Visual Abstract
Alterations and disturbances in shear stress of blood flow can induce membrane wounds in endothelial cells of blood vessels, which are repaired by early endosomal exocytosis.
Alterations and disturbances in shear stress of blood flow can induce membrane wounds in endothelial cells of blood vessels, which are repaired by early endosomal exocytosis.
The endothelial lining of blood vessels faces many mechanical challenges, including the shear stress (SS) of blood flow, which render it prone to cell membrane ruptures. Such ruptures must be repaired efficiently to maintain cellular integrity and proper vascular function. However, whether SS of blood flow can indeed affect plasma membrane integrity of endothelial cells and whether any ruptures occurring are repaired is not understood. Here, we show that alterations in the SS of fluid flow induce membrane damage in human endothelial cells, and membrane ruptures increase with increasing shear alterations. Furthermore, we show that inherent SS disturbances at aortic branches in mice are associated with endothelial membrane wounds, which are not observed in regions of laminar flow. We also show that endothelial membrane damages inflicted by shear stress alterations are repaired by a Ca2+-dependent process that involves early endosome exocytosis to provide membrane material for wound closure, suggesting conserved and robust membrane repair responses to endothelial damage in vitro and in vivo. Thus, shear stress alterations, which frequently occur at sites of endothelial dysfunction and before associated pathophysiology, cause membrane wounds in the endothelium, which are repaired efficiently to maintain a functional vasculature.
Introduction
The vascular endothelium forms an important and tightly regulated barrier controlling, among other things, the transport between blood and neighboring tissues.1 Being constantly exposed to hemodynamic forces of blood flow, endothelial cells are potentially prone to rupture.2,3 Furthermore, if such ruptures occur, the plasma membrane (PM) of endothelial cells needs to be repaired immediately to ensure cell viability and function at the single-cell level.4,5 Uncontrolled PM damage can lead to cell death,6 potentially affecting endothelial integrity and a myriad of vascular functions including hemostasis, vascular tone, immune responses, and angiogenesis.7,8 Therefore, endothelial cells must be equipped with efficient PM repair mechanisms that permit cell survival in an environment with constant mechanical challenges. The shear stress (SS) of blood flow, which is a mechanically generated force, has been shown to induce mechanosensitive responses and support many endothelial processes such as vascular tone regulation and vessel remodeling.9,10 However, various SS profiles could potentially also damage the endothelium, underscoring the need for an efficient PM repair machinery, that enables endothelial cells to function in the mechanically challenging environment within blood vessels.3,11 In this study, we analyzed how the SS of fluid flow affects the integrity of the PM of endothelial cells. By developing a pump flow setup and inducing shear alterations in vitro, we observed a direct membrane-damaging effect of altered SS on endothelial cells. Moreover, SS disturbances, which occur in vivo at branches of the mouse aorta, are also associated with enhanced endothelial membrane wounds, suggesting a damaging role of SS alterations (SSA) in endothelial cells. Importantly, we also show that wounds inflicted by SSA, both in vitro and in mice, are repaired by a Ca2+-dependent process involving the exocytosis of early endosomes (EE).
Study design
An in-house peristaltic pump with tubing was assembled to induce SSA in vitro. Cultivation and transfections of human umbilical cord vein endothelial cells (HUVEC) were performed as described before.12 The animal experiments on C57BL/6J mice received institutional review board approval from the Landesamt für Natur, Umwelt, und Verbraucherschutz Nordrhein-Westfalen (Germany) For a full description of all reagents and methods, see the supplemental Data.
Results and discussion
Alterations in SS induce PM wounds and subsequent membrane repair in human endothelial cells
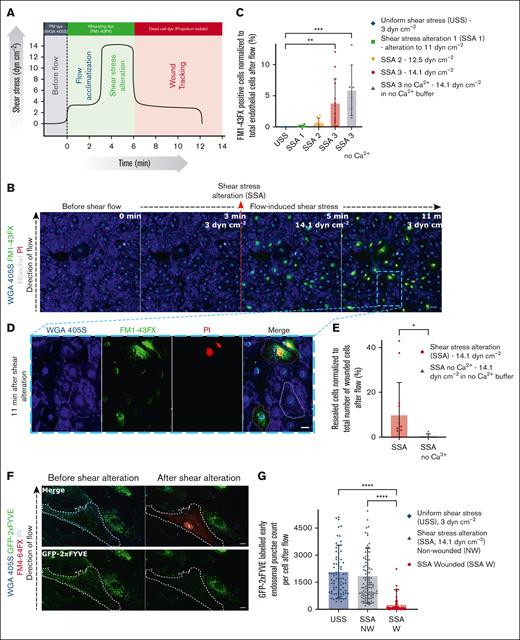
To mimic the mechanical challenge of physiological fluid SS of blood flow, we developed a pump-based flow assay and subjected a monolayer of HUVEC12,13 to varying laminar SS. A membrane-impermeable lipophilic fluorescent dye, FM1-43FX, was supplemented to the flow buffer to assess the endothelial PM integrity in response to varying SS. The cell monolayer, visualized using the membrane-binding dye wheat germ agglutinin (WGA) 405S and Hoechst nuclear stain, was first adapted to a SS of 3 dynes cm–2, which was reported for veins before.14-16 After acclimatization, the cells were exposed to alterations in SS ranging from 11 to 14.1 dynes cm–2, mimicking blood flow changes15 (Figure 1A; supplemental Figure 1A-B). No change in the integrity of the cell monolayer was observed, with an unaltered uniform SS of 3 dynes cm–2. Strikingly, immediately after SSA, PM-damaged cells were observed in the monolayer, indicated by the entry of FM1-43FX dye into injured cells (Figure 1B; see supplemental Video 1). The FM1-43FX dye is weakly fluorescent when it binds to the outer leaflet of the PM of cells but increases in fluorescence upon binding to an abundance of intracellular lipid membranes in