Key Points
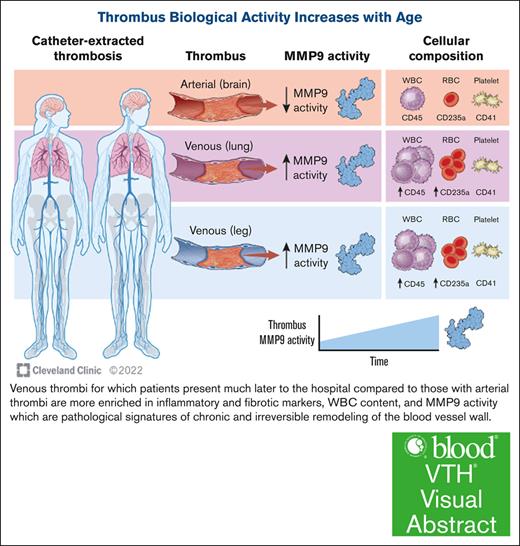
Thrombus age determines inflammatory and fibrotic capabilities through activation of enzymes that are known to damage the blood vessel wall.
Chronic thrombus in veins has greater MMP9 activity than more acute thrombus in arteries, with MMP9 appearing to be derived from macrophages trapped in thrombus.
Visual Abstract
Mechanisms behind vascular remodeling following thrombosis are unclear. Although acute arterial thrombosis in the cerebrovascular circulation has devastating consequences and requires immediate attention, the management of venous thromboembolism (VTE) varies significantly. Our goal was to determine the molecular signatures and cellular content of thrombus extracted using a catheter to gain insight into vascular remodeling. Twenty-five patients underwent catheter-directed thrombectomy (CDT); 13 for cerebrovascular accident (CVA), 8 for pulmonary embolism, and 4 for deep vein thrombosis. Protein and RNA were extracted from thrombi to enable immunoblotting, RNA sequencing, and quantification of gene expression. The time from symptom onset to thrombus extraction was 7.7 ± 1.9 hours for CVA and 109 ± 55 hours for VTE. Protein concentration, white blood cell content (monocytes), and red blood cell content were greater in venous thrombus than in arterial thrombus, whereas the platelet content was similar. Both venous and arterial thrombi contained several zinc endopeptidases belonging to the matrix metalloproteinase (MMP) family. MMP9 activity in venous thrombus was greater than arterial thrombus (61 ± 9 ng/mL per μg protein vs 25 ± 6 ng/mL per μg protein; P = .005). Arterial and venous thrombi displayed surprisingly different phenotypes, with biologically active enzymes promoting blood vessel remodeling and enzymatic activity proportional to thrombus age extracted from the veins. These mechanistic data may support the role of early CDT in venous circulation to avoid irreversible vascular remodeling.
Introduction
Venous thromboembolism (VTE) consisting of deep vein thrombosis (DVT) with or without concomitant pulmonary embolism (PE) is treated with systemic anticoagulation unless contraindicated.1 Although acute DVT is rarely immediately life-threatening, PE is a true thrombotic emergency, carrying a vascular mortality risk surpassed only by acute cerebrovascular accident (CVA) and myocardial infarction (MI).2,3 The immediate consequences of arterial and venous thrombosis are reported more frequently in clinical studies, whereas the long-term consequences of chronic thrombosis remain understudied. Most patients with acute PE and DVT enjoy substantial thrombus resolution with appropriate treatment. For patients with acute PE and high-risk features including right ventricle strain or cardiogenic shock, escalation of treatment options beyond anticoagulation includes systemic thrombolysis. Additional advanced treatment options also exist, such as catheter-directed thrombectomy (CDT) and catheter-assisted thrombolysis.4,5 Similarly, for patients with acute PE, anticoagulation is the standard of care unless contraindicated.6 Although CDT is used not infrequently in patients with critical limb ischemia secondary to arterial compression from a large thrombus burden in the veins of the lower extremity (phlegmasia cerulea dolens), CDT may be considered reasonable on a case-by-case basis for patients with iliofemoral DVT with a large thrombus burden and risk factors for postthrombotic syndrome (PTS), or in patients who clearly display significant symptoms at the time of thrombus discovery, including, but not exclusive to, pain and swelling, especially if the patient has good functional status.7,8 Patients who develop PTS can have debilitating symptoms, including pain, swelling, discoloration, limb heaviness, limb rubor, limb calor, and limb paresthesia.7-9 As such, a decision to treat patients with anticoagulation alone or in combination with CDT for thrombus extraction should be carefully considered and individualized to the patient only after careful history and physical examination.
Registry data suggest that incomplete thrombus resolution impacts ∼50% of the patients with acute DVT and PE. Pathological processes behind incomplete thrombus resolution are incompletely understood.10
In the lower extremities, the presence of persistent thrombus may irreversibly remodel blood vessels.11 Similarly, incomplete thrombus resolution in the pulmonary vasculature and vascular remodeling leads to pulmonary hypertension or the debilitating condition chronic thromboembolic pulmonary disease (CTED) in some patients.12
In patients with acute CVA, improved functional outcomes are very clear after endovascular thrombectomy.13-15 Although acute cerebrovascular insult alerts patients immediately to functional deficits, symptoms of VTE can be more subtle over days or weeks, allowing the aging thrombus to initiate vascular remodeling. In the venous circulation, prospective studies examining the impact of CDT on functional outcomes are less clear.16-19 In the case of DVT, high-quality prospective studies have shown mixed efficacy after using CDT.9,20,21 In a post hoc analysis of the ATTRACT trial, Li et al demonstrated an apparent benefit in patients who underwent early CDT within the first 48 hours and demonstrated mechanistically in a murine model of DVT that early restoration of blood flow prevented venous fibrosis.22 This implies early thrombectomy for acute iliofemoral DVT may prevent the debilitating consequences of PTS.
