ratio of the Gly1672Arg and Arg1943Cys variants was >1.6, suggesting abnormality with enhanced clearance. (B) The VWF:CB/VWF:Ag ratios of Arg1145Cys and Ala1461Val were below the threshold value of 0.7, suggesting molecular abnormalities.
We obtained plasma samples for 4 missense variants, p.Pro1266Gln, p.Gly1672Arg, p.Arg1943Cys, and p.Thr2104Ile, from each of the 3 individuals with high allele frequencies. Figure 2B compares the VWF levels of these individuals. Among the 3 samples of p.Gly1672Arg, the VWF:Rco/VWF:Ag values in 2 samples were ∼0.7, suggesting molecular abnormalities, whereas the VWF:Rco level was not below the level recorded in VWD (supplemental Table 1; Figure 2B). In 1 of these 3 cases, the VWF:CB/VWF:Ag ratio was low, indicating an abnormal collagen-binding capacity. Among the 3 with p.Arg1943Cys, only 1 individual (no. 34) had a low VWF level and a high VWF:pp/VWF:Ag ratio (ratio = 2.31). This finding is suggestive of hyper-clearance type VWD. However, the 2 samples with the same p.Arg1943Cys showed no abnormalities in all VWF-related measurements (supplemental Table 1; Figure 2B). The results demonstrate that even individuals who are heterozygous for the identical variant can exhibit dissimilar VWF phenotypes.
Nonsense variants
As mentioned earlier, 4 nonsense variants were considered pathogenic. Three of these have been identified previously in patients with type 1 or type 3 VWD and registered in the LOVD database. Analysis of the VWF levels in 8 nonsense variant carriers showed a VWF:Rco of <50% in only 2 samples (Figure 2C). Furthermore, for p.Gln1311Ter, the VWF levels were not decreased in any of the samples. In addition, as with the missense variant, there were clear individual differences in VWF levels despite sharing the same variant.
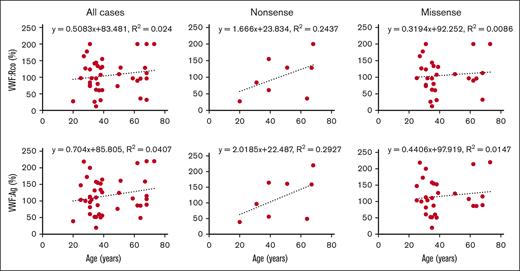
Effect of age, sex, and blood group on phenotype expression
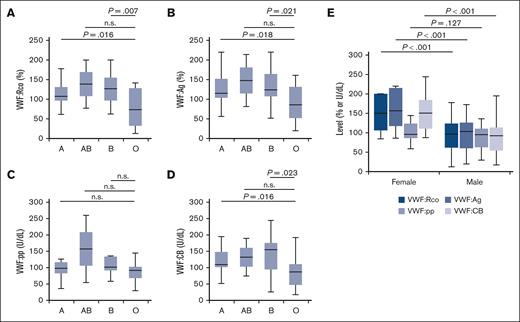
Because most VWF gene variants showed poor correlation with phenotype expression, we evaluated the combined effects of age and blood group on VWF:Rco and VWF:Ag. The average age of donors was 43.0 years (median, 37.0 years [interquartile range, 31.0-60.5]; age was unknown for 2 donors). Age did not correlate with VWF levels (Figure 4). The numbers of participants with blood groups A, B, AB, and O were 14, 9, 3, and 17, respectively. Given that the most prevalent blood group in the Japanese population is group A, representing ∼40% of the population,32 it is clear that blood group O was the most frequent among our participants (supplemental Table 1). Further analysis showed that participants with group O had significantly lower levels of VWF:Rco, VWF:Ag, and VWF:CB than the other groups (Figure 5A-B,D). A similar trend was observed for VWF:pp, although the difference was not statistically significant (Figure 5C). Further analysis showed significantly higher levels of VWF:Rco, VWF:Ag, and VWF:CB in females than males (Figure 5E).
Correlation between age and plasma VWF levels. The correlation between age and VWF levels was examined in all cases and those with missense and nonsense variants.
Correlation between age and plasma VWF levels. The correlation between age and VWF levels was examined in all cases and those with missense and nonsense variants.
Correlation of blood group and sex with plasma levels of VWF. (A-D) Correlation between blood groups and VWF-related measurements in plasma samples obtained from the donors. The number of individuals with blood groups A, AB, B, and O were 14, 3, 9, and 17, respectively. (E) Correlation between sex and VWF-related measurements. Statistical analysis was performed using a 2-tailed t test. n.s., not significant.
