TO THE EDITOR:
Next-generation sequencing (NGS) has revolutionized the diagnosis of inherited platelet disorders (IPD) by providing a rapid molecular diagnostic approach1-3; however, it has also raised discussion about the ethical implications of discovering unsolicited findings. Gene variants associated with predisposition to hematological malignancies are among such unsolicited findings when patients have not been previously informed about the potential implications of this diagnosis.4,5 Some authors maintained that while receiving a phenotypic diagnosis of IPD rarely causes distress to the patient, the identification of a mutation associated with an increased risk of leukemia may cause a major psychological burden with no benefit for patient management because currently it is neither possible to predict the individual risk of malignant transformation, nor to prevent it.6
Familial platelet disorder with associated myeloid malignancy (FPDMM) is an autosomal dominant IPD with a predisposition to hematological malignancies caused by loss-of-function germ line variants in the gene encoding RUNX1, a transcription factor involved in the differentiation of lymphoid and myeloid cells.7 FPDMM is characterized by incomplete penetrance and a broad spectrum of clinical and laboratory phenotypes, a feature that makes diagnosis very difficult. Indeed, even within same families, affected patients present with severe bleeding, while others have mild or no bleeding symptoms,8,9 thus the inherited nature of the disorder may go unnoticed. Moreover, the laboratory phenotype of FPDMM is heterogeneous, making diagnosis cumbersome. For instance, while mild/moderate thrombocytopenia is usually present, some patients have a normal platelet count. Moreover, other 2 IPDs that predispose an individual to hematological malignancies, ANKRD26- and ETV6-related thrombocytopenias, characterized like FPDMM by thrombocytopenia, normal platelet size, and the absence of major structural changes,10 enrich the scenario of inherited thrombocytopenias with normal platelet size and should be considered for differential diagnosis. On the other hand, a platelet function defect characterized by impaired platelet aggregation and granule secretion is found in most patients with FPDMM.7,11,12 Therefore, definite diagnosis can only be obtained by molecular genetic analysis because platelet function testing and clinical phenotype do not address it.
About 44% of FPDMM patients evolve to a hematological malignancy during their lifetime, primarily acute myeloid leukemia (AML) or myelodysplastic syndromes but also T- or B-cell lymphoproliferative disorders with an average age at malignant transformation of 33 years.10
Here, we report a case which suggests that the lack of a prompt identification of a RUNX1 variant in a patient with unexplained thrombocytopenia may be potentially detrimental.
All studies were approved by the responsible Institutional review boards (Bioethics Committee of University of Perugia, approval number 2014-031) and were carried out in conformance with the Declaration of Helsinki.
A 56-year-old man came to us with mild thrombocytopenia which was found during routine testing for blood donation. He did not report hemorrhagic symptoms, even after tooth extractions and the aspiration of a hepatic cyst. Thrombocytopenia was very mild (average, 139 × 106 platelets per mL [minimum to maximum (min-max), 110 × 106 to 162 × 106 platelets per mL]; controls, 237 × 106 ± 35 × 106 platelets per mL [min-max, 174 × 106 to 289 × 106 platelets per mL]), with normal platelet size (99% normal, 1% large, and 0% very large platelets; normal values: 95%-100% normal, 0%-4% large, 0%-1% very large) and morphology.