wounded cells (as observed when the dye enters membrane-ruptured cells and binds to all lipid compartments inside).12,17,18 No further damaging effect was seen when cells were maintained at the same SS or returned to the adapted SS, suggesting that membrane damage was specifically induced upon alterations of SS. Quantification of the wounded cells showed that with increasing alterations of SS, there was a dose-dependent increase in damage to the endothelial cells (Figure 1C). Upon PM damage, extracellular Ca2+ influx has been shown to act as the trigger for activating a host of repair responses in the cell, and the absence of extracellular Ca2+ influx upon damage leads to a defect in PM repair.2,19 To analyze this Ca2+ effect, the ion was depleted from the flow buffer, also by using EGTA, and the PM damage was assessed during the flow protocol used above (supplemental Figure 1C-D). Membrane wounds due to SSA were sustained upon Ca2+ depletion (Figure 1C; supplemental Figure 1E), permitting enhanced FM1-43FX dye entry (see supplemental Figure 1D), whereas no observable changes were seen in cells subjected to a constant flow without SSA in the absence of extracellular Ca2+ (supplemental Figure 1F). This indicated the involvement of Ca2+-dependent PM repair in cells wounded by altered SS.
Alterations of SS lead to the formation of membrane wounds in HUVEC that undergo repair by Ca2+-dependent early endosomal exocytosis. (A) Schematic depiction of the protocol for the pump flow assay used to induce alterations in laminar SS profiles to study the effect on endothelial membrane integrity in HUVEC. The x-axis shows the duration of the assay (in minutes), with the different phases of SS coupled to the flow experiment noted above vertically next to the black curve, and the y-axis shows the flow-induced SS values (in dynes cm–2). Fluorescent dyes used at each stage of flow are highlighted on top of the graph in the representative colors. The black curve depicts the magnitude of the SS profiles applied during the course of the highest shear alteration experiment. (B) Representative live-cell microscopy images before and after shear alterations (shown here for 3 dynes cm–2 to 14.1 dynes cm–2). The endothelium is visualized with WGA 405S (glycoprotein binding dye that labels the endothelial PM; shown in blue) and wounded cells tracked using FM1-43FX (green) and PI (red) in the flow buffer. Hoechst nuclear stain is also used to identify all endothelial cells for analysis, and the direction of the laminar flow is indicated (left). The point of SSA is marked on the image with the red dashed arrow. Note that cells were wounded only upon shear alteration and not in unaltered low or high shear. (C) Quantification of wounded cells based on the influx of FM1-43FX signal, upon varying shear alterations during flow, represented as percentages. Uniform laminar shear flow (3 dynes cm–2) served as negative control for shear alteration effects, and shear flow with the highest possible alteration achieved with the pump flow system (from 3 dynes cm–2 to 14.1 dynes cm–2) in the presence of the Ca2+ chelator EGTA served as positive wounding control. Legend on top indicates the various samples, which is the same for all further graphs. (D) Zoomed-in snapshot of the endothelial monolayer after shear alteration (at 11 minutes after flow) from panel B (indicated by a blue dashed box) shows examples of an unwounded cell with no FM1-43FX staining (no green FM1-43FX signal all over the cell; outlined in white dashes), a wounded and repaired cell identified by FM1-43FX staining only (green signal in the cytoplasm; shown in orange dashes), and wounded but nonrepaired cell, with FM1-43FX and PI staining appearing as yellow (green signal in cytoplasm and red PI signal in the nucleus appearing as yellow; shown in pink dashes). The minor nuclear signal for all cells in the FM1-43FX channel is due to the spectral bleed-through of Hoechst nuclear stain, which, however, is distinct from the FM1-43FX cytoplasmic localization that is seen only in wounded cells (also see supplemental Figure 1F). (E) Quantification of resealed cell counts performed as in panel C for shear alteration–induced wounding, based on FM1-43FX–positive and PI-negative signal (“SSA” in the graph corresponds to “SSA 3” in panel C). (F) HUVEC were transfected with the early endosomal marker, green fluorescent protein (GFP)–2xFYVE (a protein construct binding the endosomal lipid phosphatidylinositol 3-phosphate marking EE; shown in green), and subjected to the SSA-based flow assay at the maximum shear alteration (SSA 3 above). Images before and after flow alteration are shown. The wounded cell, outlined in white dashes, is identifiable by the FM4-64FX wounding dye (red) and PI (gray) signal. The GFP-2xFYVE channel alone is shown again in the bottom panels for clarity. (G) Counts of GFP-2xFYVE punctae in cells subjected to the SSA protocol are plotted for altered SS sample in wounded and nonwounded cells, along with unaltered shear negative control (low shear control with no wounded cells). SS-altered wounded cells are labeled as “SSA W” in the graph (red circles), non-wounded cells in the SS-altered sample labeled “SSA NW” (blue triangles), and cells in uniform SS control are depicted as “USS” (purple diamonds). Note that after shear alteration, the wounded cells showed fewer 2xFYVE punctae than the nearby unwounded cells or cells under unaltered shear flow. (H-I) HUVEC were