Key Points
The nonactivating β3(R760C) mutation causes macrothrombocytopenia.
Abnormal actin rearrangement and impaired αIIbβ3/RhoA pathway play major roles in β3(R760C)-related macrothrombocytopenia.
Visual Abstract
Almost all mutations of ITGA2B or ITGB3 identified in congenital macrothrombocytopenia induce constitutive activation of αIIbβ3. However, whether concomitant αIIbβ3 activation is essential for macrothrombocytopenia development remains unknown. Recently, we identified the β3(R760C) mutation that does not induce αIIbβ3 activation in a patient with macrothrombocytopenia. The family study showed that macrothrombocytopenia with reduced expression of αIIbβ3 and glycoprotein IV (GPVI) appeared to be associated with patients heterozygous for β3(R760C). We generated β3(R760C) knockin (KI) mice and investigated the effects of the mutation on platelet/megakaryote biology. Macrothrombocytopenia was decreased to 76% and 40% of platelet counts in heterozygous (Hetero) and homozygous (Homo) KI mice, respectively, when compared with the wild-type mice. Platelet αIIbβ3 and GPVI expression were decreased in KI mice, and αIIbβ3 activation was not detected in nonstimulated KI platelets. Thus, the hetero KI mice reproduced the phenotype of the human participant, indicating that the β3(R760C) mutation is responsible for the macrothrombocytopenia. Platelet aggregation, agonist-induced JON/A binding, and P-selectin expression were impaired in KI mice. Platelet spreading on fibrinogen was also impaired in Homo mice with adenosine 5′-diphosphate or thrombin stimulation. Filopodia and lamellipodia formation was impaired in fibrinogen-adhered megakaryocytes of Homo mice with significantly impaired RhoA activation. Proplatelet formation in Homo mice was impaired with abnormal morphology. In addition, platelet life span was shortened in Homo mice. These data indicate that the β3(R760C) mutation impairs the inside-out and outside-in signaling of αIIbβ3, and abnormal actin rearrangement and impaired RhoA activation may play major roles in macrothrombocytopenia.
Introduction
Integrin αIIbβ3 is a receptor for fibrinogen and von Willebrand factor (VWF) that plays essential roles in platelet adhesion and aggregation. In resting platelets, αIIbβ3 is in a low-affinity bent form, but activation signals, known as inside-out signaling, mobilize the intracellular proteins that bind to the cytoplasmic domain of αIIbβ3 and shift their conformation to a high-affinity extended form.1 Recent reports also show the intermediate state of αIIbβ3 activation between the inactive/bent form and the complete active/extended form.2,3 Ligand binding and integrin clustering lead to platelet spreading, thrombus formation, and cytoskeletal rearrangement. Many positive and negative regulators are involved in this outside-in signaling, and the cytoplasmic tails of integrin play essential roles in this process.4
Congenital abnormalities in the quantity or quality of αIIbβ3 lead to severe bleeding diathesis, which is known as Glanzmann thrombasthenia.5,6 Platelet counts and morphology are generally normal in patients with Glanzmann thrombasthenia and in αIIbβ3-deficient mice or dogs.5-8 However, some reports suggest that αIIbβ3 is involved in proplatelet formation and the release of platelets from proplatelet tips.9,10 Recently, a unique subgroup of αIIbβ3 mutations that are associated with familial macrothrombocytopenia have been reported.11-18 This subgroup was first reported by Hardisty et al in 1992.19,20 Subsequent genetic analysis found the R1026Q substitution, previously reported as R995Q because of the old numbering, in the cytoplasmic tail of αIIb.11 αIIb(R1026) forms a salt-bridge with β3(D749) and plays an essential role in keeping αIIbβ3 in the resting state. Transfection assays showed that αIIb(R1026Q) did not cause spontaneous fibrinogen binding but facilitated PAC-1 binding.11 Subsequently, Ghevaert et al reported in 2008 that patients with heterozygous β3(D749H) exhibited macrothrombocytopenia, showing constitutive activation of αIIbβ3 by the mutation.12 We also revealed the αIIb(R1026W) substitution, previously reported as αIIb(R995W), in Japanese families with macrothrombocytopenia and suggested the constitutive activation of αIIbβ3 by the mutation and aberrant outside-in signaling as potential causes for macrothrombocytopenia via abnormal cytoskeletal rearrangement using knockin (KI) mice.15,21 Almost all mutations associated with αIIbβ3-related macrothrombocytopenia are located around the transmembrane domains of αIIb or β3 and lead to the intermediate activation of αIIbβ3.13-18 However, whether this kind of constitutive intermediate activation of αIIbβ3 is essential for congenital macrothrombocytopenia remains unknown.
Recently, we identified a mutation, β3(R760C), which is also located in the cytoplasmic region of β3 in a Japanese family with macrothrombocytopenia. This mutation was recently reported in a family with macrothrombocytopenia as a likely pathogenic mutation by Morais et al.22 In this study, the platelets of β3(R760C)-bearing patients were analyzed, and it was suggested that macrothrombocytopenia with reduced expression of αIIbβ3 and impaired platelet activation was associated with the mutation. Because we found that the mutation did not induce constitutive activation of αIIbβ3, we analyzed KI mice to further investigate the effects of the nonactivating mutation, β3(R760C), on platelet/megakaryocyte (Mgk) pathophysiology.
Materials and methods
Generation of β3(R760C) KI mice and preparation of platelets and Mgks
All animal procedures were approved by the Institute of Experimental Animal Sciences, Faculty of Medicine, Osaka University. Because human β3(R760) corresponds to mouse β3(R759), mouse β3(R759) was substituted with cysteine using clustered regularly interspaced palindromic repeat (CRISPR)/CRISPR-associated protein 9 genome editing (supplemental Figure 1). We called this the β3(R760C) KI mouse to avoid confusion about the mutated residue in humans and mice. Blood was collected via cardiac puncture in acid-citrate-dextrose solution A and centrifuged at 200g to obtain the platelet-rich plasma. Washed platelets obtained via centrifugation of platelet-rich plasma at 750g for 10 minutes were suspended in Tyrode-HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) buffer (137 mM NaCl, 2.6 mM KCl, 12 mM NaHCO3, 5.5 mM d-glucose, 5 mM HEPES, 1 mM MgCl2, pH 7.4) with 1 mM CaCl2.