Understanding thrombus structure may enable better prediction of pathological sequela. It was previously shown that thrombi in the pulmonary artery have protein and cellular composition that determines stress responsiveness, which may impact the ability of the thrombus to embolize from proximal locations.23 We previously observed that thrombi extracted from coronary arteries at the time of acute MI or from patients with chronic infrarenal abdominal aortic aneurysm (AAA) secrete activated enzymes, including the zinc-endopeptidase matrix metalloproteinase (MMP) family.24,25 We hypothesized that thrombi from any vascular bed may in fact be biologically active, with unique molecular and cellular signatures based on location. To test this hypothesis, we evaluated the biological properties of thrombi extracted from patients with DVT and PE by catheter, comparing them to those of patients with CVA, each of whom underwent CTD. Comparing acute arterial thrombosis, in which patients show immediate severe neurological symptoms, with VTE, in which symptoms can appear days or weeks later, allows us to study how the cellular content of a thrombus changes over time in vivo. This may assist our understanding of thrombus formation and resolution.
Methods
Diagnosis of PTS and chronic thromboembolic pulmonary hypertension
The following was used as a guide to diagnose leg symptoms after acute DVT that constitutes PTS using the Villalta scoring system: pain, cramping, paresthesia, pruritus, leg heaviness, pretibial edema, skin hyperpigmentation or duration, redness, pain with calf compression, venous ulcers, or rubor.26 The following was used as a guide to diagnose chronic thromboembolic disease (CTED): exercise intolerance and shortness of breath without objective imaging data for thromboembolic disease and pulmonary hypertension at rest (mean pulmonary artery pressure <20 mm Hg).27
Thrombus transcriptomic analysis
Arterial thrombi (infrarenal aorta, n = 6; brain, n = 5) and venous thrombi (leg, n = 6; pulmonary artery, n = 6) were evaluated for expression of genes. The thrombus extracted at the time of clinical intervention was snap-frozen in liquid nitrogen and stored until analysis. RNA was extracted from thawed thrombus into TRIzol using a kit (Qiagen) according to the manufacturer’s instructions. RNA (100 μg) per sample was used as the template for analysis. The NanoString fibrosis and inflammation panel was customized by adding the following probes: MMP20, MMP21, CD235a, CD69, glycoprotein 1b alpha, glycoprotein 1b beta, integrin subunit alpha 2b, glycoprotein 9, glycoprotein 5, von Willebrand factor, CXCL4, and cluster differentiation 62. Data normalization and gene expression analysis were completed using nSolver version 4.0.7 and advanced analysis version 2.0.134. Adjusted P values were used to determine the expression of each gene, and pathway analysis determined the biological systems activated or suppressed in each thrombus.
Amplification of genes in thrombus
Quantitative polymerase chain reaction (qPCR) was performed for different cell types (neutrophils, macrophages, T cells, and B cells) using TaqMan primers from Thermo Fisher in 96-well plates in duplicates on QuantStudio 3 (Applied Biosystems). Each 10-μL reaction mixture consisted of 5 μL of 2× TaqMan Gene Expression Master Mix (Applied Biosystems), 0.5 μL of TaqMan Gene Expression assay, 10 ng of complementary DNA template, and volume makeup to 10 μL with nuclease-free water. The PCR conditions were programmed to hold at 50°C for 2 minutes, followed by 95°C for 10 minutes, before 40 cycles of denaturation at 95°C for 15 seconds and annealing at 60°C for 60 seconds. The primers used in the study are mentioned in Table 1.
Primers for gene marker amplification and origin of primer purchase
| Cell . | Marker . | Catalog no. . | TaqMan name . |
|---|---|---|---|
| Neutrophil | Myeloperoxidase | 4448892 | Hs00165162_m1 |
| Neutrophil | ELANE | 4453320 | Hs00236952_m1 |
| Macrophage | CD68 (M1) | 4453320 | Hs02836816_g1 |
| Macrophage | CD163 (M2) | 4453320 | Hs00174705_m1 |
| T cell | CD4 | 4448892 | Hs01065472_m1 |
| T cell | CD8A | 4453320 | Hs00233520_m1 |
| T cell | CD3E | 4453320 | Hs01062241_m1 |
| B cell | CD79A | 4448892 | Hs00998119_m1 |
| B cell | MS4A1/CD20 | 4453320 | Hs00544819_m1 |
| Cell . | Marker . | Catalog no. . | TaqMan name . |
|---|---|---|---|
| Neutrophil | Myeloperoxidase | 4448892 | Hs00165162_m1 |
| Neutrophil | ELANE | 4453320 | Hs00236952_m1 |
| Macrophage | CD68 (M1) | 4453320 | Hs02836816_g1 |
| Macrophage | CD163 (M2) | 4453320 | Hs00174705_m1 |
| T cell | CD4 | 4448892 | Hs01065472_m1 |
| T cell | CD8A | 4453320 | Hs00233520_m1 |
| T cell | CD3E | 4453320 | Hs01062241_m1 |
| B cell | CD79A | 4448892 | Hs00998119_m1 |
| B cell | MS4A1/CD20 | 4453320 | Hs00544819_m1 |
Thrombus MMP analysis
PCR
A custom-designed MMP array (Bio-Rad) was used with immobilized primers on a 96 well-plate for the following MMP isoforms: MMP1, MMP2, MMP7, MMP9, MMP10, MMP14, MMP16, MMP20, and MMP21. A commercial kit (Bio-Rad) was used for SYBR Green Fast qPCR Mix with a cycle threshold value <35, indicating fidelity of gene amplification.