transfected with the exocytosis marker, TfR-SEP (the endosomal protein transferrin receptor coupled to pH sensitive pHluorin to mark early endosomal exocytosis events; displayed in green), which shows low fluorescence at the internal acidic pH of EE but increased fluorescence after cell surface exposure due to the resulting neutralization. Still images before and after SSA are shown (H) and quantified (I) for the counts of TfR-SEP punctae in the cells after flow alteration. The wounded cell in panel H (shown in white dashes) displayed a punctate appearance of TfR-SEP all over the cell surface after flow alteration (indicated with white arrows), as opposed to the neighboring unwounded cell, which shows weakly fluorescent punctate TfR-SEP signals indicating the pre-existing TfR pool in EE. The increased counts of highly fluorescent cell surface punctae of TfR-SEP in wounded cells under shear alteration are quantified in panel I (graph legend same as panel G). Scale bars represent 50 μm in panel B; 20 μm in panel D; and 10 μm in panels F and H. Mean ± standard deviation (SD) shown for all graphs, with individual points indicating the distribution of the technical replicates for panels C and E and the cells measured for panels G and I (n = 3900-12 300 total cells in panel C; 0-404 wounded cells in panel E; 76-102 wounded cells in panel G; and 39-63 wounded cells in panel I, pooled from 3 to 4 independent experiments). Statistical analyses were performed using 1-way analysis of variance (ANOVA) with the Kruskal-Wallis test for panels C, G, and I and the Mann-Whitney U test for panel E. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
Alterations of SS lead to the formation of membrane wounds in HUVEC that undergo repair by Ca2+-dependent early endosomal exocytosis. (A) Schematic depiction of the protocol for the pump flow assay used to induce alterations in laminar SS profiles to study the effect on endothelial membrane integrity in HUVEC. The x-axis shows the duration of the assay (in minutes), with the different phases of SS coupled to the flow experiment noted above vertically next to the black curve, and the y-axis shows the flow-induced SS values (in dynes cm–2). Fluorescent dyes used at each stage of flow are highlighted on top of the graph in the representative colors. The black curve depicts the magnitude of the SS profiles applied during the course of the highest shear alteration experiment. (B) Representative live-cell microscopy images before and after shear alterations (shown here for 3 dynes cm–2 to 14.1 dynes cm–2). The endothelium is visualized with WGA 405S (glycoprotein binding dye that labels the endothelial PM; shown in blue) and wounded cells tracked using FM1-43FX (green) and PI (red) in the flow buffer. Hoechst nuclear stain is also used to identify all endothelial cells for analysis, and the direction of the laminar flow is indicated (left). The point of SSA is marked on the image with the red dashed arrow. Note that cells were wounded only upon shear alteration and not in unaltered low or high shear. (C) Quantification of wounded cells based on the influx of FM1-43FX signal, upon varying shear alterations during flow, represented as percentages. Uniform laminar shear flow (3 dynes cm–2) served as negative control for shear alteration effects, and shear flow with the highest possible alteration achieved with the pump flow system (from 3 dynes cm–2 to 14.1 dynes cm–2) in the presence of the Ca2+ chelator EGTA served as positive wounding control. Legend on top indicates the various samples, which is the same for all further graphs. (D) Zoomed-in snapshot of the endothelial monolayer after shear alteration (at 11 minutes after flow) from panel B (indicated by a blue dashed box) shows examples of an unwounded cell with no FM1-43FX staining (no green FM1-43FX signal all over the cell; outlined in white dashes), a wounded and repaired cell identified by FM1-43FX staining only (green signal in the cytoplasm; shown in orange dashes), and wounded but nonrepaired cell, with FM1-43FX and PI staining appearing as yellow (green signal in cytoplasm and red PI signal in the nucleus appearing as yellow; shown in pink dashes). The minor nuclear signal for all cells in the FM1-43FX channel is due to the spectral bleed-through of Hoechst nuclear stain, which, however, is distinct from the FM1-43FX cytoplasmic localization that is seen only in wounded cells (also see supplemental Figure 1F). (E) Quantification of resealed cell counts performed as in panel C for shear alteration–induced wounding, based on FM1-43FX–positive and PI-negative signal (“SSA” in the graph corresponds to “SSA 3” in panel C). (F) HUVEC were transfected with the early endosomal marker, green fluorescent protein (GFP)–2xFYVE (a protein construct binding the endosomal lipid phosphatidylinositol 3-phosphate marking EE; shown in green), and subjected to the SSA-based flow assay at the maximum shear alteration (SSA 3 above). Images before and after flow alteration are shown. The wounded cell, outlined in white dashes, is identifiable by the FM4-64FX wounding dye (red) and PI (gray) signal. The GFP-2xFYVE channel alone is shown again in the bottom panels for clarity. (G) Counts of GFP-2xFYVE punctae in cells subjected to the SSA protocol are plotted for altered SS sample in wounded and nonwounded cells, along with unaltered shear negative control (low shear control with no wounded cells). SS-altered wounded cells are labeled as “SSA