Mgks were obtained from the bone marrow cells of the femur and tibia of mice using a bovine serum albumin gradient after 5 days of culture with recombinant murine thrombopoietin (TPO; PeproTech, Cranbury, NJ).
Hematologic and histologic analyses
Complete blood cell counts were obtained using EDTA-anticoagulated blood and an automated hematology analyzer (KX-21NV; Sysmex, Kobe, Japan). Platelet size was determined via forward scatter in CD42b+ cells using flow cytometry.
Femurs and spleens were harvested from 8- to 10-week-old male mice, and formaldehyde-fixed paraffin sections were stained with hematoxylin and eosin and rabbit polyclonal anti-VWF antibodies (VWFpp polyclonal antibodies; Proteintech, Rosemont, IL).
Platelet GP analysis
Surface glycoprotein (GP) expression in mouse platelets and Mgks was analyzed using flow cytometry. The total GP expression in mouse platelets was analyzed using Triton X–treated cell lysates and protein blotting.
The activation state of αIIbβ3 and granular secretion with or without agonists were assessed by an activation-dependent anti-mouse αIIbβ3 monoclonal antibody, phycoerythrin-conjugated JON/A (Emfret Analytics, Würzburg, Germany), and fluorescein isothiocyanate-conjugated anti-CD62P monoclonal antibody (BD Biosciences, San Jose, CA). JON/A binding was adjusted by CD41 expression and described as the percentage of binding to wild-type (WT) platelets.
Adhesion assay
Adhesion of platelets and Mgks to immobilized fibrinogen was assessed after incubation with or without adenosine 5′-diphosphate (ADP) or thrombin for 40 minutes at 37°C. After fixation and permeabilization, platelets and Mgks were stained with teramethyl rhodamine isothiocyanate (TRITC)-conjugated phalloidin (Sigma-Aldrich, St. Louis, MO) and analyzed via confocal microscopy.
RhoA activity assay
The amount of activated RhoA was measured using G-LISA, an enzyme-linked immunosorbent assay–based assay that quantifies the amount of the active guanosine triphosphate (GTP)–bound form of RhoA, according to the manufacturer’s instructions (Cytoskeleton, Denver, CO). Briefly, cultured murine Mgks were serum starved for 24 hours and plated on fibrinogen precoated dishes. After 15 minutes of incubation, the cells were solubilized, and activated forms of RhoA in the supernatant were captured in a 96-well microtiter plate, followed by incubation with the anti-RhoA primary and horseradish peroxidase–conjugated secondary antibodies. The amount of active RhoA was determined by measuring the luminescence intensity of horseradish peroxidase reagent using an iMark microplate reader (Bio-Rad, Hercules, CA).
Platelet life span analysis
The platelet life span was analyzed as previously described.21 Briefly, NHS-biotin (biotin-N-hydroxysuccinimide ester; Thermo Scientific, Rockford, IL) was injected into the murine tail vein, and biotinylated platelets were detected using an allophycocyanin-conjugated streptavidin antibody in CD42b+ cells via flow cytometry.
Proplatelet formation analysis
Proplatelet formation was analyzed as previously described.21 Briefly, murine fetal liver cells were harvested from embryonic day 13.5 embryos and cultured with murine TPO. Proplatelet formation was monitored 4 days after the addition of TPO.
Results
Analysis of patients with congenital macrothrombocytopenia
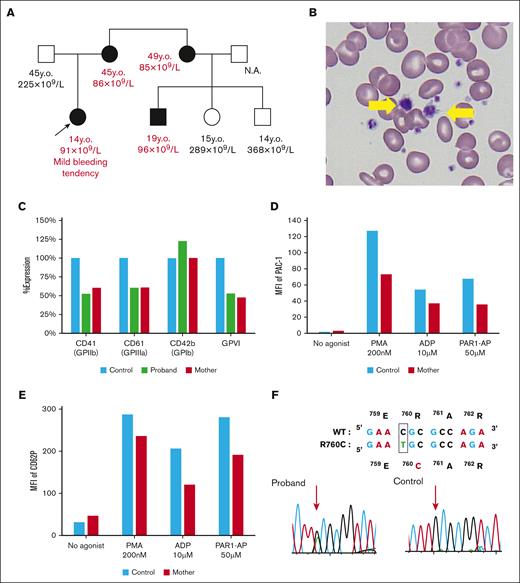
The proband was a 14-year-old Japanese girl who presented with macrothrombocytopenia with platelet counts of 60 × 109/L to 90 × 109/L and a mild bleeding tendency since birth. The family study showed that her mother, maternal sister, and cousin exhibited mild thrombocytopenia with platelet counts of 85 × 109/L to 96 × 109/L and increased mean platelet volume. Her mother also showed severe iron-deficiency anemia (Figure 1A-B; Table 1). The percentage of reticulated platelets in the affected patients was normal to slightly increased, and the serum TPO levels were within the normal range. αIIbβ3 expression on platelets was ∼50% that of healthy controls. GPVI expression was also decreased to ∼50%, whereas glycoprotein Ib expression was normal or increased (Figure 1C). We performed platelet functional analysis and found no PAC-1 binding to the nonstimulated platelets. The PAC-1 binding was decreased with ADP, protease-activated receptor-1 activating peptide, and phorbol 12-myristate 13-acetate stimulation when compared with that in the healthy control. Impaired PAC-1 binding is likely to be affected by the decrease in αIIbβ3 expression (Figure 1D). However, ADP- or protease-activated receptor-1 activating peptide–induced CD62P expression was impaired in the affected patient, suggesting that platelet cytoskeletal rearrangement may be impaired (Figure 1E). Genetic analysis revealed that all affected patients were heterozygous for ITGB3(R760C), and this mutation was not detected in the unaffected patients (Figure 1F). These results suggest that β3(R760C) substitution is responsible for macrothrombocytopenia. Because almost all ITGA2B and ITGB3 mutations associated with macrothrombocytopenia induce constitutive activation of αIIbβ3,16 we further assessed PAC-1 binding on αIIbβ3(R760C)–transfected 293T cells in this study. In contrast with the previously reported αIIb(R1026W) mutation, the mutation identified in this study did not cause the constitutive activation of αIIbβ3 (supplemental Figure 2).
