TO THE EDITOR:
In people with HIV (PWH), chronic immune activation and systemic inflammation have been identified as main drivers of cardiovascular events.1-4 The molecular pathways by which HIV drives cardiovascular risk are largely unknown and multifactorial, including alterations in the homeostasis of the immune cells, coinfections, HIV persistence, and translation of viral proteins.5-10
Atherosclerosis is the underlying pathology of cardiovascular events, and cells from the innate and adaptive immune system are involved in the development and progression of the disease. In this setting, vascular inflammation and activation of platelets promote the proliferation of progenitor cells driving differentiation to megakaryocytes (MKs; cell source of platelets), monocytes, granulocytes, and endothelial cells.11-17 Moreover, reduction of progenitor cells independently predicts adverse cardiovascular events.14-16
HIV infection affects long-lived hematopoietic stem and progenitor cells (HSPCs), and defective myeloid, erythroid, and megakaryopoiesis have been reported in treated PWH.18-22 In addition, reduced circulating progenitor cells involved in angiogenesis and vascular repair have been also reported; however, their relationship with cardiovascular risk is not well understood.16,23-25
In this study, we hypothesized that chronic systemic inflammation driven by HIV infection alters hematopoiesis. To isolate the contribution of the inflammatory component, we investigated the frequencies of circulating HSPCs in PWH and age-matched uninfected control people without HIV (PWOH) with no history of cardiovascular disease for whom pericardial fat volume measurements were available as part of a study that evaluated for subclinical cardiovascular disease (supplemental Table 1A).26
We investigated HSPCs and their in vivo differentiation commitment by flow cytometry in available samples from a subset of the study participants (supplemental Table 1B). PWH (n = 17) and control PWOH (n = 16) had a median Framingham Risk Score (FRS) of 3.7 (interquartile range [IQR] ,1.9-8) and 2.85 (IQR, 1.2-7.1), respectively. The study groups had similar serum levels of biomarkers for systemic inflammation (C-reactive protein and D-dimer) and endothelial inflammation (soluble E-selectin [sE-selectin] and soluble P-selectin [sP-selectin]) with exception of soluble VCAM1 (sVCAM1), which was elevated in PWH (supplemental Table 1B).
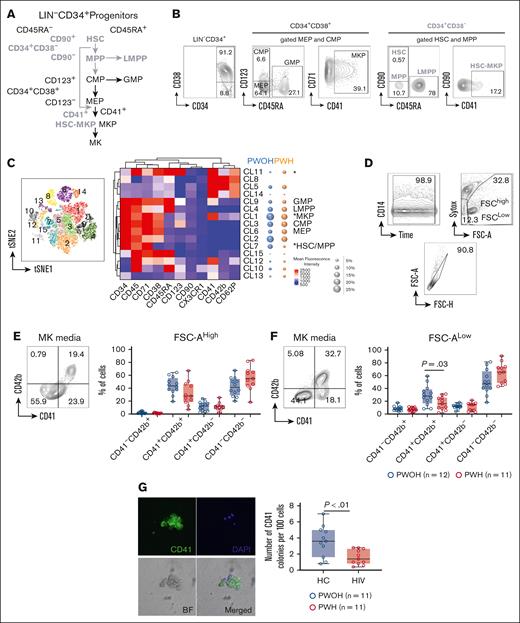
HSPCs and their multilineage differentiation pathways are shown in Figure 1A. HSPCs are identified by the exclusion of lineage cells (LIN–) and expression of progenitor marker CD34 (supplemental Figure 1). The CD34+CD38– contains multipotent cells including hematopoietic stem cells (HSCs), multipotent progenitor cells (MPPs), and lymphoid-primed multipotent progenitors. The oligopotent progenitors are contained in the CD34+CD38+ and include common myeloid progenitor, MK-erythroid progenitor (MEP), and granulocyte-monocyte progenitor (Figure 1A-B) cells.27,28