W” in the graph (red circles), non-wounded cells in the SS-altered sample labeled “SSA NW” (blue triangles), and cells in uniform SS control are depicted as “USS” (purple diamonds). Note that after shear alteration, the wounded cells showed fewer 2xFYVE punctae than the nearby unwounded cells or cells under unaltered shear flow. (H-I) HUVEC were transfected with the exocytosis marker, TfR-SEP (the endosomal protein transferrin receptor coupled to pH sensitive pHluorin to mark early endosomal exocytosis events; displayed in green), which shows low fluorescence at the internal acidic pH of EE but increased fluorescence after cell surface exposure due to the resulting neutralization. Still images before and after SSA are shown (H) and quantified (I) for the counts of TfR-SEP punctae in the cells after flow alteration. The wounded cell in panel H (shown in white dashes) displayed a punctate appearance of TfR-SEP all over the cell surface after flow alteration (indicated with white arrows), as opposed to the neighboring unwounded cell, which shows weakly fluorescent punctate TfR-SEP signals indicating the pre-existing TfR pool in EE. The increased counts of highly fluorescent cell surface punctae of TfR-SEP in wounded cells under shear alteration are quantified in panel I (graph legend same as panel G). Scale bars represent 50 μm in panel B; 20 μm in panel D; and 10 μm in panels F and H. Mean ± standard deviation (SD) shown for all graphs, with individual points indicating the distribution of the technical replicates for panels C and E and the cells measured for panels G and I (n = 3900-12 300 total cells in panel C; 0-404 wounded cells in panel E; 76-102 wounded cells in panel G; and 39-63 wounded cells in panel I, pooled from 3 to 4 independent experiments). Statistical analyses were performed using 1-way analysis of variance (ANOVA) with the Kruskal-Wallis test for panels C, G, and I and the Mann-Whitney U test for panel E. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
The contribution of PM repair was further confirmed by adding another membrane-impermeable fluorescent dye, propidium iodide (PI), after SSA and after the cells were allowed to undergo potential membrane repair for a time interval of 3 minutes (see Figure 1A; supplemental Figure 1A). PI, which binds to the DNA of membrane-ruptured cells, was thus used to identify the population of cells that were wounded during the SSA protocol and did not undergo repair later. It shows a distinct nuclear localization and thus can be distinguished from the cytoplasmic FM1-43FX signal (supplemental Figure 1A). The 2-dye experiment revealed a mixture of repaired (FM1-43FX–positive but PI-negative) and nonrepaired (FM- and PI-positive) cells (Figure 1D), with effective Ca2+ chelation leading to an enhanced defect in membrane repair (supplemental Figure 1C-D). Assessing the population of repaired cells upon maximal SSA showed that a fraction of the wounded cells was repaired, whereas depletion of Ca2+ completely inhibited repair (Figure 1E; supplemental Figure 2A). This suggested a Ca2+-dependent activation of cell-autonomous PM repair in altered SS-wounded cells.
Previously, we showed that in response to laser ablation–induced membrane wounding of single cells, endothelial cells repurpose EE to exocytose, providing membrane to reseal the wound.12 To ascertain whether HUVEC wounded by SSA repair their membranes by a similar mechanism, markers of EE, GFP–2xFYVE, and TfR-SEP were used (GFP stands for green fluorescent protein). GFP–2xFYVE is a construct that binds to phosphatidylinositol 3-phosphate, a lipid enriched in the early endosomal membrane. TfR-SEP is a fusion protein comprising the transferrin receptor (TfR) coupled to the pH-sensitive superecliptic pHluorin (SEP), which shows a marked fluorescence increase upon neutralization, that is, when TfR-SEP–loaded EE fuse with the PM.12 The markers were exogenously expressed in HUVEC, and the cells were subjected to a high-SSA protocol (14.1 dynes cm–2, in which a maximum number of wounded cells was observed) in the presence of the red fluorescent variant of the FM dye FM4-64FX. Interestingly, HUVEC wounded by SSA showed a drastic reduction of 2xFYVE-loaded EE (Figure 1F), suggesting EE exocytosis, whereas nearby undamaged cells or cells maintained at a continuous shear showed no change in EE localization (Figure 1G). Furthermore, the exocytosis of EE in cells wounded by altered SS was validated by the appearance of exocytosed TfR-SEP punctae at the cell surface after flow changes (Figure 1H). No such changes were detected in non-wounded cells at varying shear (Figure 1I). Similar results were obtained for the early endosomal exocytic SNARE (soluble N-ethylmaleimide sensitive factor attachment protein receptor), VAMP2-SEP (VAMP stands for vesicle-associated membrane protein), confirming early endosomal fusion events in shear-wounded cells (supplemental Figure 2B). Finally, the role of Ca2+ in mediating wound repair in HUVEC wounded by SSA was corroborated using a genetically encoded Ca2+ indicator, R-GECO 1.2 (red fluorescent genetically encoded Ca2+ indicator), which showed elevated Ca2+ levels solely in cells wounded after flow alteration (supplemental Figure 2C). Taken together, these results suggest that alterations in SS of fluid flow cause membrane wounds in an endothelial monolayer and these single-cell wounds are repaired via a Ca2+-dependent mechanism involving early endosomal exocytosis.