Correlation of blood group and sex with plasma levels of VWF. (A-D) Correlation between blood groups and VWF-related measurements in plasma samples obtained from the donors. The number of individuals with blood groups A, AB, B, and O were 14, 3, 9, and 17, respectively. (E) Correlation between sex and VWF-related measurements. Statistical analysis was performed using a 2-tailed t test. n.s., not significant.
Discussion
The unique and important advantages of this study are as follows: (1) VWF gene variants were not obtained from diagnosed patients but rather the general population using a Japanese genomic database; and (2) direct comparison of genotypes and phenotypes was based on analysis of stored plasma samples.
A recent international study that used an approach similar to that of our study through analysis of the gnomAD identified 505 variants (among 4313 variants) in the VWF gene that were considered VWD pathogenic or were already known to be associated with VWD.25 The study also reported that the global prevalence rates of the dominantly inherited VWD types 1, 2A, 2B, and 2M were 74, 3, 3, and 6 per 1000 persons, respectively. In addition, they stated that the prevalences of recessively inherited VWD types 2N and 3 were 0.31 and 0.7, respectively. In our study, all of the 29 variants that were considered pathogenic were identified in the heterozygous state, which brought the total number of carriers of the variants to ∼143 persons (Table 1). Therefore, the estimated prevalence of “genetic” VWD is 143 per 8300 persons, that is, ∼17 per 1000 persons. Although this is a rough estimate and the exact prevalence of VWD needs to be determined more carefully, the results suggest that the prevalence of VWD in the Japanese population is lower than that in the global population. However, this prevalence is merely the rate of carried alleles that may be pathologic variants, and the actual prevalence of VWF abnormalities is probably less than this rate. In our study, only 6 individuals had low VWF levels of <50%. Because our validation studies showed that VWF:Rco and VWF:Ag levels were 1.23 and 1.22 times higher in EDTA plasma than citrate plasma, respectively, we suspect that the true prevalence of low VWF levels is slightly higher than our estimates. Furthermore, because our laboratory-measured values in EDTA plasma were 1.04 times higher for VWF:Rco and 1.10 times higher for VWF:Ag than those of the commercial laboratory, the estimated values measured in citrate plasma by the private laboratory were 1.28 (1.23 × 1.04) times lower for VWF:Rco and 1.34 (1.22 × 1.1) times lower than our EDTA-based values. This means that the VWF:Rco measurements in up to 64% and VWF:Ag measurements in up to 67% were likely <50%. Even when we applied these adjustment values to all samples, the values of VWF:Rco and VWF:Ag were <50% in only 10 of 43 samples. Thus, the estimated “phenotypic” VWD prevalence is ∼0.72 or 1.2 per 1000 persons, because the rate is 6 or 10 per 8300 persons. Moreover, the percentage of those who actually manifest a bleeding tendency and require treatment is even lower.33,34 Based on the earlier discussion, it seems that calculating the true prevalence rate is a challenging endeavor. Type 1 VWD may be particularly challenging because of the large number of cases and the difficulty of diagnosis. In any case, the prevalence of VWD in Japan as currently understood, either genetically or phenotypically, is remarkably low compared to other racial groups.
VWD is well-known for incomplete penetrance and variable expressivity, probably due to its multigenic or polygenic disease nature. In addition, the clinical presentation is often different despite the same disease type. Especially in type 1 VWD, incomplete penetrance and variable expressivity often make it difficult to ascertain the phenotype.35-37 In contrast to type 1, the phenotype of type 2 VWD is usually considered fully penetrant.36,37 In our study, however, complete penetration was observed only in a limited number of cases, regardless of the type of VWD. There are already several reports of similar findings, for example, family members with the same pathogenic variant presenting with different phenotypes and bleeding tendencies.12,38 This variation may be due to factors other than VWF gene variants, such as blood group, age, hormones, exercise, menstrual cycle, and stress, all of which contribute to the expression of VWF.12,35 Among these factors, blood type O has a significant impact on VWD, because this group reduces the level of VWF by ∼30%.39 It should be noted that the activity and antigen levels of VWF measured using plasma EDTA-2Na overestimates the level of VWF, and it may also influence other parameters. Further work is needed to determine the extent of penetration and variability of expression.
We also assessed the correlation between age and VWF levels. Previous studies reported a close correlation between age and plasma VWF levels, with 20% to 30% increase in VWF levels per decade with advancing age.39-41 In this study, the number of individuals exhibiting a clear reduction in VWF levels despite carrying pathogenic variants was smaller than anticipated. This outcome may be related to the age of the study participants (average age, 43 years). The inclusion of younger population in the study could have influenced the results; the number of individuals presenting with abnormal VWF values would have been somewhat higher. Furthermore, there was no relation between age and either VWF:Ag or VWF:Rco. In this regard, 2 scenarios could be considered. First, a positive correlation exists between age and VWF levels; however, the presence of pathogenic variants may disrupt this correlation. Comparison of the missense and nonsense cases showed that the latter exhibited a slightly stronger correlation, albeit a marginal one. This may be attributed to the fact that simple quantitative abnormalities, which do not give rise to qualitative abnormalities, are more indicative of age-related increases. Second, the timing of blood sampling may have not been conducted at the donor's native VWF level. Thus, there is a need to determine VWF level several times in the same individual.