Analysis of a family with β3(R760C)-related macrothrombocytopenia. (A) Family tree of the proband. Squares represent the male and circles represent the female family members. Arrow indicates the proband. Clinical status is indicated by the open (unaffected) or solid (affected) symbol. Age and platelet count are listed under each symbol. (B) May-Grünwald Giemsa staining of the proband peripheral blood film. Giant platelets are indicated by yellow arrows. (C) Surface GP expression levels in platelets were determined via flow cytometry. Data are shown as percentage expression relative to the father’s platelets (healthy control). (D) PAC-1 binding on platelets with or without agonists was detected via flow cytometry. (E) P-selectin (CD62P) expression with or without agonists was determined via flow cytometry. Representative results of 2 to 3 independent experiments are shown in panels C-E. (F) Genetic analysis of the proband and her father (control). Sequencing of platelet complementary DNA and genomic DNA revealed that the affected family members exhibited the heterozygous β3(R760C) mutation. MFI, mean fluorescence intensity; N.A., not available; y.o., years old.
Analysis of a family with β3(R760C)-related macrothrombocytopenia. (A) Family tree of the proband. Squares represent the male and circles represent the female family members. Arrow indicates the proband. Clinical status is indicated by the open (unaffected) or solid (affected) symbol. Age and platelet count are listed under each symbol. (B) May-Grünwald Giemsa staining of the proband peripheral blood film. Giant platelets are indicated by yellow arrows. (C) Surface GP expression levels in platelets were determined via flow cytometry. Data are shown as percentage expression relative to the father’s platelets (healthy control). (D) PAC-1 binding on platelets with or without agonists was detected via flow cytometry. (E) P-selectin (CD62P) expression with or without agonists was determined via flow cytometry. Representative results of 2 to 3 independent experiments are shown in panels C-E. (F) Genetic analysis of the proband and her father (control). Sequencing of platelet complementary DNA and genomic DNA revealed that the affected family members exhibited the heterozygous β3(R760C) mutation. MFI, mean fluorescence intensity; N.A., not available; y.o., years old.
Hematologic parameters of the proband and her family
| Parameter . | Proband . | Mother . | Aunt . | Cousin 1 . | Cousin 2 . | Cousin 3 . | Father . |
|---|---|---|---|---|---|---|---|
| (19 y.o.) . | (15 y.o.) . | (14 y.o.) . | |||||
| WBC (× 109/L) | 6.63 | 5.01 | 5.08 | 6.25 | 7.11 | 5.89 | 4.86 |
| RBC (× 1012/L) | 4.93 | 5.04 | 4.13 | 5.33 | 4.83 | 4.46 | 4.91 |
| Hb (g/dL) | 14.3 | 8.7 | 12.3 | 16.9 | 14.9 | 12.7 | 13.3 |
| Hct (%) | 41.5 | 32.3 | 37.8 | 48.9 | 43.6 | 38.4 | 41.3 |
| MCV (fL) | 84.2 | 64.1 | 91.5 | 91.7 | 90.3 | 86.1 | 84.1 |
| MPV (fL) | 12.7 | N.D. | 12.3 | 13.1 | 10.5 | 9.5 | 9.7 |
| PLT (× 109/L) | 91 | 86 | 85 | 96 | 289 | 368 | 225 |
| RP% | 11.1 | 7.9 | 9.7 | 10.3 | 9.8 | 8.7 | 4.9 |
| TPO (pg/mL) | 9.0 | 34.0 | 16.0 | 12.0 | 9.0 | 8.4 | 30.0 |
| Parameter . | Proband . | Mother . | Aunt . | Cousin 1 . | Cousin 2 . | Cousin 3 . | Father . |
|---|---|---|---|---|---|---|---|
| (19 y.o.) . | (15 y.o.) . | (14 y.o.) . | |||||
| WBC (× 109/L) | 6.63 | 5.01 | 5.08 | 6.25 | 7.11 | 5.89 | 4.86 |
| RBC (× 1012/L) | 4.93 | 5.04 | 4.13 | 5.33 | 4.83 | 4.46 | 4.91 |
| Hb (g/dL) | 14.3 | 8.7 | 12.3 | 16.9 | 14.9 | 12.7 | 13.3 |
| Hct (%) | 41.5 | 32.3 | 37.8 | 48.9 | 43.6 | 38.4 | 41.3 |
| MCV (fL) | 84.2 | 64.1 | 91.5 | 91.7 | 90.3 | 86.1 | 84.1 |
| MPV (fL) | 12.7 | N.D. | 12.3 | 13.1 | 10.5 | 9.5 | 9.7 |
| PLT (× 109/L) | 91 | 86 | 85 | 96 | 289 | 368 | 225 |
| RP% | 11.1 | 7.9 | 9.7 | 10.3 | 9.8 | 8.7 | 4.9 |
| TPO (pg/mL) | 9.0 | 34.0 | 16.0 | 12.0 | 9.0 | 8.4 | 30.0 |
Normal ranges of RP% and TPO are 1.4% to 9.1% and <106 pg/mL, respectively.23
Hb, hemoglobin; Hct, hematocrit; MCV, mean corpuscular volume; MPV, mean platelet volume; N.D., not detected; PLT, platelet count; RBC, red blood cell count; RP%, percentage of reticulated platelets; WBC, white blood cell count; y.o., years old.
Macrothrombocytopenia with reduced expression of αIIbβ3 and GPVI in β3(R760C) KI mice
We generated KI mice with the β3(R760C) mutation and investigated the underlying molecular defects to examine whether this nonactivating mutation could be responsible for the macrothrombocytopenia. KI mice were fully viable and showed no apparent bleeding. Platelet counts decreased to 76% and 40% in heterozygous (Hetero) and homozygous (Homo) mice, respectively, when compared with that in the WT mice (Figure 2A; Table 2). No significant differences were found in the red blood cell counts, hemoglobin levels, and white blood cell counts (Table 2). Peripheral blood films showed an increase in large platelets in Homo mice when compared with that in WT mice, and the increased size of KI platelets was confirmed via flow cytometry (Figure 2A). These results indicate that the β3(R760C) mutation is responsible for the macrothrombocytopenia.