DSS causes PM wounds and associated repair in endothelial cells of the mouse aorta
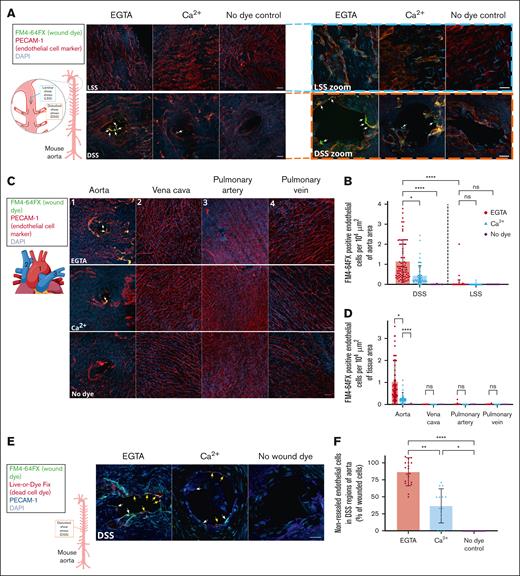
The observations using induced SSA of fluid flow prompted us to assess whether SSA in blood flow are indeed associated with endothelial PM wounds. The SS of blood flow is intrinsically prone to alterations and disturbances depending on the region of the blood vessel. Specifically, we investigated whether inherent SS variations in the mouse vasculature are associated with endothelial membrane damages. Therefore, C57BL/6J wild-type mice after euthanasia were immediately perfused with buffer containing membrane-impermeant FM4-64FX dye, and various regions of the aorta, the largest blood vessel, were examined for indications of membrane damage in situ. Intriguingly, an accumulation of FM4-64FX–positive dye signal correlating to PECAM-1 (platelet endothelial cell adhesion molecule 1)–stained endothelial cells was observed around branches and curved regions in the aorta (Figure 2A, bottom panels; supplemental Figure 3A). Such branches or curved regions seen in the aortic arch and at intercostal arteries depict regions characteristic of disturbed SS (DSS). In contrast, no endothelial cell–associated FM4-64FX signal was detectable in regions of the aorta with no curvature, which are exposed to laminar SS (Figure 2A, top panels; supplemental Figure 3B). Furthermore, mice perfused with wound-indicating FM dye in the absence of Ca2+ using the Ca2+ chelator EGTA showed an increased accumulation of wounded cells around branches, as opposed to aortas perfused in the presence of Ca2+ (Figure 2A-B; supplemental Figure 3). These results indicated that regions of DSS in the mouse aorta are prone to endothelial PM damage, and the PM wounds inflicted are likely repaired by a Ca2+-dependent mechanism.
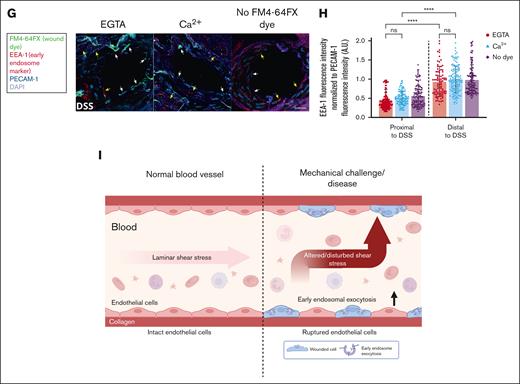
Regions of DSS in mice are prone to endothelial membrane wounds, which undergo Ca2+-mediated repair using EE. (A) C57BL/6J mice were perfused immediately after euthanasia with a buffer containing the wounding dye FM4-64FX (displayed in green), washed, and fixed. The aorta was prepared, stained for endothelial cells by anti–PECAM-1 antibodies (red) and for all cells by DAPI (4′,6-diamidino-2-phenylindole) nuclear stain (blue), and imaged by confocal microscopy. FM4-64FX was perfused either in the presence of Ca2+ or the Ca2+ chelator, EGTA, to study the role of Ca2+-dependent membrane repair, and perfusions without FM4-64FX served as the no dye negative control. Representative images of regions of laminar SS (LSS; top) and DSS in the aorta (bottom) are shown, also marked in the schematic of the mouse aorta on the left. The schematic of the aorta shows the regions of LSS (blue line) and DSS (orange line) examined, with the potential flow profiles in these regions depicted with gray arrows. The FM4-64FX signal was considered to indicate wounded cells, only if it correlated to PECAM-1–stained endothelial cells and a corresponding DAPI signal. Note that enhanced FM4-64FX signal (labeled with white arrows) was specifically observed in regions of DSS around the branches of arteries, and no staining was seen in the no dye control. High magnification images of LSS (blue dashed box) and DSS regions (orange dashed box) are shown on the right. The FM4-64FX localizations around the branches are clearly distinct from the background punctae observed in all cells, and some are indicated with white arrows (also see supplemental Figure 3). (B) Quantifications of FM4-64FX positive endothelial cells in DSS and LSS regions of the aorta are represented as cell counts per 104 μm2 of aorta area. Wounded endothelial cells were only observed in regions of DSS (separated from LSS regions by a dashed black line in the graph). Note the wounded endothelial cell counts are significantly higher in perfusions with EGTA (red circles) than Ca2+ (blue triangles) or no dye controls (purple diamonds). Legend on the right indicates the various perfusion conditions, also for