Our study included 20 plasma samples from 9 different variants that are already known to be associated with VWD and are deposited in the LOVD database. These were 3 types of nonsense and 6 types of missense variants. Among these, only 2 nonsense samples showed a clear decrease in VWF. This result is surprising and raises concerns about the reliability of our measurements. Admittedly, the condition of the plasma and time of collection may not be optimal. However, to date, the general analysis of VWD has so far been performed in persons with abnormal phenotypes. Therefore, although there are reports of families of specific variants,42 the phenotype of persons with pathogenic variants in the general population is not known.
The VWF molecule contains 234 cysteine residues, and this number is 4 times higher than that of a typical human protein.43 Although there are different views on the number of free cysteine residues, there is agreement on their importance in intramolecular conformation and intermolecular interactions. The loss or gain of cysteine residues in VWF genetic variants is likely to have a significant effect on the formation of Weibel-Palade bodies and the secretion and clearance of VWF.44 In this study, we identified 6 missense variants with a clear reduction in VWF, and 3 showed substitution to cysteine residues. Furthermore, the 3 variants that showed substitution to cysteine exhibited a larger difference between VWF:Rco and VWF:Ag than the other 3 missense variants, suggesting that they exhibit molecular abnormality (Figure 2A). p.Tyr301Cys is located in the D1 domain of VWF. This domain is part of the prepropeptide, and only a few pathologic variants are located in this part of the VWF. Although p.Tyr301Cys is not listed in the LOVD, it has been reported as a variant causing quantitative abnormalities of both type 1 and type 3.45 However, in our cases, the VWF:Rco/VWF:Ag ratio was low at 0.61, suggesting that there may be some other factor(s) causing the qualitative abnormalities. p.Arg1145Cys is located in the D3 domain of VWF. This variant is also not listed in LOVD; however, it has been detected in type 2A VWD.45 Because the D3 domain, together with the D' domain, is the binding site of FVIII, it was thought that this variant might possibly show abnormal binding to FVIII. However, confirmation of the D'D3 domain in complex with FVIII confirmed that the site of this variant is different from the site of direct binding to FVIII (data not shown). Instead, abnormal binding to collagen was suspected. Because the collagen-binding domains of VWF are A1 and A3,46,47 cysteine arising from this variant may affect the structure of the A1 domain. p.Arg1943Cys is located in the D4 domain of VWF and is a novel pathogenic variant. The D4 domain has no documented interactions with other molecules. Although variants that cause quantitative abnormalities have been documented in this domain, this region has been relatively uncommon in pathogenic variant reports. We checked the phenotype of 3 persons with this variant, and only 1 showed an abnormality. A low VWF:Rco/VWF:Ag ratio and a high VWF:pp/VWF:Ag ratio were observed in this person, suggesting the combination of type 2 and type 1C elements. In the variants in which the amino acid is substituted with cysteine, molecular aberrant features may be more likely to appear in the phenotype.
This study has certain limitations. First, the number of registered population in jMorp/TMM was not very large. In addition, most participants were residents of the Tohoku region, which could introduce some bias and may limit the generalization of the data to all Japanese. Second, the plasma used for phenotypic analysis may not be truly appropriate for this study, mainly due to the use of EDTA-2Na as an anticoagulant. Third, the study was based on a single time point. Accurate characterization of the phenotype of an individual requires multiple measurements using blood samples withdrawn at different time points. Fourth, because no information regarding the bleeding phenotype was available in the biobank data, our study did not diagnose VWD subtypes but solely determined VWF levels. Furthermore, VWF activity was evaluated by VWF:Rco instead of VWF:GPIbM and VWF:GPIbR, which were not available in Japan. We cannot deny the impact of polymorphisms affecting VWF binding to ristocetin, such as p.Asp1472His. Although information on this well-known variant in the individuals analyzed in this study was not available in the biobank data, its allele frequency in the Japanese is 0.111847 (accessed March 2025) which is close to that in the European population. Fifth, we cannot exclude the possibility of c.3931C>T; p.Gln1311Ter variant as a pseudogenic sequence. Finally, several tools were used to predict pathogenicity, which resulted in the identification of a somewhat higher proportion of disease phenotypes.