Gel zymography
Homogenized thrombus lysates from 7 mg of starting material per sample were centrifuged for 15 minutes at 4°C, and the supernatants were placed in 50% volume-to-volume 2× nonreducing sample buffer at the following final concentration: Tris-HCl 250 mM, 0.5% sodium dodecyl sulfate (SDS), 1% glycerol, and 0.05% bromophenol blue for 10 minutes without boiling before loaded onto a precast gel with a gelatin matrix (10% zymogram, no. ZY00102BOX, Invitrogen). The gel was renatured by gentle rocking in 2.5% Triton-X-100 for 30 minutes at room temperature, and then allowed to equilibrate at room temperature in zymogram buffer for 30 minutes at the following final concentrations: Tris-base 50 mM, NaCl 0.2 M, CaCl2 5 mM, Tween-20 0.02% before decanting, and incubating in fresh zymogram buffer for 12 hours overnight at 37°C. Zymogram buffer was decanted, and the gel was rocked at room temperature for 4 hours in SimplyBlue SafeStain (Invitrogen). MMP activity was noted by clear bands in the final gel (reverse image). The total MMP activity in each lane was quantified by densitometry using the ImageJ software (National Institutes of Health [NIH]).
MMP activity assay
Proteins extracted from arterial and venous thrombi were quantified. A final concentration of 600 μg/mL per sample was used to determine MMP9 activity in a substrate-specific chromogenic assay according to the manufacturer’s recommendations (catalog no. F9M00, R&D Systems).
Immunoblotting
Homogenized thrombus lysates were centrifuged for 15 minutes at 4°C, and the supernatants were placed in 50% volume-to-volume 2× denaturing sample buffer at the following final concentration: Tris-HCl 250 mM, 0.5% SDS, 1% glycerol, and 0.05% bromophenol blue for 10 minutes without boiling. Forty milligrams of protein per lane was separated by SDS-polyacrylamide gel electrophoresis (5%-20% gradient; Bio-Rad) at 125 V (constant voltage and room temperature) on commercially available gradient gels (4%-20%; Invitrogen). The separated proteins were transferred to nitrocellulose membranes (Bio-Rad) at 105 V for 1 hour with an ice pack at room temperature. The blocking buffer was 3% bovine serum albumin/Tris buffered saline Tween-20 for 60 minutes at room temperature with agitation. Tris buffered saline with Tween 20-T Primary antibody incubation (1:1000 dilution in 3% bovine serum albumin/Tris buffered saline Tween-20) for 12 hours at 4°C with agitation. The secondary antibody (GE Healthcare, Buckinghamshire, United Kingdom) was used at a 1:2000 titer in 5% milk/Tris buffered saline Tween-20 for 1 hour at room temperature with agitation. Final autoradiographic films (Bioblot BXR, Laboratory Product Sales, Rochester, NY) were quantified by densitometry using ImageJ software (NIH).
Statistics
Graphical data are shown as mean ± standard error of the mean unless stated otherwise. The equal variance between groups was evaluated using the Shapiro-Wilk test. For normally distributed data between 2 groups, a 2-tailed Student t test was used. For nonparametric data, the Mann-Whitney U test was used. Significance was accepted as a P value of <.05. Statistical analyses were conducted using GraphPad Prism 7 (GraphPad Software).
This study complies with the Declaration of Helsinki and was approved by the University of Rochester Research Subjects Review Board for the analysis of thrombus that would otherwise be biological waste from patients presenting to neurological and vascular surgery services. The study participants provided written informed consent for the use of the tissues for biomedical research.
Results
Patient demographics and procedural details
Based on comorbidities, 25 broadly representative patients (13 with arterial thrombus, 8 with PE, and 4 with DVT) were enrolled in this study. Four of the 12 samples from patients with VTE represented in this study were extracted from patients with DVT (2 from the left common iliac vein, 1 from the right common iliac vein and, 1 from the left femoral vein). The ClotTriever catheter was used to extract thrombi from patients with DVT and the FlowTriever Catheter was used to extract thrombi from patients with acute PE. All patients with PE and DVT received systemic heparin, whereas 50% of the stroke patients who received full-dose tissue plasminogen activator did not (P = .016). PTS developed in 25% of the patients with VTE, whereas 16.7% of the patients with acute PE had clinical documentation consistent with CTED at follow-up (Table 2). Patients with stroke were slightly older than those with VTE (74 vs 62 years; P = .053) and with a lower body mass index (24.0 kg/m2 for stroke vs 35.0 kg/m2 for VTE; P = .0006). Most patients with acute stroke received either IV or intra-arterial tissue plasminogen activator (71% vs 0% patients; P = .001). The time from symptom onset to thrombus extraction was 14-fold greater in patients with VTE than in those with stroke (109 vs 7.7 hours, respectively; P < .0001; Figure 1A). Medications that may impact thrombus formation or resolution, including antiplatelet agents, anticoagulants, and statins, were similar between both groups (supplemental Table 1).