all further graphs. (C) Wound dye perfusions into mice were performed as above, and along with the aorta, the superior vena cava, pulmonary artery, and pulmonary vein were examined (marked on the images and in the schematic on the left as “1” indicating the aorta, “2” marking vena cava, and “3” and “4” labeling the pulmonary artery and vein, respectively). No FM4-64FX signal corresponding to PECAM-1–stained cells could be seen in vessels other than aorta (white arrows) in all conditions, indicating no inherent membrane damage in these other vessels. (D) Quantifications of the wounded cell counts in panel C. (E) Mice were perfused with FM4-64FX wound dye (shown in green), washed, and then perfused again with a second dye, Live-or-Dye Fix 488 (red), washed, fixed and aorta sections were stained for PECAM-1 to label endothelial cells (dark blue) and with DAPI to label all cells (light blue), as above. Wound-tracking dyes were perfused either in the presence of Ca2+ or the Ca2+ chelator, EGTA, to study the population of cells undergoing Ca2+-dependent membrane repair, and perfusion without both wound dyes served as the no wound dye negative control. High magnification images of DSS regions are shown here (also see supplemental Figure 4A for a fluorescence overview). Along with the FM4-64FX signal around branches (white arrows marking wounded endothelial cells), Live-or-Dye signal was also noted (yellow arrows) in PECAM-1–labeled cells, indicative of wounded and non-repaired endothelial cells. This Live-or-Dye signal is significantly higher in EGTA conditions, suggesting an active Ca2+ dependent repair mechanism. (F) Percentages of the non-repaired endothelial cells (Live-or-Dye positive) as in panel E are represented relative to the total number of wounded (FM4-64FX–positive) endothelial cells across the perfusion conditions. Note that cells under EGTA perfusion show a major defect in membrane repair, as opposed to those cells in the presence of Ca2+ (see supplemental Figure 4D for the repaired cell analyses). (G) FM4-64FX wound dye perfusions into mice were performed as before, and after fixation and dissection, the aorta was stained for EE with anti–EEA-1 (early endosome antigen 1) antibodies (red), with anti–PECAM-1 antibodies to identify endothelial cells (dark blue), and with DAPI to label all cells (light blue). Zoomed-in regions of DSS are displayed (see supplemental Figure 5A for low zooms). Punctate EEA-1 staining can be observed in all PECAM-1–stained endothelial cells. FM4-64FX–positive wounded cells around branches (indicated with white arrows in zooms) showed reduced EEA-1 staining as compared to unwounded PECAM-1–positive cells without FM4-64FX stain or PECAM-1 cells far from the curvature (both indicated with yellow arrows). (H) EEA-1 fluorescence intensities were measured in endothelial cells around the DSS exposed branches (as in panel G; labeled “Proximal to DSS” on the x-axis) and in cells far away from the branches (“Distal to DSS”), normalized to the corresponding PECAM-1 endothelial cell signals, and plotted. Note that the cells around the bifurcation show significantly reduced EEA-1 staining (separated from distal region measurements in the graph by a dashed black line), indicating early endosomal involvement in membrane wound repair. (I) Model showing the membrane damaging effect of SS of blood flow on the endothelial lining of a blood vessel, which occurs upon mechanically challenging alterations or disturbances in SS of blood flow. Scale bars represent 20 μm in panels A and C and 10 μm in zoom-ins in panels A, E, and G. Mean ± SD with the distribution of all samples shown for the graphs (n = 36-103 fields of view pooled from 14 mice in panel B; 13-73 regions from 6 mice in panel D; 10-20 regions each combined from 3 mice in panel F; and 133-191 cells from 3 mice in panel H). Statistical comparisons using 1-way ANOVA with the Kruskal-Wallis test were performed for all analyses. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. ns, not significant.