Reduced circulating MKP in PWH. Circulating hematogenic progenitor cells were analyzed in PBMCs from PWH (n = 17) and PWOH (n = 16) by flow cytometry. (A) Hematopoietic cell differentiation. CD34+CD38– contains multipotent cells (bold gray text): HSC, MPP, and lymphoid-primed multipotent progenitors (LMPPs). The oligopotent progenitors CD34+CD38+ (black text): common myeloid progenitor (CMP), MEP, and granulocyte-monocyte progenitor (GMP) cells. MKPs originate from the bipotent progenitor MEP (black arrows). HSC-MKPs (stem-cell like) that originate from a subset of HSC-CD41+ (gray arrows). (B) Manual gating strategy. Free platelets (SSClowCD42b+) were excluded from the analysis followed by gating FSC vs SSC, live and single cells. The lineage negative cells (LIN–) were gated by the exclusion of lineage positive cells using a cocktail of mAbs (CD2, CD3, CD4, CD7, CD8, CD10, CD11b, CD14, CD19, CD20, CD56, and CD235a). An additional CD14 mAb was used to eliminated potential contaminant events on the LIN– population (supplemental Figure 1A). The LIN– (gated as LIN–CD14– cells) were analyzed for progenitor marker expression CD34 and CD38: LIN–CD34+CD38– cells (bold gray text) contains multipotent progenitors: HSC, CD45RA–CD90+; MPP, CD45RA–CD90–; and LMPP, CD45RA+. The LIN–CD34+CD38+ cells (black text) contain oligopotent progenitors: CMP, CD45RA–CD123+; MEP, CD45RA–CD123–; and GMP, CD45RA+ cells. MKPs derived from MEP were gated as MEPCD41+. The MKPs derived from HSC (“stem-cell like” or HSC-MKP) were defined as HSC-CD41+. Because of the low number of events, the analysis of CD41+ was performed in the MEP-CMP and HSC-MPP together. (C) Unsupervised clustering analysis using t-SNE and PhenoGraph of the total LIN– cells. The heat map represents mean fluorescence intensity of markers (CD34, CD45, CD71, CD38, CD45RA, CD123, CD90, CX3CR1, CD41, CD42b, and CD62P). Bubble plots represent the median frequency of each cluster in PWOH (blue) and PWH (orange). (D) In vitro MK differentiation. LIN–CD34+ and LIN–CD34low were sorted from PBMCs from PWH (n = 11) and PWOH (n = 12) and cultured in MK conditioned media. After 12 days of culture, differentiation was evaluated with a cocktail of mAbs: CD34, CD38, and CD42b. Sytox staining was used for exclusion of dead cells and differentiation was evaluated by positive expression of CD41 and CD42b. (E) MKs were gated on the high FSC-A and (F) platelets in the low FSC-A and negative nuclei staining (sytox–) population. The graphs represented by box and whisker show the median value with first and third quartiles in the box, with whiskers extending to the minimum and maximum values. (G) Colony-forming unit assay: 500 sorted LIN–CD34+ cells were cultured in MegaCult-C Collagen and Medium with Lipid in the presence of recombinant human thrombopoietin, recombinant human interleikin-3, and recombinant human interleikin-6. Colonies were evaluated at days 13 to 14 of culture by CD41 expression and Hoechst 33324 was used as nuclei staining. Representative individual images of MK colony including CD41 (green), Hoechst 33324 (blue), and differential interference contrast of the BF and merged images. MK colonies were identified by CD41 expression and represented in the graph as CD41+ colonies per 100 seeding cells (LIN–CD34+ cells). Comparison between the groups was performed using a nonparametric unpaired Mann-Whitney test. P < .05 was considered significant. BF, brightfield image; DAPI, 4',6-diamidino-2-phenylindolem; FSC, forward scatter; FSC-A, forward scatter-area; mAb, monoclonal antibody; MKP, megakaryocyte progenitor; PBMC, peripheral blood mononuclear cells; SSC, side scatter; t-SNE, t-distributed stochastic neighbor embedding.