Characteristics of the study population
| . | Venous thrombus (n = 12) . | Arterial thrombus (n = 13) . | P value . |
|---|---|---|---|
| Demographics | |||
| Age, mean ± SD (y) | 62 ± 17 | 74 ± 11 | .053 |
| Female (%) | 6 (50) | 8 (61.5) | .561 |
| BMI, mean ± SD (kg/m2) | 35 ± 9 | 24 ± 4 | .0006 |
| Race | |||
| Asian (%) | 1 (8.3) | 0 (0) | .30 |
| Black (%) | 2 (17.7) | 2 (15.4) | .93 |
| White (%) | 9 (75) | 10 (76.9) | .93 |
| Unknown (%) | 0 (0) | 1 (7.7) | .30 |
| Thrombus type (%) | |||
| Cerebrovascular | 0 (0) | 13 (100) | |
| DVT | 4 (33.3) | 0 (0) | |
| PE | 8 (66.7) | 0 (0) | |
| Massive PE | 5 (41.7) | 0 (0) | |
| Vessel occluded (%) | |||
| MCA | 0 (0) | 8 (61.5) | |
| ICA | 0 (0) | 4 (30.8) | |
| Basilar | 0 (0) | 1 (7.7) | |
| RCIV | 1 (8.3) | 0 (0) | |
| LSIV | 1 (8.3) | 0 (0) | |
| LCIV | 2 (16.7) | 0 (0) | |
| PA (main) | 4 (33) | 0 (0) | |
| RV | 1 (8.3) | 0 (0) | |
| PA (distal) | 1 (8.3) | 0 (0) | |
| PA (left) | 1 (8.3) | 0 (0) | |
| PA (right) | 1 (8.3) | 0 (0) | |
| PTS symptoms (%) | |||
| Yes | 3 (25) | 0 (0) | |
| No | 6 (50) | 0 (0) | |
| Unknown | 3 (35) | 0 (0) | |
| CTED symptoms (%) | |||
| Yes | 2 (16.7) | 0 (0) | |
| No | 7 (58.3) | 0 (0) | |
| Unknown | 2 (25) | 0 (0) |
| . | Venous thrombus (n = 12) . | Arterial thrombus (n = 13) . | P value . |
|---|---|---|---|
| Demographics | |||
| Age, mean ± SD (y) | 62 ± 17 | 74 ± 11 | .053 |
| Female (%) | 6 (50) | 8 (61.5) | .561 |
| BMI, mean ± SD (kg/m2) | 35 ± 9 | 24 ± 4 | .0006 |
| Race | |||
| Asian (%) | 1 (8.3) | 0 (0) | .30 |
| Black (%) | 2 (17.7) | 2 (15.4) | .93 |
| White (%) | 9 (75) | 10 (76.9) | .93 |
| Unknown (%) | 0 (0) | 1 (7.7) | .30 |
| Thrombus type (%) | |||
| Cerebrovascular | 0 (0) | 13 (100) | |
| DVT | 4 (33.3) | 0 (0) | |
| PE | 8 (66.7) | 0 (0) | |
| Massive PE | 5 (41.7) | 0 (0) | |
| Vessel occluded (%) | |||
| MCA | 0 (0) | 8 (61.5) | |
| ICA | 0 (0) | 4 (30.8) | |
| Basilar | 0 (0) | 1 (7.7) | |
| RCIV | 1 (8.3) | 0 (0) | |
| LSIV | 1 (8.3) | 0 (0) | |
| LCIV | 2 (16.7) | 0 (0) | |
| PA (main) | 4 (33) | 0 (0) | |
| RV | 1 (8.3) | 0 (0) | |
| PA (distal) | 1 (8.3) | 0 (0) | |
| PA (left) | 1 (8.3) | 0 (0) | |
| PA (right) | 1 (8.3) | 0 (0) | |
| PTS symptoms (%) | |||
| Yes | 3 (25) | 0 (0) | |
| No | 6 (50) | 0 (0) | |
| Unknown | 3 (35) | 0 (0) | |
| CTED symptoms (%) | |||
| Yes | 2 (16.7) | 0 (0) | |
| No | 7 (58.3) | 0 (0) | |
| Unknown | 2 (25) | 0 (0) |
Patients with acute thrombotic stroke (n = 13) and VTE (DVT and PE; n = 12) were included in this study. Data are reported as the mean ± SD unless otherwise stated, with differences between the groups as noted. Group comparisons by for dichotomous and t test for continuous variables.
BMI, body mass index; CIV, common iliac vein; ICA, internal carotid artery; LCIV, left common iliac vein; LEIV, left external iliac vein; MCA, middle cerebral artery; PA, pulmonary artery; RCIV, right common internal vein; RV, right ventricle; SD, standard deviation.
Features of thrombi in patients with thrombotic stroke and VTE. (A) Time from symptom onset for acute thrombotic stroke (n = 13) and VTE (DVT and PE; n = 12) was reported as mean ± standard error of the mean (SEM). ∗P < .0001 (Mann-Whitney U test [MWU]). (B) Arterial thrombi (n = 13) and venous thrombi (DVT and PE, n = 12) by catheter and the concentration of protein in 7 mg of tissue were assessed. The venous thrombi were more heterogeneous than the arterial thrombi. Protein concentration was measured using the Bradford method and reported as the mean ± SEM. ∗P = .031 between groups by MWU.
Features of thrombi in patients with thrombotic stroke and VTE. (A) Time from symptom onset for acute thrombotic stroke (n = 13) and VTE (DVT and PE; n = 12) was reported as mean ± standard error of the mean (SEM). ∗P < .0001 (Mann-Whitney U test [MWU]). (B) Arterial thrombi (n = 13) and venous thrombi (DVT and PE, n = 12) by catheter and the concentration of protein in 7 mg of tissue were assessed. The venous thrombi were more heterogeneous than the arterial thrombi. Protein concentration was measured using the Bradford method and reported as the mean ± SEM. ∗P = .031 between groups by MWU.