Considering that immune thrombocytopenia was barely likely, because reticulated platelets were normal (8.7%; normal, 7.6%-9.9%) and antiplatelet antibodies absent, and after exclusion of other autoimmune disorders or infectious diseases, we carried out platelet function tests.11 Indeed, many inherited thrombocytopenias, especially when mild to moderate, are associated with a platelet function defect.12
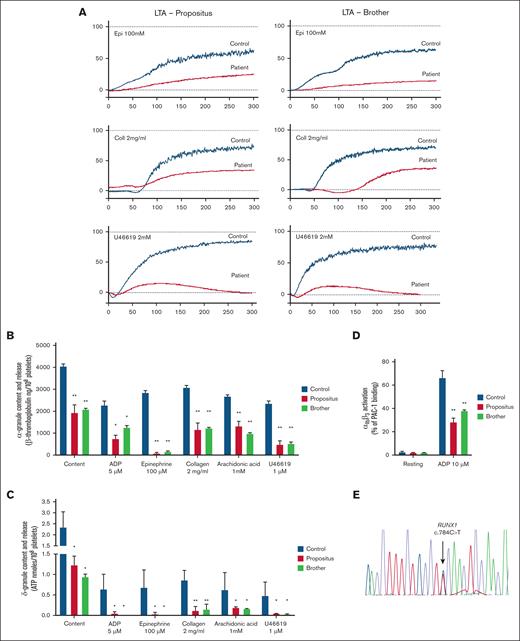
Platelet function testing11,12 revealed defective aggregation in response to epinephrine, U46619 and collagen (Figure 1A) and reduced content and release of both α and δ granules (Figure 1B-C). The expression of platelet αIIbβ3 and glycoprotein Ib/IX/V was normal, while αIIbβ3 activation in response to adenosine 5′-diphosphate was impaired (Figure 1D). A provisional diagnosis of combined α/δ-granule deficiency was formulated, and we asked the patient for consent to undergo genetic testing.
Platelet function testing and sequencing. (A) Human platelet aggregation in response to epinephrine (Epi; 100 μM), collagen (Coll; 2 mg/mL), U46619 (1 μM) in platelet-rich plasma from the patient (left panels), his brother (right panels), and parallel healthy controls. Traces are representative of 4 independent experiments. (B) α-granule secretion in platelets from the patient, his brother, and parallel healthy controls, as assessed by the measurement of β-thromboglobulin content and secretion by enzyme-linked immunosorbent assay. Agonists were adenosine 5′-diphosphate (ADP; 5 μM), Epi (100 μM), Coll (2 mg/mL), arachidonic acid (1mM), and U46619 (1 μM). β-thromboglobulin levels are reported as ng/108 platelets. Data are presented as means ± standard error of the mean (SEM) from 4 independent experiments. ∗P < .05 vs control; ∗∗P < .01 vs control; 2-way analysis of variance (ANOVA). (C) δ-granule secretion in platelets from the patient, his brother and parallel healthy controls as assessed by the measurement of adenosine triphosphate (ATP) content and secretion by lumiaggregometry. Agonists were ADP (5 μM), Epi (100 μM), Coll (2 mg/mL), arachidonic acid (1 mM), and U46619 (1 μM). ATP is reported as nanomoles per 108 platelets. Data are presented as means ± SEM from 4 independent experiments. ∗P < .05 vs control; ∗∗P < .01 vs control; 2-way ANOVA. (D) Integrin αIIbβ3 activation (PAC-1 binding) as assessed by flow cytometry in response to ADP (10 μM) in whole blood from the patient, his brother, and parallel healthy controls. PAC-1 binding is reported as percentage of positive platelets, calculated as the percentage of platelets (gated for their forward scatter and side scatter values, and for their positivity to the platelet marker CD42b) that bound PAC-1 over the total platelet population after setting of nonspecific binding. Data are presented as means ± SEM from 4 independent experiments. ∗∗P < .01 vs control; 2-way ANOVA. (E) Sequencing of DNA from the finger nails of patient brother, showing the heterozygous c.784C>T variant in RUNX1, leading to p.Gln262Ter.