Number, morphology, and GP expression in β3(R760C) KI platelets. (A) Peripheral blood was obtained from 8- to 10-week-old male mice. Giant platelets are indicated by yellow arrows in murine peripheral blood films stained with the May-Grünwald Giemsa stain (upper panel). Platelet counts in each phenotype are presented as the mean and standard deviation (SD; n = 6 each; lower left panel). Platelet size in each phenotype was determined as the mean of forward scatter (FSC) of CD42b+ cells via flow cytometry (lower right panel). Data are presented as the mean and SD (n = 6 each). (B) Surface GP expression was determined via flow cytometry. Data are shown as percentage expression in comparison with the mean of the WT. Data are presented as mean and SD (n = 6 each). (C). Soluble GPVI concentration in murine plasma was measured using an enzyme-linked immunosorbent assay (ELISA) kit. Measurements were taken from 3 mice of each phenotype in 2 independent experiments. The data are presented as the mean and SD. (D) Total GP expression in KI mouse platelets. Lysates obtained from the washed platelets were separated via sodium dodecyl sulfate-polyacrylamide gel electrophoresis, followed by immunoblotting using specific antibodies. GPV expression levels were additionally determined as the internal control in the same membrane. ∗∗∗P < .001, ∗∗P < .01, and ∗P < .05 (1-way analysis of variance [ANOVA]). MFI, mean fluorescence intensity; ns, not significant.
Number, morphology, and GP expression in β3(R760C) KI platelets. (A) Peripheral blood was obtained from 8- to 10-week-old male mice. Giant platelets are indicated by yellow arrows in murine peripheral blood films stained with the May-Grünwald Giemsa stain (upper panel). Platelet counts in each phenotype are presented as the mean and standard deviation (SD; n = 6 each; lower left panel). Platelet size in each phenotype was determined as the mean of forward scatter (FSC) of CD42b+ cells via flow cytometry (lower right panel). Data are presented as the mean and SD (n = 6 each). (B) Surface GP expression was determined via flow cytometry. Data are shown as percentage expression in comparison with the mean of the WT. Data are presented as mean and SD (n = 6 each). (C). Soluble GPVI concentration in murine plasma was measured using an enzyme-linked immunosorbent assay (ELISA) kit. Measurements were taken from 3 mice of each phenotype in 2 independent experiments. The data are presented as the mean and SD. (D) Total GP expression in KI mouse platelets. Lysates obtained from the washed platelets were separated via sodium dodecyl sulfate-polyacrylamide gel electrophoresis, followed by immunoblotting using specific antibodies. GPV expression levels were additionally determined as the internal control in the same membrane. ∗∗∗P < .001, ∗∗P < .01, and ∗P < .05 (1-way analysis of variance [ANOVA]). MFI, mean fluorescence intensity; ns, not significant.
Hematologic parameters of β3(R760C) KI mice
| Parameter . | WT . | Hetero . | Homo . |
|---|---|---|---|
| WBC (×109/L) | 5.46 (2.40-8.60) | 6.58 (2.40-12.70) | 4.38 (2.90-6.00) |
| RBC (×1012/L) | 10.07 (9.56-10.46) | 9.85 (9.30-10.78) | 9.79 (8.46-10.93) |
| Hb (g/dL) | 14.5 (13.9-14.8) | 14.3 (13.4-15.4) | 14.0 (12.3-15.5) |
| PLT (×109/L) | 1095 (1043-1183) | 834 (689-949)∗ | 445 (360-496)∗ |
| RP% | 8.04 (7.68-8.24) | 8.39 (7.73-8.87) | 8.62 (8.30-8.78) |
| TPO (pg/mL) | 283 (233-330) | 208 (203-219) | 270 (233-345) |
| Parameter . | WT . | Hetero . | Homo . |
|---|---|---|---|
| WBC (×109/L) | 5.46 (2.40-8.60) | 6.58 (2.40-12.70) | 4.38 (2.90-6.00) |
| RBC (×1012/L) | 10.07 (9.56-10.46) | 9.85 (9.30-10.78) | 9.79 (8.46-10.93) |
| Hb (g/dL) | 14.5 (13.9-14.8) | 14.3 (13.4-15.4) | 14.0 (12.3-15.5) |
| PLT (×109/L) | 1095 (1043-1183) | 834 (689-949)∗ | 445 (360-496)∗ |
| RP% | 8.04 (7.68-8.24) | 8.39 (7.73-8.87) | 8.62 (8.30-8.78) |
| TPO (pg/mL) | 283 (233-330) | 208 (203-219) | 270 (233-345) |
Peripheral blood was obtained from 8- to 10-week-old male mice. The average (range) is shown (n = 6).
Hb, hemoglobin; Hct, hematocrit; MCV, mean corpuscular volume; PLT, platelet count; RBC, red blood cell count; RP%, percentage of reticulated platelets; WBC, white blood cell count.
P < .05 vs WT.
Flow cytometry analysis revealed that surface αIIbβ3 expression levels in Hetero and Homo platelets were reduced to ∼70% and 30%, respectively, relative to those in WT platelets (Figure 2B). Surface GPVI expression levels in Hetero and Homo platelets were reduced to 88% and 66% of that in WT platelets, respectively, consistent with the phenotype of the platelets from the human participants (Figure 2B). C-type lectin-like receptor 2 (CLEC-2) expression was slightly, but significantly, decreased in KI platelets (Figure 2B). In contrast, glycoprotein Ib expression was increased in KI platelets probably because of an increase in platelet size (Figure 2B). Immunoblotting revealed that whole αIIbβ3 and GPVI expression levels in Homo platelets were decreased when compared with those in WT platelets (Figure 2C). In addition, a significant increase in soluble GPVI levels was observed in Homo plasma, suggesting that an increase in GPVI shedding contributes, at least in part, to decreased GPVI expression in KI platelets (Figure 2D). RNA-sequencing analysis showed no major differences between Homo and WT platelets in the messenger RNA levels of platelet GPs, including GPVI (supplemental Figure 3).