Regions of DSS in mice are prone to endothelial membrane wounds, which undergo Ca2+-mediated repair using EE. (A) C57BL/6J mice were perfused immediately after euthanasia with a buffer containing the wounding dye FM4-64FX (displayed in green), washed, and fixed. The aorta was prepared, stained for endothelial cells by anti–PECAM-1 antibodies (red) and for all cells by DAPI (4′,6-diamidino-2-phenylindole) nuclear stain (blue), and imaged by confocal microscopy. FM4-64FX was perfused either in the presence of Ca2+ or the Ca2+ chelator, EGTA, to study the role of Ca2+-dependent membrane repair, and perfusions without FM4-64FX served as the no dye negative control. Representative images of regions of laminar SS (LSS; top) and DSS in the aorta (bottom) are shown, also marked in the schematic of the mouse aorta on the left. The schematic of the aorta shows the regions of LSS (blue line) and DSS (orange line) examined, with the potential flow profiles in these regions depicted with gray arrows. The FM4-64FX signal was considered to indicate wounded cells, only if it correlated to PECAM-1–stained endothelial cells and a corresponding DAPI signal. Note that enhanced FM4-64FX signal (labeled with white arrows) was specifically observed in regions of DSS around the branches of arteries, and no staining was seen in the no dye control. High magnification images of LSS (blue dashed box) and DSS regions (orange dashed box) are shown on the right. The FM4-64FX localizations around the branches are clearly distinct from the background punctae observed in all cells, and some are indicated with white arrows (also see supplemental Figure 3). (B) Quantifications of FM4-64FX positive endothelial cells in DSS and LSS regions of the aorta are represented as cell counts per 104 μm2 of aorta area. Wounded endothelial cells were only observed in regions of DSS (separated from LSS regions by a dashed black line in the graph). Note the wounded endothelial cell counts are significantly higher in perfusions with EGTA (red circles) than Ca2+ (blue triangles) or no dye controls (purple diamonds). Legend on the right indicates the various perfusion conditions, also for all further graphs. (C) Wound dye perfusions into mice were performed as above, and along with the aorta, the superior vena cava, pulmonary artery, and pulmonary vein were examined (marked on the images and in the schematic on the left as “1” indicating the aorta, “2” marking vena cava, and “3” and “4” labeling the pulmonary artery and vein, respectively). No FM4-64FX signal corresponding to PECAM-1–stained cells could be seen in vessels other than aorta (white arrows) in all conditions, indicating no inherent membrane damage in these other vessels. (D) Quantifications of the wounded cell counts in panel C. (E) Mice were perfused with FM4-64FX wound dye (shown in green), washed, and then perfused again with a second dye, Live-or-Dye Fix 488 (red), washed, fixed and aorta sections were stained for PECAM-1 to label endothelial cells (dark blue) and with DAPI to label all cells (light blue), as above. Wound-tracking dyes were perfused either in the presence of Ca2+ or the Ca2+ chelator, EGTA, to study the population of cells undergoing Ca2+-dependent membrane repair, and perfusion without both wound dyes served as the no wound dye negative control. High magnification images of DSS regions are shown here (also see supplemental Figure 4A for a fluorescence overview). Along with the FM4-64FX signal around branches (white arrows marking wounded endothelial cells), Live-or-Dye signal was also noted (yellow arrows) in PECAM-1–labeled cells, indicative of wounded and non-repaired endothelial cells. This Live-or-Dye signal is significantly higher in EGTA conditions, suggesting an active Ca2+ dependent repair mechanism. (F) Percentages of the non-repaired endothelial cells (Live-or-Dye positive) as in panel E are represented relative to the total number of wounded (FM4-64FX–positive) endothelial cells across the perfusion conditions. Note that cells under EGTA perfusion show a major defect in membrane repair, as opposed to those cells in the presence of Ca2+ (see supplemental Figure 4D for the repaired cell analyses). (G) FM4-64FX wound dye perfusions into mice were performed as before, and after fixation and dissection, the aorta was stained for EE with anti–EEA-1 (early endosome antigen 1) antibodies (red), with anti–PECAM-1 antibodies to identify endothelial cells (dark blue), and with DAPI to label all cells (light blue). Zoomed-in regions of DSS are displayed (see supplemental Figure 5A for low zooms). Punctate EEA-1 staining can be observed in all PECAM-1–stained endothelial cells. FM4-64FX–positive wounded cells around branches (indicated with white arrows in zooms) showed reduced EEA-1 staining as compared to unwounded PECAM-1–positive cells without FM4-64FX stain or PECAM-1 cells far from the curvature (both indicated with yellow arrows). (H) EEA-1 fluorescence intensities were measured in endothelial cells around the DSS exposed branches (as in panel G; labeled “Proximal to DSS” on the x-axis) and in cells far away from the branches (“Distal to DSS”), normalized to the corresponding PECAM-1 endothelial cell signals, and plotted. Note that the cells around the bifurcation show significantly reduced EEA-1 staining (separated from distal region measurements in the graph by a dashed black line), indicating early endosomal involvement in membrane wound repair. (I) Model showing the membrane damaging effect of SS of blood flow on the endothelial lining of a blood vessel, which occurs upon mechanically challenging alterations or disturbances in SS of blood flow. Scale bars represent 20 μm in panels A and C and 10 μm in zoom-ins in panels A, E, and G. Mean ± SD with the distribution of all samples shown for the graphs (n = 36-103 fields of view pooled from 14 mice in panel B; 13-73 regions from 6 mice in panel D; 10-20 regions each combined from 3 mice in panel F; and 133-191 cells from 3 mice in panel H). Statistical comparisons using 1-way ANOVA with the Kruskal-Wallis test were performed for all analyses. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. ns, not significant.