Platelet function testing and sequencing. (A) Human platelet aggregation in response to epinephrine (Epi; 100 μM), collagen (Coll; 2 mg/mL), U46619 (1 μM) in platelet-rich plasma from the patient (left panels), his brother (right panels), and parallel healthy controls. Traces are representative of 4 independent experiments. (B) α-granule secretion in platelets from the patient, his brother, and parallel healthy controls, as assessed by the measurement of β-thromboglobulin content and secretion by enzyme-linked immunosorbent assay. Agonists were adenosine 5′-diphosphate (ADP; 5 μM), Epi (100 μM), Coll (2 mg/mL), arachidonic acid (1mM), and U46619 (1 μM). β-thromboglobulin levels are reported as ng/108 platelets. Data are presented as means ± standard error of the mean (SEM) from 4 independent experiments. ∗P < .05 vs control; ∗∗P < .01 vs control; 2-way analysis of variance (ANOVA). (C) δ-granule secretion in platelets from the patient, his brother and parallel healthy controls as assessed by the measurement of adenosine triphosphate (ATP) content and secretion by lumiaggregometry. Agonists were ADP (5 μM), Epi (100 μM), Coll (2 mg/mL), arachidonic acid (1 mM), and U46619 (1 μM). ATP is reported as nanomoles per 108 platelets. Data are presented as means ± SEM from 4 independent experiments. ∗P < .05 vs control; ∗∗P < .01 vs control; 2-way ANOVA. (D) Integrin αIIbβ3 activation (PAC-1 binding) as assessed by flow cytometry in response to ADP (10 μM) in whole blood from the patient, his brother, and parallel healthy controls. PAC-1 binding is reported as percentage of positive platelets, calculated as the percentage of platelets (gated for their forward scatter and side scatter values, and for their positivity to the platelet marker CD42b) that bound PAC-1 over the total platelet population after setting of nonspecific binding. Data are presented as means ± SEM from 4 independent experiments. ∗∗P < .01 vs control; 2-way ANOVA. (E) Sequencing of DNA from the finger nails of patient brother, showing the heterozygous c.784C>T variant in RUNX1, leading to p.Gln262Ter.
Variants of genes involved in bleeding and platelet disorders were assessed by NGS on DNA extracted from white blood cells, performing targeted sequencing of 91 TIER-1 genes.13
NGS identified a nonsense variant in RUNX1 (c.784C>T; p.Gln262Ter, transcript NM_001754.5),14 classified as pathogenic in ClinVar, absent from gnomAD. The variant was confirmed by Sanger sequencing and a diagnosis of FPDMM was made, which was also consistent with the results of the platelet function testing.
The patient referred to a bone marrow donation to his brother for the treatment of an acute leukemia 31 years earlier, when he was 25. On that occasion, he was found to have very mild thrombocytopenia but no further investigation was performed.
The patient’s brother, a 41-year old man who did not have any hemorrhagic manifestations, was diagnosed with AML in the 1990s and underwent his first bone marrow transplantation at the age of 10 years. He relapsed 7 months later, and therefore he had a second bone marrow transplantation. Since then, he has been in remission. In both cases the bone marrow donor was his HLA-haploidentical brother, our propositus. Therefore, we investigated whether the brother of our propositus was also a carrier of the same gene variant. Given that his circulating white blood cells would necessarily carry the mutation because it was derived from the bone marrow of our propositus, we analyzed DNA extracted from his finger nails and found the same RUNX1 variant (Figure 1E). Platelet testing revealed the same pattern of platelet function defect observed in our propositus (Figure 1A-D). Expression of 2 typical biomarkers of FPDMM, α2β1 and MYH10,15,16 was assessed. Platelet expression of α2β1, which is positively regulated by RUNX1, was reduced (Figure 2A), while MYH10, which is negatively regulated by RUNX1 and is absent in healthy control platelets, was increased (Figure 2B). To assess the presence of acquired somatic variants in genes typically involved in myeloid neoplasms, we used a NGS myeloid panel. We analyzed DNA from the blood taken at the time of platelet function testing, 31 years after the bone marrow donation. Both subjects had the same somatic TET2 variants with substantial difference in clonality, higher in the propositus (Figure 2C). In addition, the propositus had another TET2 variant whereas his brother had a KRAS variant (Figure 2C). It is likely that the bone marrow of the propositus had already acquired the 2 TET2 variants at the time of bone marrow donation, and that this clone was engrafted in his brother. It is worth noting that TET2 and KRAS are involved in clonal hematopoiesis of indeterminate potential (CHIP) which has been frequently described in patients with FPDMM.17,18
Expression of FPDMM biomarkers and assesment of acquired somatic variants. (A) Integrin α2β1 expression, as assessed by flow cytometry using anti-integrin α2 (CD49b) and anti-integrin β1 (CD29) fluorescein isothiocyanate–conjugated antibodies. CD49b and CD29 binding are reported as median fluorescence intensity of platelets (gated for their forward scatter and side scatter values, and for their positivity to the platelet marker CD42b) that bound CD49b and CD29, relative to the total platelet population after setting of nonspecific binding. Data are presented as means ± SEM from 4 independent experiments for controls, and n = 1 for the propositus and the brother. (B) MYH10 expression was assessed by nested polymerase chain reaction (PCR). Total RNA was isolated from platelets and HeLa cells (used as a positive control because they express MYH10) using the TRIzol reagent (Invitrogen). A total of 500 ng of total RNA served as the template for complementary DNA (cDNA) synthesis. The RNA was treated with DNase (Invitrogen), and cDNA was then synthesized using the iScript kit (Bio-Rad, Hercules, CA). Specific primers were designed to amplify human MYH10 (primer set for primary PCR was MYH10 forward [For]: 5′-AGTTCAAGGCCACCATCTCA-3′; MYH10 reverse [Rev]: 5′-TGGAAGAGAAGCTGATGGGG-3′; primer set for secondary PCR was MYH10 For: 5′-AGTTCAAGGCCACCATCTCA-3′; MYH10 Rev: 5′-GCTCATCCTCAACCTGCATG-3′). The PCR was performed using the AccuPrime GC-rich DNA Polymerase kit (Thermo Fisher). The PCR was performed as follows: denaturation at 95°C for 3 minutes, followed by 35 cycles of 95°C for 30 minutes, 57°C for 30 seconds, and 72°C for 30 seconds and by a final elongation step at 72°C for 7 minutes. (C) Targeted custom NGS. The Myeloid Solution (MYS, SOPHiA GENECTIC, Arrow Diagnostics) was used to investigate hot spot mutations of the full coding sequence of 30 genes typically involved in myeloid neoplasms, including ABL1, ASXL1, BRAF, CALR, CBL, CEBPA, CSF3R, DNMT3A, ETV6, EZH2, FLT3, HRAS, IDH1, IDH2, JAK2, KIT, KRAS, MPL, NPM1, NRAS, PTPN11, RUNX1, SETBP1, SF3B1, SRSF2, TET2, TP53, U2AF1, WT1, and ZRSR2. Libraries were prepared using 200 ng of peripheral blood genomic DNA, following manufacturer’s instructions. Pooled libraries were sequenced using the MiSeq Reagent kit v3 on the Illumina MiSeq Sequencer (Illumina, San Diego, CA). FASTQ files were analyzed with SOPHiA DDM software (version 5.10.54.1). Exonic, splice site, and noncoding target gene variants were taken into consideration and filtered retaining those with a global minor allele frequency <0.01 and with a variant allele frequency (VAF) >1%. For the interpretation of putative germ line variants, we referred to the American College of Medical Genetics and Genomics. Somatic variants were classified by means of the FATHMM web server (http://fathmm.biocompute.org.uk). Benign/likely benign variants were excluded from analysis. Beyond the somatic variants, the germ line RUNX1 c.784C>T p.(Gln262∗) variant was found in the 2 samples. INDEL, insertion/deletion; SNP, single nucleotide variant; NTC, no template control; OD, optical density.