Cellular characteristics and biological activity of thrombus
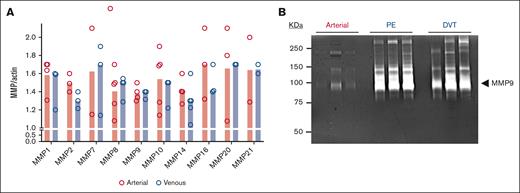
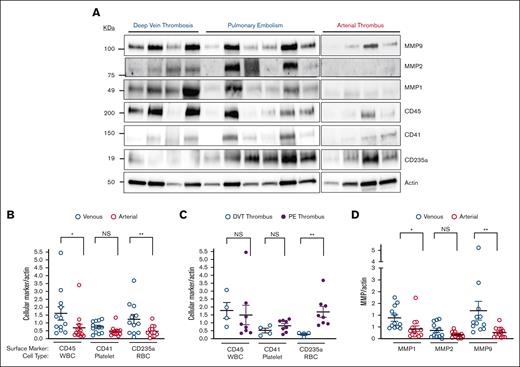
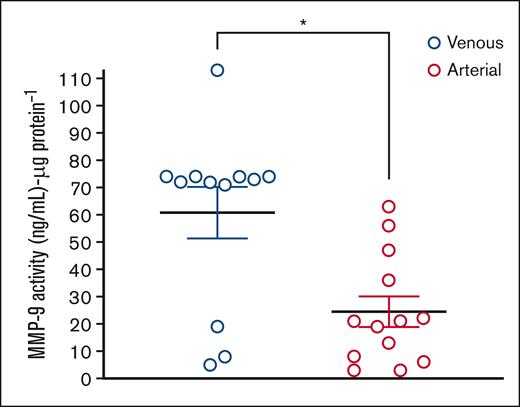
Thrombi extracted from venous circulation that had aged longer than those extracted from arterial circulation had a higher protein concentration per 7 mg of dry tissue (3.1 vs 2.1 μg/mL; P = .03) (Figure 1B). We previously demonstrated that arterial thrombi extracted from patients with acute MI or AAA were enriched in activated MMPs by gel zymography.24,25 As such, a bespoke MMP gene array evaluated the presence of MMPs in arterial and venous thrombi. MMP isoforms were quantitatively similar in arterial and venous thrombi, with notable exceptions of MMP7 and MMP21, which remained undetectable in some arterial thrombi evaluated. Gene expression of the following MMP isoforms was detected in all thrombi extracted from patients with VTE and CVA: MMP1, MMP2, MMP8, MMP9, MMP10, and MMP14 (Figure 2A). The protein extracted from the thrombus was then evaluated by gel zymography, which revealed a dominant MMP isoform of ∼95 kDa corresponding to the migration location of activated MMP9 (Figure 2B). This signal was more notable in venous thrombi. Immunoblotting for cell surface biomarkers specific for white blood cells (WBCs; CD45), platelets (CD41), and erythrocytes (CD235a) revealed that venous thrombus was enriched in leukocytes and erythrocytes compared with arterial thrombi. The platelet content was similar in both arterial and venous thrombi. Focusing on 2 distinct locations in the venous circulation, we observed that platelet and WBC contents were similar in thrombi extracted from the pulmonary artery compared with thrombi extracted from patients with iliofemoral DVT (Figure 3A-B). Interestingly, erythrocyte content was threefold higher in thrombi extracted from the pulmonary artery than in thrombi extracted from deep veins in the leg (Figure 3C). Supporting the observation of higher MMP activity in venous thrombi by gel zymography, immunoblotting protein extract from thrombus revealed MMPs were present in greater quantities in the venous circulation, with MMP9 being the dominant MMP isoform expressed. MMP2 was similarly expressed in arterial and venous thrombi (Figure 3D). An MMP9-specific chromogenic substrate further confirmed that MMP9 peptidase activity was significantly greater in venous than in arterial thrombi (61 ± 9 ng/mL per μg protein vs 25 ± 6 ng/mL per μg protein; P = .0051; Figure 4).
MMPs in thrombus from patients with thrombotic stroke and VTE. (A) RNA extracted from 7 mg of thrombus was quantified using reverse transcriptase PCR (quantitative reverse transcription PCR) for MMPs. Where RNA was found in at least 3 thrombi from each group, the data are reported as the mean ± SEM. The absence of data points indicated that RNA in the thrombus (but not in the positive control) was not detected. (B) Protein extracted from 7 mg thrombus was isolated and analyzed for separation by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) then activity assessed by in-gel zymography. MMP9 is the dominant isoform at 96 kDa seen most prominently in venous thrombi. A representative zymogram for n = 3 thrombi evaluated in each group is shown. Missing data points indicated that the gene was not present in the analyzed sample.
MMPs in thrombus from patients with thrombotic stroke and VTE. (A) RNA extracted from 7 mg of thrombus was quantified using reverse transcriptase PCR (quantitative reverse transcription PCR) for MMPs. Where RNA was found in at least 3 thrombi from each group, the data are reported as the mean ± SEM. The absence of data points indicated that RNA in the thrombus (but not in the positive control) was not detected. (B) Protein extracted from 7 mg thrombus was isolated and analyzed for separation by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) then activity assessed by in-gel zymography. MMP9 is the dominant isoform at 96 kDa seen most prominently in venous thrombi. A representative zymogram for n = 3 thrombi evaluated in each group is shown. Missing data points indicated that the gene was not present in the analyzed sample.
Circulating cellular markers in thrombus from patients with thrombotic stroke and VTE. (A) Protein extracted from 7 mg thrombus was isolated and separated by SDS-PAGE before immunoblotting using protein markers found on WBCs (CD45), platelets (CD41), and red blood cells (RBCs) (CD235a). Actin was used as a loading control. Immunoreactive bands were quantified by densitometry and reported as mean ± SEM. ∗P = .01 (MWU); ∗∗P = .02 (t test); ∗∗∗P = .040 (MWU). (B) Protein extracted from 7 mg thrombus was isolated and separated by SDS-PAGE and then assessed for standard cell surface markers used for identification of WBCs, RBCs, and platelets. Immunoreactive bands were quantified by densitometry and reported as mean ± SEM. ∗P = .01 (MWU); ∗∗P = .02 (t test). (C) Protein extracted from 7 mg thrombus was isolated and separated by SDS-PAGE and then assessed for standard cell surface markers used for identification of WBCs, RBCs, and platelets. Immunoreactive bands were quantified by densitometry and reported as mean ± SEM. ∗∗P = .04 (MWU). (D) Protein extracted from 7 mg thrombus was isolated and analyzed, separated by SDS-PAGE, and assessed for MMP1, MMP2, and MMP9 protein content. Actin was used as a loading control. Immunoreactive bands were quantified by densitometry and reported as mean ± SEM. ∗P = .0044 (MWU); ∗∗P = .0003 (MWU). NS, not significant.