Impaired platelet functions in KI mice
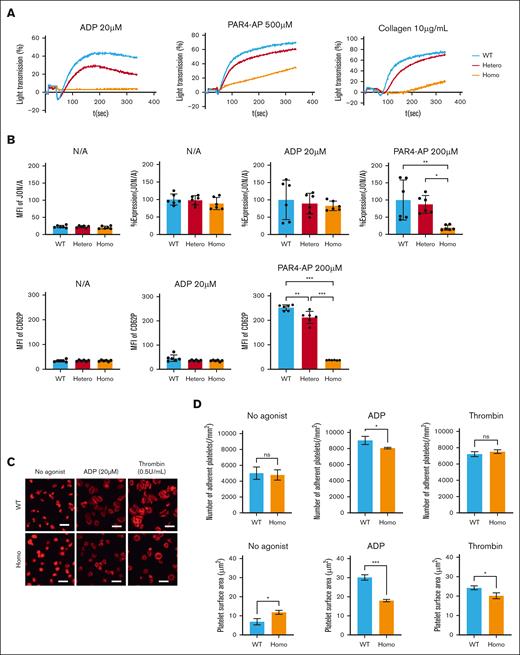
Platelet aggregation induced by collagen, PAR4-AP, and ADP was markedly impaired in Homo platelets relative to Hetero and WT platelets (Figure 3A). Because the expression level of αIIbβ3 may affect the results of platelet aggregation, the affinity state of αIIbβ3 was assessed by binding of an activation-dependent anti-αIIbβ3 antibody, JON/A. After adjustment of αIIbβ3 expression, there was no difference in JON/A binding without agonists between KI and WT platelets, confirming that β3(R760C) does not cause spontaneous activation of αIIbβ3 (Figure 3B). ADP-induced JON/A binding in KI platelets did not differ from that in WT platelets, but PAR4-induced JON/A binding was significantly reduced in Homo platelets (Figure 3B). Accordingly, the impaired ADP-induced platelet aggregation is mainly caused by the reduction in αIIbβ3 expression. In addition, PAR4-induced CD62P expression was reduced in the Homo platelets (Figure 3B). These results indicated that platelet activation, especially PAR4-induced activation signaling, was impaired in KI mice.
Functional analysis of KI platelets. (A) Platelet aggregation study was assessed using platelet-rich plasma diluted to a concentration of 250 × 109/L. Data are representative of three independent experiments. (B) Activation state of αIIbβ3 and granular secretion were assessed by determining the JON/A binding (upper) and CD62P (lower) expression levels on platelets, respectively, with or without an agonist via flow cytometry. JON/A binding was adjusted by the expression level of CD41 and shown as %expression compared to that of WT. Data are presented as the mean and SD (n = 6 each). ∗∗∗P < .001 and ∗∗P < .01 (one-way ANOVA). (C) Washed platelets (2.5 × 105) from WT or Homo were added to the fibrinogen-coated coverslips with or without 20 μM ADP or 0.5 U/mL thrombin and incubated at 37°C for 20 min. Then platelets were fixed, permeabilized, and stained with TRITC-conjugated phalloidin. Scale bars represent 10μm. Representative results of 2 independent experiments are shown. (D) The number of adherent platelets (upper) and covered area (lower) were analyzed using the ImageJ software (National Institutes of Health). Results are presented as the mean and SD. ∗∗∗P < .001, ∗∗P < .01, and ∗P < .05 (unpaired t test).
Functional analysis of KI platelets. (A) Platelet aggregation study was assessed using platelet-rich plasma diluted to a concentration of 250 × 109/L. Data are representative of three independent experiments. (B) Activation state of αIIbβ3 and granular secretion were assessed by determining the JON/A binding (upper) and CD62P (lower) expression levels on platelets, respectively, with or without an agonist via flow cytometry. JON/A binding was adjusted by the expression level of CD41 and shown as %expression compared to that of WT. Data are presented as the mean and SD (n = 6 each). ∗∗∗P < .001 and ∗∗P < .01 (one-way ANOVA). (C) Washed platelets (2.5 × 105) from WT or Homo were added to the fibrinogen-coated coverslips with or without 20 μM ADP or 0.5 U/mL thrombin and incubated at 37°C for 20 min. Then platelets were fixed, permeabilized, and stained with TRITC-conjugated phalloidin. Scale bars represent 10μm. Representative results of 2 independent experiments are shown. (D) The number of adherent platelets (upper) and covered area (lower) were analyzed using the ImageJ software (National Institutes of Health). Results are presented as the mean and SD. ∗∗∗P < .001, ∗∗P < .01, and ∗P < .05 (unpaired t test).
Next, we assessed platelet adhesion to immobilized fibrinogen. Although the number of adherent platelets in Homo samples without the agonist was not different from that in WT, filopodia formation and shape change in Homo platelets were impaired when compared with those in WT platelets (Figure 3C). Moreover, the spreading of Homo platelets on fibrinogen with ADP or thrombin was impaired in KI platelets (Figure 3C-D). We also observed significantly impaired focal adhesion kinase phosphorylation in fibrinogen-adhered Homo platelets (supplemental Figure 4).
These results indicate that KI mouse platelets exhibit impaired platelet functions with impaired inside-out and outside-in signaling of αIIbβ3.
Morphologic abnormalities and impaired RhoA activation in fibrinogen-adhered KI Mgks
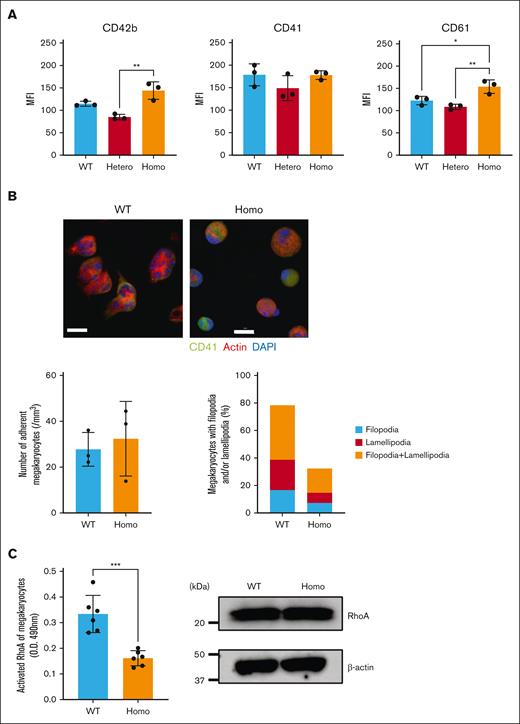
The impaired platelet functions in KI mice may be a consequence of the decreased αIIbβ3 expression in KI platelets. To verify this, we conducted experiments using Mgks. The surface expression level of αIIbβ3 on KI Mgks was comparable with that on WT Mgks (Figure 4A). No apparent differences were observed in the adhesion cell counts of Mgks on fibrinogen, but filopodia and lamellipodia formation of fibrinogen-adhered Mgks were impaired in Homo mice when compared with that in WT mice (Figure 4B). We also observed impaired RhoA activation in fibrinogen-adhered Homo Mgks despite the equivalent expression of RhoA in KI and WT Mgks (Figure 4C). These results indicate that outside-in signaling through αIIbβ3 is impaired by the β3(R760C) mutation, which leads to impaired actin rearrangement with impaired RhoA activation.