Expression of FPDMM biomarkers and assesment of acquired somatic variants. (A) Integrin α2β1 expression, as assessed by flow cytometry using anti-integrin α2 (CD49b) and anti-integrin β1 (CD29) fluorescein isothiocyanate–conjugated antibodies. CD49b and CD29 binding are reported as median fluorescence intensity of platelets (gated for their forward scatter and side scatter values, and for their positivity to the platelet marker CD42b) that bound CD49b and CD29, relative to the total platelet population after setting of nonspecific binding. Data are presented as means ± SEM from 4 independent experiments for controls, and n = 1 for the propositus and the brother. (B) MYH10 expression was assessed by nested polymerase chain reaction (PCR). Total RNA was isolated from platelets and HeLa cells (used as a positive control because they express MYH10) using the TRIzol reagent (Invitrogen). A total of 500 ng of total RNA served as the template for complementary DNA (cDNA) synthesis. The RNA was treated with DNase (Invitrogen), and cDNA was then synthesized using the iScript kit (Bio-Rad, Hercules, CA). Specific primers were designed to amplify human MYH10 (primer set for primary PCR was MYH10 forward [For]: 5′-AGTTCAAGGCCACCATCTCA-3′; MYH10 reverse [Rev]: 5′-TGGAAGAGAAGCTGATGGGG-3′; primer set for secondary PCR was MYH10 For: 5′-AGTTCAAGGCCACCATCTCA-3′; MYH10 Rev: 5′-GCTCATCCTCAACCTGCATG-3′). The PCR was performed using the AccuPrime GC-rich DNA Polymerase kit (Thermo Fisher). The PCR was performed as follows: denaturation at 95°C for 3 minutes, followed by 35 cycles of 95°C for 30 minutes, 57°C for 30 seconds, and 72°C for 30 seconds and by a final elongation step at 72°C for 7 minutes. (C) Targeted custom NGS. The Myeloid Solution (MYS, SOPHiA GENECTIC, Arrow Diagnostics) was used to investigate hot spot mutations of the full coding sequence of 30 genes typically involved in myeloid neoplasms, including ABL1, ASXL1, BRAF, CALR, CBL, CEBPA, CSF3R, DNMT3A, ETV6, EZH2, FLT3, HRAS, IDH1, IDH2, JAK2, KIT, KRAS, MPL, NPM1, NRAS, PTPN11, RUNX1, SETBP1, SF3B1, SRSF2, TET2, TP53, U2AF1, WT1, and ZRSR2. Libraries were prepared using 200 ng of peripheral blood genomic DNA, following manufacturer’s instructions. Pooled libraries were sequenced using the MiSeq Reagent kit v3 on the Illumina MiSeq Sequencer (Illumina, San Diego, CA). FASTQ files were analyzed with SOPHiA DDM software (version 5.10.54.1). Exonic, splice site, and noncoding target gene variants were taken into consideration and filtered retaining those with a global minor allele frequency <0.01 and with a variant allele frequency (VAF) >1%. For the interpretation of putative germ line variants, we referred to the American College of Medical Genetics and Genomics. Somatic variants were classified by means of the FATHMM web server (http://fathmm.biocompute.org.uk). Benign/likely benign variants were excluded from analysis. Beyond the somatic variants, the germ line RUNX1 c.784C>T p.(Gln262∗) variant was found in the 2 samples. INDEL, insertion/deletion; SNP, single nucleotide variant; NTC, no template control; OD, optical density.
Considering that 44% of patients with FPDMM develop hematological malignancies during their lifetime,10 the decision to subject a patient with a suspected IPD to genetic testing, with the consequent possibility of diagnosing an IPD predisposing them to hematological malignancies, must be carefully considered. Moreover, the existence of IPDs predisposing individuals to extrahematological complications, such as MYH9-RD which predisposes to cataract, renal dysfunction, and hearing loss at a young age, must be considered and discussed.10 Ethical issues for the possible detection of secondary findings5,6 can be solved by administering to the patient an informed consent form that explicitly mentions the possible discovery of unsolicited findings, included an unsolicited predisposition to leukemia, asking whether the patient wants to be informed of them in case they are found, and clarifying that he/she has been made aware that there are no current means to prevent leukemia.4
In our case, genetic testing of the bone marrow donor with thrombocytopenia could have prevented the transplantation of the bone marrow carrying a RUNX1 pathogenic variant, and possibly CHIP-related variants, to his leukemic brother. It is worth noting that the brother received bone marrow carrying the RUNX1 variant when he was 10 years old and even now, 30 years later, he is in remission. The relapse he had 7 months after the first transplantation was considered a recovery of the original leukemic clone. However, chimerism was not tested at that time, while a recently performed analysis showed complete chimerism, indicating that only donor hematopoietic cells are now present in the bone marrow. Thus, a donor cell–derived new leukemia cannot be excluded.19
Our case raises a series of considerations as follows: (1) RUNX1 germ line variants predisposing to leukemia require the acquisition of additional somatic mutations driving leukemic transformation, according to the “two-hit hypothesis.”20 Indeed, of the 2 brothers carrying the same pathogenic RUNX1 variant only 1 developed leukemia. (2) Somatic mutations may occur randomly both among individuals within a family and within the same individual in different moments of their life. Indeed the brother of our propositus, still carrying a bone marrow with a predisposing RUNX1 variant, developed leukemia only once. (3) The acquisition of somatic mutations and also the evolution of CHIP clones does not depend only on the presence of a germ line RUNX1 variant but also on other factors, such as environment, which may modify the FPDMM outcome.