Next, potential membrane wounds in endothelial cells were analyzed in various blood vessels of the mouse that are not prone to SSA, such as the vena cava, pulmonary artery, and pulmonary vein (Figure 2C). No discernible FM4-64FX–positive cells were detected in the different vessels (Figure 2D), suggesting that regions of DSS as seen in the aorta are more susceptible to PM wounds in vivo. We further probed the wounds observed at the sites of aorta branches by perfusion of a second membrane-impermeable dye, Live-or-Dye Fix, after the initial perfusion with FM4-64FX and a repair period. This dye, similar to PI in the pump flow assays, would only enter endothelial cells that remained damaged even after the repair period and thereby labels the non-repaired wounded cells. The experiment revealed a mixture of wounded and repaired as well as wounded and non-repaired cells around these regions (Figure 2E; supplemental Figure 4A-B). Again, the wounds were repaired in a Ca2+-dependent manner, because the number of non-repaired cells was higher under Ca2+ depletion (Figure 2F; supplemental Figure 4C-D). Finally, inspection of the mechanism of PM wound repair at the aorta branches showed that there was a reduction of EE staining (corresponding to early endosomal exocytosis as in Figure 1F-G) observed in wounded endothelial cells at these DSS sites, as opposed to cells at sites distal from DSS (Figure 2G-H; supplemental Figure 5A-C). Thus, similar to the pump flow assay in HUVEC, a conserved PM repair mechanism of EE exocytosis is activated in the DSS-wounded endothelial cells of the mouse aorta.
Our findings indicate that alterations and disturbances in the SS of blood flow, which is a constant mechanical challenge present in blood vessels, lead to PM damage of single endothelial cells. This triggers a PM repair response that involves the wounding-induced and Ca2+-dependent fusion of EE with the PM, likely to provide additional membrane for resealing of the wounds and aid in membrane repair (Figure 2I). Alterations in SS are observed at the branches of blood vessels, and using the abundant EE of endothelial cells to provide membrane permits a fast and robust response for the repair of PM-wounded cells. The extra membrane delivered by the EE potentially also serves to reduce the tension of the PM, aiding efficient repair.12,13,20
SSA are also associated with endothelial activation, for example, in response to injury or inflammation.21 Interestingly, endothelial activation after inflammation triggers Weibel-Palade body (endothelial storage granule) exocytosis, which has been linked to PM repair; furthermore, PM damage was shown to be elevated in patients with von Willebrand disease (a blood clotting disorder).22 Endothelial dysfunction and overactivation are also observed at regions of DSS,14,23 where inherent PM wounds in mice are observed in our study. PM repair of endothelial cells, thus, is most likely necessary to prevent uncontrolled cellular damage in these hot spots of endothelial dysfunction.24 This is vital because uncontrolled membrane damage in various cells has been shown to lead to unregulated cell death, affecting tissue integrity as a whole.5,8 In the long term, the elevated Ca2+ deposition in the damaged cells, due to inefficient membrane repair, likely leads to an apoptotic cell phenotype, resulting in paracellular activation of nearby cells and thereby promoting a possible pro-inflammatory overactivated response. Together, our study identifies, to our knowledge, for the first time an early stage of endothelial irregularity in the form of PM damage that manifests itself specifically in regions of DSS that are later prone to pathology.25,26 PM damage of individual endothelial cells is thus a potential early-stage trigger for endothelial dysfunction, which so far has been largely neglected in studies of endothelial integrity.27,28 Given the importance of endothelial dysfunction in pathophysiological processes such as atherogenesis, which predominantly occurs in regions of DSS,23,29 it will be interesting in the future to study whether the initiation and/or progression of atherogenesis is associated with endothelial PM damage and impaired repair.
Acknowledgments
The authors are grateful to Sarah Weischer and Thomas Zobel (Imaging Network, University of Münster) for help with microscopy and image analysis. They acknowledge Alexander Hackstein, Olga Schengel, and Luise Wander for support with the mouse experiments. They thank Mara Pitulescu (Max Planck Institute for Molecular Biomedicine, Münster) for providing them with the anti-mouse EEA-1 antibody. They also thank members of the Gerke and Söhnlein laboratories for reagents and support. N.R. was a member of Cells-in-Motion Interfaculty Centre-International Max Planck Research School, the joint graduate school of the Cells-in-Motion Interfaculty Centre, University of Münster, Germany, and the International Max Planck Research School-Molecular Biomedicine, Münster, Germany.
This work was supported by grants from the German Research Foundation (DFG) (CRC1009, project A06, and GE514/6-3; V.G.). O.S. receives support from the DFG (CRC TRR3332 A2 & Z1, CRC1009 A13, CRC1123 A6, CRU342 P1) and the Interdisciplinary Centre for Clinical Research (IZKF) and the Innovative Medical Research (IMF) at the University of Münster.
Authorship
Contribution: N.R. and V.G. conceived the study; N.R. performed the experiments; A.M.S. contributed to the pump flow assay experiments; C.P. and A.L.L.M. contributed to the mouse perfusion experiments; V.G., N.R., and O.S. supervised the project; and N.R. and V.G. wrote the original manuscript, with inputs from C.P. and A.M.S.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Volker Gerke, Institute of Medical Biochemistry, Centre for Molecular Biology of Inflammation, University of Münster, 48149 Münster, Germany; email: gerke@uni-muenster.de; and Nikita Raj, Institute of Medical Biochemistry, Centre for Molecular Biology of Inflammation, University of Münster, 48149 Münster, Germany; email: nikita.raj@ukmuenster.de.
References
Author notes
All data are available in the main text or the supplemental Data. Additional data related to this manuscript are available on request from the corresponding authors, Volker Gerke (gerke@uni-muenster.de) and Nikita Raj (nikita.raj@ukmuenster.de).
The full-text version of this article contains a data supplement.