Circulating cellular markers in thrombus from patients with thrombotic stroke and VTE. (A) Protein extracted from 7 mg thrombus was isolated and separated by SDS-PAGE before immunoblotting using protein markers found on WBCs (CD45), platelets (CD41), and red blood cells (RBCs) (CD235a). Actin was used as a loading control. Immunoreactive bands were quantified by densitometry and reported as mean ± SEM. ∗P = .01 (MWU); ∗∗P = .02 (t test); ∗∗∗P = .040 (MWU). (B) Protein extracted from 7 mg thrombus was isolated and separated by SDS-PAGE and then assessed for standard cell surface markers used for identification of WBCs, RBCs, and platelets. Immunoreactive bands were quantified by densitometry and reported as mean ± SEM. ∗P = .01 (MWU); ∗∗P = .02 (t test). (C) Protein extracted from 7 mg thrombus was isolated and separated by SDS-PAGE and then assessed for standard cell surface markers used for identification of WBCs, RBCs, and platelets. Immunoreactive bands were quantified by densitometry and reported as mean ± SEM. ∗∗P = .04 (MWU). (D) Protein extracted from 7 mg thrombus was isolated and analyzed, separated by SDS-PAGE, and assessed for MMP1, MMP2, and MMP9 protein content. Actin was used as a loading control. Immunoreactive bands were quantified by densitometry and reported as mean ± SEM. ∗P = .0044 (MWU); ∗∗P = .0003 (MWU). NS, not significant.
MMP9 increases in venous compared with arterial thrombus. The activity of MMP9 in proteins extracted from venous thrombus (DVT or PE) compared with arterial thrombus (acute stroke) using a chromogenic substrate. A MMP9-specific chromogenic substrate confirms that MMP9 activity is markedly higher in venous than arterial thrombi (61 ± 9 ng/mL per μg protein vs 25 ± 6 ng/mL per μg protein). ∗∗P = .0051 (MWU).
MMP9 increases in venous compared with arterial thrombus. The activity of MMP9 in proteins extracted from venous thrombus (DVT or PE) compared with arterial thrombus (acute stroke) using a chromogenic substrate. A MMP9-specific chromogenic substrate confirms that MMP9 activity is markedly higher in venous than arterial thrombi (61 ± 9 ng/mL per μg protein vs 25 ± 6 ng/mL per μg protein). ∗∗P = .0051 (MWU).
Molecular characteristics of thrombus in different vascular beds
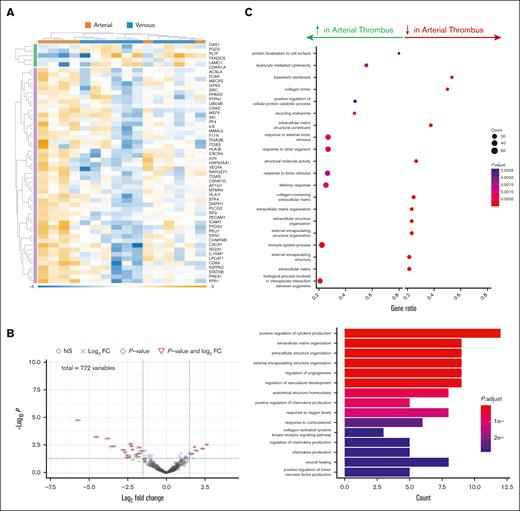
RNA extracted from various vascular beds was used to ascertain whether the site of thrombus initiation determines its phenotype. Capitalizing on the NanoString platform, which amplifies lower-yield or fragmented RNA template common to aged tissue, the gene expression profile of thrombi in 2 distinct arterial beds (brain and aorta) was surprisingly dissimilar, with only the antiapoptotic marker bcl-2 expression in aortic thrombus exceeding expression in brain thrombus (by fivefold). Generally, pathways for the organization of the extracellular matrix, fibrosis, and adhesion, reflecting permanence in the arterial wall, were upregulated, and pathways for cellular communication and resolution were downregulated in aortic thrombus (older and chronic) compared with brain thrombus (younger and acute) (Figure 5).
Transcriptomic analysis of arterial thrombus from different vascular beds. RNA was extracted and the expression of genes involved in inflammation and fibrosis was determined in arterial thrombi extracted from patients with infrarenal (n = 6) at the time of surgical repair or from the brain after acute thrombotic stroke (n = 5) at the time of catheter thrombectomy. (A) Heat map and (B) Volcano plot and Gene Ontology analysis for biological pathways in the thrombus. (C) Cellular and biochemical processes increased or decreased in aortic thrombus with respect to brain thrombus. FC, fold change.
Transcriptomic analysis of arterial thrombus from different vascular beds. RNA was extracted and the expression of genes involved in inflammation and fibrosis was determined in arterial thrombi extracted from patients with infrarenal (n = 6) at the time of surgical repair or from the brain after acute thrombotic stroke (n = 5) at the time of catheter thrombectomy. (A) Heat map and (B) Volcano plot and Gene Ontology analysis for biological pathways in the thrombus. (C) Cellular and biochemical processes increased or decreased in aortic thrombus with respect to brain thrombus. FC, fold change.
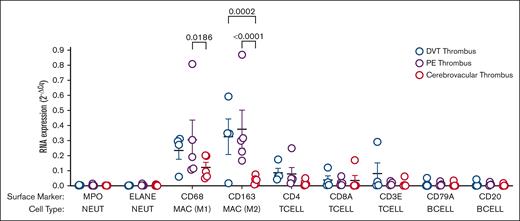
Comparing the thrombi from the precapillary venous vasculature (pulmonary artery) with those from the postcapillary arterial vasculature (brain), marked differences were noted in the expression of genes involved in angiogenesis, vascular inflammation and repair, apoptosis, and oxidative stress (Figure 6; supplemental Figure 1). More predictably, comparing thrombi from the venous vasculature but from 2 different vascular beds (the pulmonary artery and proximal veins of the lower extremity), there were fewer differences than between different arterial beds. However, some differences in gene expression were still observed, especially for B lymphocyte and T lymphocyte signaling as well as general inflammation (greater in thrombi from the deep veins in the leg than in the pulmonary artery; supplemental Tables 2-4). Using RNA extracted from arterial and venous thrombi and cell-specific genes, we determined that the identity of CD45 positive cells identified as leukocytes that are enriched in venous thrombus compared with arterial thrombus were macrophages, both inflammatory (CD68, M1) and reparative (CD163, M2). Interestingly, but perhaps predictably, arterial thrombus leukocytes identified were only macrophages and inflammatory. No difference was observed in neutrophil or lymphocyte content when comparing arterial and venous thrombi (Figure 7). MMP9 specific activity was threefold higher in catheter-extracted cerebrovascular (arterial) thrombi in women compared with men (29.6 ± 6.3 ng/mL per μg protein vs 10.4 ± 3.9 ng/mL per μg protein; P = .020) and numerically greater but not reaching statistical significance in women compared with men with venous thrombi (63.96 ± 14 ng/mL per μg protein vs 41.7 ± 14.0 ng/mL per μg protein; P = .38) (supplemental Figure 2).