A similar case of a man with AML, who was later diagnosed with FPDMM and who received allogeneic bone marrow transplantation from his FPDMM-affected sister, was previously reported. Twenty-one months after transplantation, abnormal blasts of donor origin were found in this patient and concurrently a myelodysplastic syndrome was diagnosed in his donor sister. Both underwent bone marrow transplantation from matched unrelated donors. The patient died of Epstein-Barr virus–related lymphoma 1 year later, the sister remained in remission until the date of the report.21
The study of our family suggests that the brother of our propositus developed AML because he was a carrier of FPDMM and received 2 hematopoietic stem cell transplantations from our propositus, who was a carrier of FPDMM too. This poses the patient at risk of further leukemic transformation. At the time of bone marrow transplantation (in the 1990s) FPDMM was still unknown, but it is now known that IPDs predisposing individuals to hematological malignancies are more common than expected, representing 19% of all inherited thrombocytopenias.22 Therefore, (1) a careful examination of the family history with questions aimed at assessing phenotypes different from bleeding (eg, hematological malignancies) should be part of the anamnestic investigation for IPD; (2) after obtaining explicit informed consent, patients with a suspected IPD should be genetically screened; (3) mutational screening of siblings of patients affected by an IPD predisposing them to hematological malignancies should be performed; and (4) leukemic surveillance protocols of patients diagnosed with an IPD predisposing them to hematological malignancies should be applied. According to recent recommendations,23,24 surveillance testing with complete blood count with differential and reticulocyte count is recommended annually, while bone marrow aspirate/biopsies with morphology and cytogenetic analysis should be done every 1 to 3 years23,24; genomic studies should also include NGS for myeloid genes.
Acknowledgments: The authors thank C. Mecucci (University of Perugia) for useful comments and advice on CHIP variant testing.
This work was supported by a Telethon grant (GMR22T1086 [P.G.]).
Contribution: L.B., E.F., E.G., G.C.T., A.M.M., C.M., and R.L.S. performed platelet function studies and DNA analysis, and analyzed and interpreted data; A.C. and P.G. contributed the patients for the study; P.G. designed and supervised the study; L.B. and P.G. wrote the manuscript; and P.G. critically revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Loredana Bury, Division of Internal and Cardiovascular Medicine Centro Didattico, Department of Medicine and Surgery, University of Perugia, Edificio B piano 1 06132 Perugia, Perugia, Italy; email: loredana.bury@unipg.it.
References
Author notes
Renewable materials, data sets, and protocols will be available to other investigators without unreasonable restrictions from the corresponding author, Loredana Bury (loredana.bury@unipg.it), on request.