Morphology and RhoA activation in fibrinogen-adhered Mgks. (A) Mgks were isolated from the bone marrow cells of the femur and/or tibia of mice using a bovine serum albumin gradient after 5 days of culture with recombinant murine TPO. GP expression was determined via flow cytometry. The data are presented as the mean and SD (n = 3 each). ∗∗P < .01 and ∗P < .05 (1-way ANOVA). (B) Cultured murine Mgks (3.0 × 104) were added to fibrinogen-coated coverslips and incubated at 37°C for 20 minutes. The cells were then fixed, permeabilized, and stained with TRITC-conjugated phalloidin (red), Alexa Fluor 488–labeled CD41 (green), and DAPI (4',6-diamidino-2-phenylindole; blue), followed by observation under a confocal microscope (Olympus FV3000) (40×). Scale bars represent 50 μm. (C) Cultured murine Mgks were serum starved for 24 hours and plated on fibrinogen-coated dishes. After 15 minutes of incubation, the cells were solubilized with lysis buffers provided by the manufacturer. The amount of activated RhoA was measured using an ELISA-based assay kit, G-LISA, that quantifies the amount of the active GTP-bound form of RhoA. Measurements were taken for 3 mice of each phenotype in 2 independent experiments (left). The total RhoA expression in murine Mgks was measured via immunoblotting (right). Data are representative of 2 independent experiments. MFI, mean fluorescence intensity.
Morphology and RhoA activation in fibrinogen-adhered Mgks. (A) Mgks were isolated from the bone marrow cells of the femur and/or tibia of mice using a bovine serum albumin gradient after 5 days of culture with recombinant murine TPO. GP expression was determined via flow cytometry. The data are presented as the mean and SD (n = 3 each). ∗∗P < .01 and ∗P < .05 (1-way ANOVA). (B) Cultured murine Mgks (3.0 × 104) were added to fibrinogen-coated coverslips and incubated at 37°C for 20 minutes. The cells were then fixed, permeabilized, and stained with TRITC-conjugated phalloidin (red), Alexa Fluor 488–labeled CD41 (green), and DAPI (4',6-diamidino-2-phenylindole; blue), followed by observation under a confocal microscope (Olympus FV3000) (40×). Scale bars represent 50 μm. (C) Cultured murine Mgks were serum starved for 24 hours and plated on fibrinogen-coated dishes. After 15 minutes of incubation, the cells were solubilized with lysis buffers provided by the manufacturer. The amount of activated RhoA was measured using an ELISA-based assay kit, G-LISA, that quantifies the amount of the active GTP-bound form of RhoA. Measurements were taken for 3 mice of each phenotype in 2 independent experiments (left). The total RhoA expression in murine Mgks was measured via immunoblotting (right). Data are representative of 2 independent experiments. MFI, mean fluorescence intensity.
Impaired proplatelet formation and shortened platelet life span in KI mice
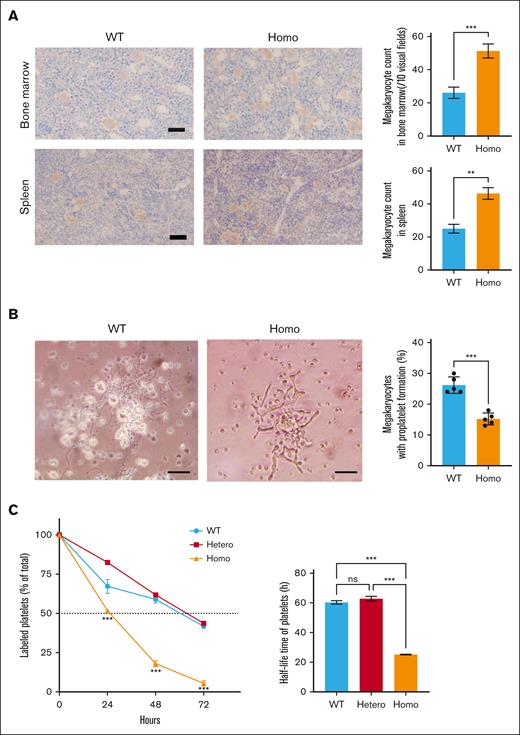
To further investigate the pathologic mechanism of macrothrombocytopenia in KI mice, we analyzed the numbers of Mgks in the bone marrow and spleen. The number of Mgks was unexpectedly increased in KI mice (Figure 5A). However, no difference was observed in the ploidy of Mgks in KI and WT mice (supplemental Figure 5A), and no splenomegaly was observed in KI mice (supplemental Figure 5B). Because KI mice did not show apparent bleeding diathesis, these results suggest that failure at the terminal stage of platelet production affects macrothrombocytopenia in KI mice. Subsequently, proplatelet formation by Mgks was assessed using fetal liver–derived Mgks. The number of proplatelet-formed Mgks was significantly decreased in Homo samples, and Homo proplatelets exhibited a shortened and bold morphological abnormality when compared with WT proplatelets (Figure 5B). Next, we analyzed the platelet life span by injecting NHS-biotin and detecting streptavidin-binding platelets over time. Although no differences were observed in the percentage of labeled platelets in WT and Hetero mice, this percentage was significantly decreased 24 and 48 hours after injection in Homo mice when compared with that in WT mice (Figure 5C).