Transcriptomic analysis of arterial thrombus from different vascular beds. RNA was extracted, and the expression of genes involved in inflammation and fibrosis was determined in arterial thrombus extracted from patients with infrarenal AAA (n = 6) at the time of surgical repair or from the brain following acute thrombotic stroke (n = 5) at the time of catheter thrombectomy. (A) Heat map and (B) Volcano plot and Gene Ontology analysis for biological pathways in the thrombus. (C) Cellular and biochemical processes increased or decreased in aortic thrombus with respect to brain thrombus. FC, fold change.
Transcriptomic analysis of arterial thrombus from different vascular beds. RNA was extracted, and the expression of genes involved in inflammation and fibrosis was determined in arterial thrombus extracted from patients with infrarenal AAA (n = 6) at the time of surgical repair or from the brain following acute thrombotic stroke (n = 5) at the time of catheter thrombectomy. (A) Heat map and (B) Volcano plot and Gene Ontology analysis for biological pathways in the thrombus. (C) Cellular and biochemical processes increased or decreased in aortic thrombus with respect to brain thrombus. FC, fold change.
WBC population in thrombus from different vascular beds. RNA was extracted from the whole thrombus from different vascular beds. Gene expression (numerator) is shown relative to 2 housekeeping genes (actin and glyceraldehyde-3-phosphate dehydrogenase) (denominator) and reported as mean ± SEM. Differences between groups by 2-way analysis of variance (n = 4-5). BCELL, B Lymphocyte; Cq, cycle threshold; MAC, macrophage; MPO, myeloperoxidase; NEUT, neutrophil; TCELL, T lymphocyte.
WBC population in thrombus from different vascular beds. RNA was extracted from the whole thrombus from different vascular beds. Gene expression (numerator) is shown relative to 2 housekeeping genes (actin and glyceraldehyde-3-phosphate dehydrogenase) (denominator) and reported as mean ± SEM. Differences between groups by 2-way analysis of variance (n = 4-5). BCELL, B Lymphocyte; Cq, cycle threshold; MAC, macrophage; MPO, myeloperoxidase; NEUT, neutrophil; TCELL, T lymphocyte.
Discussion
We discovered that the molecular and cellular compositions of thrombi in 4 distinct vascular beds differ, which may impact how thrombi remodel blood vessels in those locations. The most striking observation of this investigation is that the thrombus retains clear biological activity for MMP9, even after several days, with enzymatic activity proportional to thrombus age and macrophage content (see the visual abstract). Contact time between the thrombus and the vessel well and early restoration of blood flow with thrombus resolution (or extraction) have already been reported as important events in the pathogenesis of and protection against PTS, respectively. Our study provides mechanistic insight and adds to this body of literature.28,29
A closer examination of the ATTRACT trial revealed that subjectively reported symptoms of PTS were less in patients treated with pharmacomechanical thrombolysis than in those treated with conservative therapy with anticoagulation, whereas the CAVENT trial data revealed a lower incidence of PTS 2 years after the primary thrombotic event with the addition of pharmacomechanical thrombolysis.20,21 This suggests that the underlying mechanism for irreversible venous remodeling continues even after treating patients according to established guidelines. Consistent with prior studies, we found an increased representation of WBC and red blood cell content in proximal lower extremity venous thrombus, especially in those extracted from the pulmonary artery.30-32 Chernysh et al capitalized on the temporal resolution afforded by scanning electron microscopy to study venous thrombus (DVT, PE) and arterial thrombus (coronary artery) showing enriched red blood cell content in venous thrombus.30 This finding is ultimately supported by the data in our present investigation. Consistent with the findings of Chernysh et al, thrombi extracted from patients with VTE contained more WBCs than arterial thrombus. A notable difference in our study is that we also observed thrombus extracted by catheter from deep veins in the leg to be more enriched in WBCs than in arterial thrombus. Venous thrombi from deep leg veins in the study by Chernysh et al appear to have been extracted by open embolectomy whereas PE thrombus was removed from decreased individuals at the time of autopsy.30 Each venous thrombus extracted by catheter came from living individuals in our study. These distinctions suggest that the mechanism of thrombus extraction may also impact thrombus quality and composition, and biological insights from thrombus should be concluded with this in mind.
Although human platelets are known to secrete MMPs under pathological cardiovascular conditions,24,33,34 WBCs generally harbor and secrete more MMPs into the systemic circulation. Given the similar platelet content in arterial and venous thrombi, the platelet contribution to the markedly higher MMP9 activity in venous thrombus is likely WBC derived. Because CD45 is a general marker for leukocytes, phenotyping leukocyte subtypes in thrombus would be an insightful and important next step for future studies. Using RNA extracted from whole thrombus, it is potentially revealing that macrophages, rather than neutrophils, T lymphocytes, or B lymphocytes, are the dominant leukocytes. Furthermore, the expression of macrophages in extracted thrombus was much higher in venous than arterial thrombus and suggested that macrophages may mediate MMP9 release in humans, as has been suggested for patients with vascular remodeling in the context of AAA.25,35 Macrophages have been observed in many studies in the vein wall after acute venous thrombus, although their relative contribution to deleterious vascular remodeling and thrombus resolution remains in question.36-38 We identified surface markers for both M1 and M2 macrophages in venous thrombus, suggesting that the balance of these 2 subtypes likely determines thrombus resolution or ongoing, aberrant venous remodeling, and potentially PTS. This discovery makes the macrophage a natural target for pharmacological intervention to prevent MMP-mediated venous remodeling, which may precede the development of PTS. The venous vascular bed also has lower shear stress, which affords continuous cellular recruitment and WBC enrichment of venous thrombus, as noted in our study. Although several MMP isoforms are detected in human thrombus, MMP9, also known as gelatinase B, or type IV collagenase, based on its ability to digest and penetrate the basement membrane,39 is enriched with the greatest enzymatic activity by chromogenic substrate-specific studies.