Reduced circulating MKP in PWH. Circulating hematogenic progenitor cells were analyzed in PBMCs from PWH (n = 17) and PWOH (n = 16) by flow cytometry. (A) Hematopoietic cell differentiation. CD34+CD38– contains multipotent cells (bold gray text): HSC, MPP, and lymphoid-primed multipotent progenitors (LMPPs). The oligopotent progenitors CD34+CD38+ (black text): common myeloid progenitor (CMP), MEP, and granulocyte-monocyte progenitor (GMP) cells. MKPs originate from the bipotent progenitor MEP (black arrows). HSC-MKPs (stem-cell like) that originate from a subset of HSC-CD41+ (gray arrows). (B) Manual gating strategy. Free platelets (SSClowCD42b+) were excluded from the analysis followed by gating FSC vs SSC, live and single cells. The lineage negative cells (LIN–) were gated by the exclusion of lineage positive cells using a cocktail of mAbs (CD2, CD3, CD4, CD7, CD8, CD10, CD11b, CD14, CD19, CD20, CD56, and CD235a). An additional CD14 mAb was used to eliminated potential contaminant events on the LIN– population (supplemental Figure 1A). The LIN– (gated as LIN–CD14– cells) were analyzed for progenitor marker expression CD34 and CD38: LIN–CD34+CD38– cells (bold gray text) contains multipotent progenitors: HSC, CD45RA–CD90+; MPP, CD45RA–CD90–; and LMPP, CD45RA+. The LIN–CD34+CD38+ cells (black text) contain oligopotent progenitors: CMP, CD45RA–CD123+; MEP, CD45RA–CD123–; and GMP, CD45RA+ cells. MKPs derived from MEP were gated as MEPCD41+. The MKPs derived from HSC (“stem-cell like” or HSC-MKP) were defined as HSC-CD41+. Because of the low number of events, the analysis of CD41+ was performed in the MEP-CMP and HSC-MPP together. (C) Unsupervised clustering analysis using t-SNE and PhenoGraph of the total LIN– cells. The heat map represents mean fluorescence intensity of markers (CD34, CD45, CD71, CD38, CD45RA, CD123, CD90, CX3CR1, CD41, CD42b, and CD62P). Bubble plots represent the median frequency of each cluster in PWOH (blue) and PWH (orange). (D) In vitro MK differentiation. LIN–CD34+ and LIN–CD34low were sorted from PBMCs from PWH (n = 11) and PWOH (n = 12) and cultured in MK conditioned media. After 12 days of culture, differentiation was evaluated with a cocktail of mAbs: CD34, CD38, and CD42b. Sytox staining was used for exclusion of dead cells and differentiation was evaluated by positive expression of CD41 and CD42b. (E) MKs were gated on the high FSC-A and (F) platelets in the low FSC-A and negative nuclei staining (sytox–) population. The graphs represented by box and whisker show the median value with first and third quartiles in the box, with whiskers extending to the minimum and maximum values. (G) Colony-forming unit assay: 500 sorted LIN–CD34+ cells were cultured in MegaCult-C Collagen and Medium with Lipid in the presence of recombinant human thrombopoietin, recombinant human interleikin-3, and recombinant human interleikin-6. Colonies were evaluated at days 13 to 14 of culture by CD41 expression and Hoechst 33324 was used as nuclei staining. Representative individual images of MK colony including CD41 (green), Hoechst 33324 (blue), and differential interference contrast of the BF and merged images. MK colonies were identified by CD41 expression and represented in the graph as CD41+ colonies per 100 seeding cells (LIN–CD34+ cells). Comparison between the groups was performed using a nonparametric unpaired Mann-Whitney test. P < .05 was considered significant. BF, brightfield image; DAPI, 4',6-diamidino-2-phenylindolem; FSC, forward scatter; FSC-A, forward scatter-area; mAb, monoclonal antibody; MKP, megakaryocyte progenitor; PBMC, peripheral blood mononuclear cells; SSC, side scatter; t-SNE, t-distributed stochastic neighbor embedding.
Two sources of MK progenitors (MKPs) have been identified. Those MKPs derived from the bipotent progenitor MEP and those that originate from a subset of HSC expressing CD41 called “stem cell–like” or HSC-MKP (Figure 1A, black and gray arrows, respectively). The latter supports the replenishment of platelets during increased demand induced by inflammatory insults such as viral infections.29,30
Using an unsupervised cluster analysis, we found that compared with the control group, PWH has significantly reduced frequencies in clusters (CL) with phenotypes corresponding to HSC-MPP (CL7, P = .01) and MKP (CL1, P = .03; Figure 1C, bubble plots). In contrast, CL11 (LIN–CD34low) was significantly increased in PWH and expressed markers of myeloid differentiation (CD38, CD123, and CD45RA) and MK (CD41) suggesting a mixed cell type at more differentiated stages (Figure 1C). Manual gating analysis confirmed that reduction of total LIN–CD34+ in PWH was associated with lower frequencies of MKP derived from both MEP and HSC (supplemental Figure 1B-C). These observations suggest that chronic inflammation during HIV infection may promote in vivo increased demand for platelet production that is reflected in low frequencies of both types of MKP.