![Expression of FPDMM biomarkers and assesment of acquired somatic variants. (A) Integrin α2β1 expression, as assessed by flow cytometry using anti-integrin α2 (CD49b) and anti-integrin β1 (CD29) fluorescein isothiocyanate–conjugated antibodies. CD49b and CD29 binding are reported as median fluorescence intensity of platelets (gated for their forward scatter and side scatter values, and for their positivity to the platelet marker CD42b) that bound CD49b and CD29, relative to the total platelet population after setting of nonspecific binding. Data are presented as means ± SEM from 4 independent experiments for controls, and n = 1 for the propositus and the brother. (B) MYH10 expression was assessed by nested polymerase chain reaction (PCR). Total RNA was isolated from platelets and HeLa cells (used as a positive control because they express MYH10) using the TRIzol reagent (Invitrogen). A total of 500 ng of total RNA served as the template for complementary DNA (cDNA) synthesis. The RNA was treated with DNase (Invitrogen), and cDNA was then synthesized using the iScript kit (Bio-Rad, Hercules, CA). Specific primers were designed to amplify human MYH10 (primer set for primary PCR was MYH10 forward [For]: 5′-AGTTCAAGGCCACCATCTCA-3′; MYH10 reverse [Rev]: 5′-TGGAAGAGAAGCTGATGGGG-3′; primer set for secondary PCR was MYH10 For: 5′-AGTTCAAGGCCACCATCTCA-3′; MYH10 Rev: 5′-GCTCATCCTCAACCTGCATG-3′). The PCR was performed using the AccuPrime GC-rich DNA Polymerase kit (Thermo Fisher). The PCR was performed as follows: denaturation at 95°C for 3 minutes, followed by 35 cycles of 95°C for 30 minutes, 57°C for 30 seconds, and 72°C for 30 seconds and by a final elongation step at 72°C for 7 minutes. (C) Targeted custom NGS. The Myeloid Solution (MYS, SOPHiA GENECTIC, Arrow Diagnostics) was used to investigate hot spot mutations of the full coding sequence of 30 genes typically involved in myeloid neoplasms, including ABL1, ASXL1, BRAF, CALR, CBL, CEBPA, CSF3R, DNMT3A, ETV6, EZH2, FLT3, HRAS, IDH1, IDH2, JAK2, KIT, KRAS, MPL, NPM1, NRAS, PTPN11, RUNX1, SETBP1, SF3B1, SRSF2, TET2, TP53, U2AF1, WT1, and ZRSR2. Libraries were prepared using 200 ng of peripheral blood genomic DNA, following manufacturer’s instructions. Pooled libraries were sequenced using the MiSeq Reagent kit v3 on the Illumina MiSeq Sequencer (Illumina, San Diego, CA). FASTQ files were analyzed with SOPHiA DDM software (version 5.10.54.1). Exonic, splice site, and noncoding target gene variants were taken into consideration and filtered retaining those with a global minor allele frequency <0.01 and with a variant allele frequency (VAF) >1%. For the interpretation of putative germ line variants, we referred to the American College of Medical Genetics and Genomics. Somatic variants were classified by means of the FATHMM web server (http://fathmm.biocompute.org.uk). Benign/likely benign variants were excluded from analysis. Beyond the somatic variants, the germ line RUNX1 c.784C>T p.(Gln262∗) variant was found in the 2 samples. INDEL, insertion/deletion; SNP, single nucleotide variant; NTC, no template control; OD, optical density.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodvth/2/1/10.1016_j.bvth.2024.100043/2/m_bvth_vth-2024-000193-gr2.jpeg?Expires=1767727855&Signature=Vab8jhWJwSH5BKo5y92HssQRNvegr9Slxik9w7Zb~NJwGiEaknk7jkkyyzXg2VAV4Ob7iWC8hljjLIaDa2V7544oxPZ~~wXcmclSVg30JbwrJkV5Qe0xrb-cKVpT7DCSGZbRiYrMM1PGQk4IkyLgbm7V~IyVHGIN4J0N5rQR4Jd5tFsXoc0j1AHjJiQpK5hzLniJ4mP72nOFinnTWmwCGqidb2EoiHXLlk~LHM1BN096UHpU9vvcM8yTEdzn8XY-ZfTh0b0FNpCbPUzMTrtZSrOU02~vJvQIs20gpY9JST2GYNiIX0pnUhYe48B7wu64-8v-FHIJmB9fdKRjRi7lxg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)