In conclusion, our analysis of the Japanese database demonstrated incomplete penetrance and variable expressivity of VWF gene variants. A high frequency of the variant allele that can cause VWD was noted; however, a limited number of variant carriers exhibited low levels of VWF. Careful interpretation of the pathogenicity of VWF gene variants is necessary. Estimation of VWD prevalence directly based on variant allele frequency may risk overestimation.
Acknowledgments
The authors express their sincere gratitude to the people of Japan and those worldwide for their support to the Great East Japan Earthquake–affected areas after the disaster. The authors thank the members of the Tohoku Medical Megabank Organization (ToMMo), including the Genome Medical Research Coordinators, and the office and administrative personnel for their assistance. The authors are grateful to everyone who participated in or worked with the cohort to make this study possible. A complete list of members is available at https://www.megabank.tohoku.ac.jp/english/a220901/.
The ToMMo and Tohoku Medical Megabank projects were supported, in part, by grants from the Reconstruction Agency; Ministry of Education, Culture, Sports, Science and Technology; and Japan Agency for Medical Research and Development (JP17 km0105001, JP 21tm0124005, and JP21tm0424601).
Authorship
Contribution: T.A. performed the analysis, interpreted the results, and drafted the manuscript; H.I. designed the study, performed the analysis, interpreted the results, and drafted the manuscript; S.O. and K.K. contributed to the distribution of plasma samples; A.M. and K.S. provided support for blood coagulation experiments; R.M., T.Y., M.B., Y.C., T.H., and K.A. participated in the discussion and interpretation of the results from a clinical perspective; E.N.K. monitored the progress of the study and provided important discussion and suggestions about the study and results; and E.K. supervised the entire study, interpreted the results, and provided critical suggestions in the preparation of the manuscript.
Conflict-of-interest disclosure: T.A. is an employee of BioMarin Pharmaceutical Japan K.K. H.I. has received speaker honoraria from Bayer, Sanofi, Novo Nordisk Pharma, and CSL Behring. A.M. is the holder of an endowed chair at the Department of Gene Research of Coagulation Disorders at Tokyo Medical University, which received funding from CSL Behring. R.M. has received honoraria from Chugai Pharmaceutical, Sanofi, Bayer, Takeda Pharmaceutical, Novo Nordisk Pharma, KM Biologics, Fujimoto Pharmaceutical, and CSL Behring. T.Y. has received honoraria from Chugai Pharmaceutical, Novo Nordisk Pharma, Bayer, Takeda Pharmaceutical, CSL Behring, Fujimoto Pharmaceutical, and Sanofi. Y.C. has received honoraria from Chugai Pharmaceutical, Sanofi, Bayer, Takeda Pharmaceutical, Novo Nordisk Pharma, Pfizer, KM Biologics, Japan Blood Products Organization, Fujimoto Pharmaceutical, and CSL Behring. M.B. has received funding for research as a principal investigator from Chugai Pharmaceutical; and honoraria from Chugai Pharmaceutical, Sanofi, Bayer, Takeda Pharmaceutical, Novo Nordisk Pharma, Pfizer, KM Biologics, Fujimoto Pharmaceutical, and CSL Behring. K.S. has received honoraria from Sanofi, Bayer, Takeda Pharmaceutical, Novo Nordisk Pharma, Chugai Pharmaceutical, CSL Behring, Pfizer, and Japan Blood Products Organization. T.H. has received research grant from Sanofi; and honoraria from Chugai Pharmaceutical, Sanofi, Takeda Pharmaceutical, Pfizer, KM Biologics, Japan Blood Products Organization, and CSL Behring. K.A. has received honoraria from Chugai Pharmaceutical, Sanofi, Bayer, Takeda Pharmaceutical, Novo Nordisk Pharma, Pfizer, KM Biologics, Japan Blood Products Organization, Fujimoto Pharmaceutical, and CSL Behring. E.K. has received research grants from Chugai Pharmaceutical and CSL Behring; and honoraria from Chugai Pharmaceutical, Sanofi, Bayer, Takeda Pharmaceutical, Novo Nordisk Pharma, and CSL Behring. The remaining authors declare no competing financial interests.
Correspondence: Hiroshi Inaba, Department of Laboratory Medicine, Tokyo Medical University, 6-7-1 Nishishinjuku, Shinjuku-ku, Tokyo 160-0023, Japan; email: hrsinb@tokyo-med.ac.jp.
References
Author notes
Data are available on request from the corresponding author, Hiroshi Inaba (hrsinb@tokyo-med.ac.jp). The authors confirm that the data supporting the findings of this study are available within the article and the supplemental Materials.
The full-text version of this article contains a data supplement.