We next investigated the ability of sorted LIN–CD34+ to undergo in vitro differentiation into MK and myeloid cells. We also investigated whether LIN–CD34low was primed into MK or myeloid lineage cells (supplemental Figure 1). The sorted populations were stained with May-Grünwald-Giemsa and evaluated by microscopy before and after culture (supplemental Figure 2). At day 12 of culture, LIN–CD34+ progenitors from PWH cultured in MK but not in myeloid conditioned media showed a delayed trend of CD38 downregulation in some donors compared with PWOH, although this was not statistically significant (supplemental Figure 2D-E). No cells were covered from the LIN–CD34low cultures.
MK differentiation was monitored by the expression CD42b (platelet glycoprotein Ibα chain) and CD41 (integrin α-IIb). Live cells were gated for high forward scatter (FSC) to analyze MK and low FSC and nuclei staining negative to analyze platelets (Figure 1D). In PWH, the differentiation of MK showed a reduced trend (although this was not statistically significant), and a significant reduction in platelet production was observed compared with PWOH (Figure 1E-F, respectively). The reduced platelet yield in the liquid culture is consistent with the lower frequency of MKP observed in circulation (Figure 1C).
MKP’s capacity to proliferate and form colonies was significantly decreased in PWH (Figure 1G) and colonies tended to be smaller than those observed in PWOH (supplemental Figure 3A). In contrast, levels of myeloid cell differentiation analyzed by expression of CD11b and CD14 (supplemental Figure 2F) and colony formation including erythroid were similar between the groups (supplemental Figure 3B) suggesting that chronic HIV infection influences MKP differentiation and may affect platelet production.
In PWH, platelet counts inversely correlated with the frequencies of circulating MKP (R = −0.560; P = .019) and those that rise from the HSC-MKP (R = −0.621; P = .008). Similarly, the frequencies of MPP were inversely associated with platelet counts (R = −0.587; P = .013; supplemental Table 2; supplemental Figure 4A).
In addition, the frequencies of MEP and HSC-MKP showed relationships with biomarkers of platelet activation and endothelial inflammation, sP-selectin (R = 0.647; P = .007) and sVCAM1 (R = 0.628; P = .009), respectively. In contrast, in PWOH, serum levels of sVCAM1 showed a positive correlation with the frequencies of HSC (supplemental Table 3; supplemental Figure 4B). No associations were noted between progenitors and biomarkers of systemic inflammation and coagulation (supplemental Table 4). These data suggest that in PWH sVCAM1 may be involved in the recruitment and homing of progenitor cells to the site of vascular injury.31
To better understand potential clinical implications, we investigated the relationship between platelet counts and cardiovascular risk, pericardial fat deposition as a factor of subclinical cardiovascular disease in all study participants including PWH (n = 90) and PWOH (n = 29) (supplemental Table 1A).26 Platelet counts were inversely associated with FRS and epicardial and pericardial adipose tissues and were also negatively correlated with serum levels of biomarkers of endothelial inflammation sVCAM1 (supplemental Figure 4C). The multivariate analysis adjusting for smoking status, diabetes, and HIV status showed that sVCAM1 was the main factor associated with platelet counts (Table 1).
Multivariate linear regression analysis of platelet counts and biomarkers of inflammation and cardiovascular risk
| Biomarker . | Regression coefficient (95% CI)∗, P value† . | |||
|---|---|---|---|---|
| Total study participants (n = 119) . | PWH (n = 90) . | |||
| Coefficient (95% CI)∗ . | P value† . | Coefficient (95% CI)∗ . | P value† . | |
| D-dimer | 0.00 (0.00-0.00) | .12 | 0.00 (0.00-0.00) | . 24 |
| CRP | 0.02 (−0.01 to 0.04) | .15 | 0.02 (−0.02 to 0.04) | .42 |
| sE-selectin | 0.01 (−0.01 to 0.03) | .12 | −0.01 (−0.03 to 0.02) | .63 |
| sP-selectin | 0.04 (−0.03 to 0.07) | .44 | 0.00 (−0.06 to 0.08) | .96 |
| sVCAM1 | −1.60 (−2.5 to −0.66) | <.001‡ | −1.90 (−3.2 to −0.58) | .005‡ |
| FRS | −0.02 (−0.03 to 0.00) | .041 | −0.01 (−0.04 to 0.01) | .22 |
| Paracardial fat volume | −0.21 (−0.42 to −0.00) | .054 | −0.21 (−0.51 to 0.10) | .18 |