Mgks in bone marrow, proplatelet formation, and platelet life span. (A) Mgks in the bone marrow from the femur and spleen were assessed under an optical microscope (left). Mgks were detected by staining with anti-VWF antibodies. The counts of Mgks in 10 visual fields (200× original magnification) were determined (right). (B) Murine fetal liver cells were harvested from embryonic day 13.5 embryos and cultured with murine TPO. Proplatelet formation was monitored 4 days after the addition of TPO. Images were obtained using the BX51 microscope (Olympus) with 20× objective (left). The scale bars represent 50 μm. The percentages of proplatelet-forming Mgks are demonstrated in the right panel. The results are presented as the mean and SD. Representative results of 2 independent experiments are shown (n = 6). (C) Mice were injected with 600 μg of NHS-biotin via the murine tail vein on day 0, and the percentages of biotinylated platelets at various time points were assessed via flow cytometry. The results are presented as the mean and SD (n = 3 each). ∗∗∗P < .001 (unpaired t test). ns, not significant.
Mgks in bone marrow, proplatelet formation, and platelet life span. (A) Mgks in the bone marrow from the femur and spleen were assessed under an optical microscope (left). Mgks were detected by staining with anti-VWF antibodies. The counts of Mgks in 10 visual fields (200× original magnification) were determined (right). (B) Murine fetal liver cells were harvested from embryonic day 13.5 embryos and cultured with murine TPO. Proplatelet formation was monitored 4 days after the addition of TPO. Images were obtained using the BX51 microscope (Olympus) with 20× objective (left). The scale bars represent 50 μm. The percentages of proplatelet-forming Mgks are demonstrated in the right panel. The results are presented as the mean and SD. Representative results of 2 independent experiments are shown (n = 6). (C) Mice were injected with 600 μg of NHS-biotin via the murine tail vein on day 0, and the percentages of biotinylated platelets at various time points were assessed via flow cytometry. The results are presented as the mean and SD (n = 3 each). ∗∗∗P < .001 (unpaired t test). ns, not significant.
These results indicate that failure in the terminal stage of platelet production, including abnormal proplatelet formation and shortened platelet life span, may be responsible for macrothrombocytopenia in KI mice.
Discussion
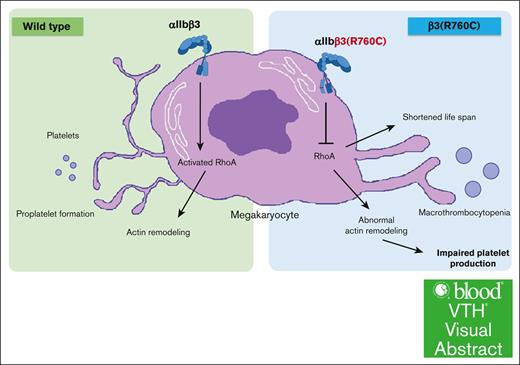
We found the nonactivating β3(R760C) mutation in a patient with macrothrombocytopenia. The family study showed that macrothrombocytopenia with reduced expression of αIIbβ3, GPVI, and impaired platelet activation was associated with heterozygous participants. The KI mice study demonstrated that the mutation (1) causes macrothrombocytopenia, (2) reduces the expression of αIIbβ3 and GPVI on platelets, (3) leads to impairment of agonist-induced inside-out signaling, especially PAR4-induced signaling, (4) leads to impaired outside-in signaling of αIIbβ3, that is, impaired cytoskeletal rearrangement with reduced RhoA activation, and (5) impairs proplatelet production from Mgks that seemed to be the main cause of β3(R760C)-related macrothrombocytopenia; shortened platelet life span may exacerbate thrombocytopenia.
Several mutations in ITGA2B and ITGB3 have been reported in αIIbβ3-related macrothrombocytopenia, and almost all mutations are located around the transmembrane domain of αIIb or β3.16 These regions are especially important for control of the αIIbβ3 affinity state. For example, disruption of the salt-bridge between αIIb(R1026) and β3(D749) by the αIIb(R1026W) or β3(D749H) mutation causes spontaneous intermediate activation of αIIbβ3, rather than full activation of αIIbβ3, with macrothrombocytopenia.12,15 In other mutations associated with αIIbβ3-related macrothrombocytopenia, such as αIIb(G1022C), αIIb(F1024del), β3(D647-E686del), β3(L744P), and β3(T746del), spontaneous intermediate αIIbβ3 activation was also detected.13,14,16-18 It may be possible that a constitutive intermediate state of αIIbβ3 activation, per se, might play a critical role in abnormal proplatelet formation and shortened platelet life span, thus contributing to the development of the thrombocytopenia. However, the validity of this hypothesis remains unclear. The β3(R760C) mutation was reported by Morais et al in 2020; platelets heterozygous in the mutation increased PAC-1 binding without agonist stimulation.22 We independently found β3(R760C) in a Japanese family with macrothrombocytopenia. Contrary to the results of Morais et al, we did not detect spontaneous PAC-1 binding to β3(R760C)-bearing platelets and confirmed that β3(R760C) was a nonactivating mutation by the transfection assay. The KI mice study demonstrated that β3(R760C) causes macrothrombocytopenia without inducing constitutive activation of αIIbβ3. Our results also showed that the mutation led to impaired inside-out and outside-in signaling through αIIbβ3. Specifically, abnormalities in spreading on fibrinogen and reduced proplatelet formation indicate that abnormal platelet production with impaired cytoskeletal rearrangement seems to be the major mechanisms of β3(R760C)-related macrothrombocytopenia, indicating that constitutive activation of αIIbβ3 is dispensable but abnormal outside-in signaling and cytoskeletal rearrangement are essential for developing macrothrombocytopenia.
Abnormal proplatelet formation of Mgks with morphologic abnormalities has been demonstrated using Mgks derived from patients with congenital macrothrombocytopenia, KI mice, and αIIbβ3 mutant-transfected Mgks.10,15,21 Other studies suggested that downregulation of RhoA activation caused by an αIIbβ3 mutation may be responsible for abnormal platelet production.18,24 Activation of RhoA is involved in the terminal stage of platelet production.25,26 Importantly, Mgk-specific RhoA-knockout (KO) mice exhibit macrothrombocytopenia.27 Consistent with these lines of evidence, our results suggest that impaired outside-in signaling caused by β3(R760C) leads to macrothrombocytopenia via impairment of RhoA activation. Interestingly, we noticed marked similarities between β3(R760C) KI mice and RhoA KO mice; that is, both mice showed not only macrothrombocytopenia but also impaired platelet aggregation, impaired PAR4-induced αIIbβ3 activation, and CD62P expression. Moreover, RhoA KO mice showed a shortened platelet life span and an increased number of Mgks in the bone marrow.27 These results raise a hypothesis that RhoA activation by αIIbβ3 outside-in signaling may affect unexpectedly broad aspects of platelet biology. However, some discrepancies were observed between β3(R760C) KI and RhoA KO mice. The decrease in GPVI and CLEC-2 expression and morphologic abnormalities on fibrinogen-adhered platelets and Mgks in β3(R760C) KI mice were not described in RhoA KO mice,27 suggesting that β3(R760C) may also affect a RhoA independent signaling pathway. For example, Rac and Cdc42 are well known regulators of lamellipodia and filopodia formation, respectively, in various cell types,28 and β3(R760C) may affect activation of not only RhoA but also other small GTPases.