| Epicardial fat volume | −0.31 (−0.60 to −0.02) | .035 | −0.30 (−0.70 to 0.10) | .14 |
| Pericardial fat volume§ | −0.52 (−0.99 to −0.05) | .032 | −0.51 (−1.2 to 0.16) | .14 |
| Biomarker . | Regression coefficient (95% CI)∗, P value† . | |||
|---|---|---|---|---|
| Total study participants (n = 119) . | PWH (n = 90) . | |||
| Coefficient (95% CI)∗ . | P value† . | Coefficient (95% CI)∗ . | P value† . | |
| D-dimer | 0.00 (0.00-0.00) | .12 | 0.00 (0.00-0.00) | . 24 |
| CRP | 0.02 (−0.01 to 0.04) | .15 | 0.02 (−0.02 to 0.04) | .42 |
| sE-selectin | 0.01 (−0.01 to 0.03) | .12 | −0.01 (−0.03 to 0.02) | .63 |
| sP-selectin | 0.04 (−0.03 to 0.07) | .44 | 0.00 (−0.06 to 0.08) | .96 |
| sVCAM1 | −1.60 (−2.5 to −0.66) | <.001‡ | −1.90 (−3.2 to −0.58) | .005‡ |
| FRS | −0.02 (−0.03 to 0.00) | .041 | −0.01 (−0.04 to 0.01) | .22 |
| Paracardial fat volume | −0.21 (−0.42 to −0.00) | .054 | −0.21 (−0.51 to 0.10) | .18 |
| Epicardial fat volume | −0.31 (−0.60 to −0.02) | .035 | −0.30 (−0.70 to 0.10) | .14 |
| Pericardial fat volume§ | −0.52 (−0.99 to −0.05) | .032 | −0.51 (−1.2 to 0.16) | .14 |
CRP, C-reactive protein.
Multivariable linear regression analysis adjusting for smoking status (ever vs never), diabetes, and HIV status.
Raw P value was reported.
If raw P <.005, statistical significance holds under Bonferroni-corrected type I error.
Pericardial fat volume is a composite of paracardial and epicardial fat volume.
In conclusion, we found that PWH (low FRS) have reduced circulating progenitors, and ongoing inflammation may promote platelet production demand that is supported by the differentiation of MEP and “stem-like” HSC-MKP. The PWH study group had a trend of lower platelet count, and it may reflect an early impact of systemic inflammation in HSCP in the bone marrow. Chronic inflammation and cardiovascular risk factors have been associated with reduced frequencies and functional exhaustion of progenitors.32-34 Moreover, clonal hematopoiesis, an age-associated increased somatic mutation in HSC that drives the expansion of mutated clones, has been associated with cardiovascular disease in PWH.35,36 Future studies should address the contribution of HIV-driven inflammation and HIV persistence in HSCP function and its potential impact on the increased cardiovascular risk.
Acknowledgments: The authors thank the District of Columbia Center for AIDS Research, an National Institutes of Health (NIH)–funded program (P30AI117970) that is supported by the following NIH cofunding and participating institutes and centers: National Institute of Allergy and Infectious Diseases (NIAID); National Cancer Institute; National Institute of Child Health and Human Development; National Heart, Lung, and Blood Institute; National Institute on Drug Abuse; National Institute of Mental Health; National Institute on Aging; National Institute of Diabetes and Digestive and Kidney Diseases; National Institute on Minority Health and Health Disparities; National Institute of Dental and Craniofacial Research; National Institute of Nursing Research, Fogarty International Center, and Office of AIDS Research.
Research reported in this publication was supported by the NIAID of the NIH (NIH R01AI145549).
Contribution: M.C. designed the study; T.L. performed the experiments; M.C. and T.L. analyzed and interpreted the data and wrote the manuscript; C.H. provided the study cohort samples and clinical data and wrote the manuscript; J.A. performed statistical analysis; C.M. and M.J.P. contributed with some flow cytometry experiments; D.M. and P.K. were involved in recruitment of some participants of the study at MedStar Georgetown University Hospital; M.O. was involved in the staining and evaluation of the progenitor cells; C.J. contributed with unsupervised clustering analysis and editing of the manuscript; and all authors listed critically reviewed the manuscript and approved it for submission.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marta Catalfamo, Department of Microbiology and Immunology, Georgetown University School of Medicine, 3970 Reservoir Rd NW, New Research Building, Room EG19A, Washington, DC 20057; email: mc2151@georgetown.edu.
References
Author notes
Original data are available from the corresponding author, Marta Catalfamo (mc2151@georgetown.edu).
The full-text version of this article contains a data supplement.