Another unique feature of platelets with the β3(R760C) mutation is reduced expression of GPVI, a feature that was not observed in αIIb(R1026W) human (data not shown) or mouse platelets21 or not examined for other mutations that are responsible for macrothrombocytopenia. The expression levels of both GPVI and CLEC-2 were reduced in β3(R760C) KI mice. RNA-sequencing analysis revealed no major differences in the GPVI messenger RNA expression levels between WT and KI platelets (supplemental Figure 5), suggesting that reduction of these membrane receptors in β3(R760C) KI platelets is caused by a posttranscriptional mechanism. The increase in soluble GPVI in KI mouse plasma (Figure 2D) suggests that GPVI shedding is increased in KI mice. Platelet surface GPVI and CLEC-2 expression levels are decreased via metalloprotease–mediated ectodomain shedding with the release of their soluble forms and/or internalization/degradation pathways upon platelet activation or antibody binding.29-31 However, apparent platelet activation was not observed in β3(R760C) KI platelets by the assessment of granular release, αIIbβ3 affinity, and platelet morphology. Our KI mice may suggest the existence of yet unidentified αIIbβ3-GPVI-axis signaling. However, further studies are required to investigate this possibility.
β3(R760) is located at or near the binding site of α-actinin and Gα13.4 Although the role of α-actinin in outside-in signaling remains unclear, it seems to regulate the αIIbβ3 affinity state.32 It is also known that α-actinin1 mutations lead to macrothrombocytopenia.33 The association of Gα13 with not only integrin signaling but also RhoA activation has been reported.34-36 Because Gα13 binds to the 757ExE motif of β337 located just next to the R760C mutation, it might be possible that the mutation affects the interaction between Gα13 and β3. However, we could not detect a clear difference between the KI and WT samples in the interaction of Gα13 or α-actinin with αIIbβ3 by the pull-down assay (data not shown). Details of the molecular mechanism that leads to impaired outside-in signaling and RhoA activation by the β3(R760C) mutation remains to be determined.
In summary, we demonstrated that the nonactivating β3(R760C) mutation in the cytoplasmic region of β3 causes macrothrombocytopenia, indicating that constitutive αIIbβ3 activation is not essential for the development of macrothrombocytopenia. The KI mice study suggests abnormal cytoskeletal rearrangement via impaired RhoA activation as the potential mechanism of β3(R760C)-related macrothrombocytopenia.
Acknowledgments
This study was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (22K08476) and the Ministry of Health, Labour and Welfare of Japan (23FC1022).
Authorship
Contribution: K.N. contributed to the study by performing the experiments, analyzing the data, and writing the manuscript; H. Kashiwagi and Y.T. designed the study and drafted the manuscript; K.A., T.E., M.K., D.M., D.O., and H. Kato analyzed the data and reviewed the manuscript; and N.H. supervised the study and reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hirokazu Kashiwagi, Department of Hematology and Oncology, Graduate School of Medicine, Osaka University, 2-2 Yamadaoka, Suita, 565-0871, Japan; email: kashi@hp-blood.med.osaka-u.ac.jp.
References
Author notes
Raw RNA-sequencing data related to this study have been deposited in the Gene Expression Omnibus database (accession number GSE264702).
The full-text version of this article contains a data supplement.

![Number, morphology, and GP expression in β3(R760C) KI platelets. (A) Peripheral blood was obtained from 8- to 10-week-old male mice. Giant platelets are indicated by yellow arrows in murine peripheral blood films stained with the May-Grünwald Giemsa stain (upper panel). Platelet counts in each phenotype are presented as the mean and standard deviation (SD; n = 6 each; lower left panel). Platelet size in each phenotype was determined as the mean of forward scatter (FSC) of CD42b+ cells via flow cytometry (lower right panel). Data are presented as the mean and SD (n = 6 each). (B) Surface GP expression was determined via flow cytometry. Data are shown as percentage expression in comparison with the mean of the WT. Data are presented as mean and SD (n = 6 each). (C). Soluble GPVI concentration in murine plasma was measured using an enzyme-linked immunosorbent assay (ELISA) kit. Measurements were taken from 3 mice of each phenotype in 2 independent experiments. The data are presented as the mean and SD. (D) Total GP expression in KI mouse platelets. Lysates obtained from the washed platelets were separated via sodium dodecyl sulfate-polyacrylamide gel electrophoresis, followed by immunoblotting using specific antibodies. GPV expression levels were additionally determined as the internal control in the same membrane. ∗∗∗P < .001, ∗∗P < .01, and ∗P < .05 (1-way analysis of variance [ANOVA]). MFI, mean fluorescence intensity; ns, not significant.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodvth/2/1/10.1016_j.bvth.2024.100036/3/m_bvth_vth-2024-000191-gr2.jpeg?Expires=1765039798&Signature=uxICW87xqzQVZpWMZ5VETpYhLETFdwmiYxBAvkZ0kTRb9DH2SwAgY4XIPjSb2kFKiuijqIVZLYuN~DBeBbBIgxpiDnwbKPDF0eOdNIh401HqoAJlmnp7sI1~HOyW1W3PH6VXi-NaAG8kbm9Mvrc46U7JDxkCJQPUd1PItGoMTlM-KwvhD1P2ukh6xRrwbo7qfh4cvPTZ7PZRqPC3Tuebpm33y1gz-zkFNR4N8KbJK0Sn6Nl3svBc-sCORo2W9ivyH-xt4CSQmMW-A3rcPVOmtwqFUJx783Wpw3fqoJGFoN8ZGF8ErLczq75ft8GB2mBCnwzXwS383V3ncIbLOENIfA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)