MMP9 activity in thrombi extracted from patients who ultimately developed PTS and CTED was among the highest of all thrombi examined in our study, potentially revealing a mechanistic insight into vascular remodeling in patients with these disorders. Among the zinc-dependent metalloproteinases, MMP9 is known to remodel the extracellular matrix and tissues both in close proximity and distant from the origin of secretion, with a pathogenic role in infarct expansion and ventricular rupture after MI,40 in hemorrhagic transformation after CVA,41 and in AAA growth, dissection, and rupture.25,42 It is therefore not surprising that, for the first time, we demonstrate significant biological activity of venous thrombus ex vivo after catheter extraction. Thrombus biological activity persists even after a freeze/thaw cycle and implicates persistent thrombus abutting the blood vessel wall as a potential mediator of ongoing inflammation, scarring, and intimal angiogenesis preceding irreversible vascular damage that opposes thrombus resolution common to both PTS and CTED.43-45
In relevant animal models of DVT, MMP9 is released into the circulation in the early and intermediate periods after acute venous thrombosis and then normalizes in the setting of thrombus resolution. Genetic MMP9 deletion prevents venous remodeling, biomechanical tissue dysfunction, and permanent venous fibrosis.46-48 Furthermore, medications that suppress MMP9 activity in vivo in animals with experimental PE are protected from vascular remodeling and pulmonary hypertension.49 These studies suggest incomplete thrombus resolution and continued MMP release may be factors that promote irreversible venous remodeling.
By their embolic nature and disparate origin of embolization, cerebral arterial thrombi intuitively have a more heterogeneous cellular composition than venous thrombi.50 Tutwiler et al elegantly demonstrated that clot contraction kinetics, which is determined by normal platelet function, is greater in thrombi from stroke patients that sustain an atheroembolic CVA compared with those with more aged thrombi from the right atrial appendage that precipitate cardioembolic CVA in patients with atrial fibrillation.51 It is noteworthy that only 4 (29%) of the patients with arterial thrombi extracted from patients in our study had atrial fibrillation which may constitute a representation of less-aged thrombi, and this may be another explanation for the lower MMP9 activity we observed in arterial thrombi compared with venous thrombi.
Recent studies have emphasized the architectural patterns shared by thrombi in VTE and embolic CVA, as well as the potential to evaluate thrombus-derived blood biomarkers to assist clinical decision-making for catheter thrombectomy and predict thrombus susceptibility to thrombolytic drugs.52,53 To further highlight the potential importance of thrombus-derived MMP not only as an adverse mediator of vascular remodeling, Zhong et al elegantly showed that blood MMP in the context of acute thrombotic CVA predicts short-term death and major disability.54 Although we identified threefold higher MMP9 activity in arterial thrombi extracted from women compared with men after acute CVA, the biological consequences of this remain unclear. The notion that MMP9 secretion may be a driver of disease etiology in a sexual dimorphic manner was previously suggested by activity in the blood vessel wall of patients with AAA, which was greater in males who have a predilection for this disease.55 It was also revealed that macrophages were the dominant leukocyte in arterial thrombus extracted from patients with acute stroke in our present study; furthermore, it was the M1 macrophage marker (CD68, inflammatory) and not the M2 (CD168, reparative) macrophage found mostly in arterial thrombus from patients at the time of acute stroke. Certainly, although the NIH stroke scale, time from symptom onset to presentation, and use of intravascular thrombolysis are variables predicting hemorrhagic transformation, sex does not appear to be one of them.56 More broadly, however, the estrous cycle increases MMP9 activity in human cells,57 and MMP9 concentration appears to promote pathological vascular processes including arterial hypertension and vascular stiffening.58
Conclusions
Although the CAVENT and ATTRACT trials disagree regarding the ability of catheter-directed intervention to consistently reduce the incidence of PTS in patients with DVT, a 15% decrease in the incidence of PTS after catheter-directed intervention at 24 months follow-up was nonetheless observed.20,59 We provide mechanistic insight that thrombus composition and enzymatic activity differ substantially based on the vascular bed and the duration of contact between the thrombus and the blood vessel wall. Focused translational research efforts on understanding how thrombus-derived mediators affect vascular function and thrombus resolution are required to prevent the devastating symptoms of PTS and CTED.
Limitations
This investigation, although conducted only in humans and using human tissues, is observational in nature and relies on patients’ recollection of symptom onset time. An important consideration when comparing cellular content and enzymatic activity of thrombus extracted from the leg and thrombus extracted from the pulmonary artery is that they did not come from the same patient in this study. Simultaneous iliofemoral thrombectomy and pulmonary artery thrombectomy are rarely clinically justified. However, from a scientific perspective, paired thrombus from the same patient would be more biologically meaningful. Furthermore, although blood vessels contain substrates for thrombus-derived enzymes, we propose but do not directly demonstrate that thrombus-derived MMP9 directly remodels blood vessels.
Acknowledgments
The authors thank Karen Keslar for technical assistance and quality control and Naseer Sangwan for his outstanding additions to the Nanostring transcriptomic analysis. NanoString assays were performed using National Institute of Allergy and Infectious Disease–sponsored Clinical Trials in Organ Transplantation NanoString Core (U01AI063594).
This study was supported by National Institutes of Health grant HL158801 (S.J.C.) and American Heart Association grant 24POST1191727 (A.A.).
Authorship
Contribution: M.T.B., S.J.C., D.M., W.M.B. III, and R.L.F. designed the study; D.M., M.T.B., J.C., I.H., M.T., and A.N. collected and preserved clinical material used as research substrates; M. Godwin, A.A., S.J.C., S.G, performed experiments; S.J.C. designed and supervised the study; S.J.C., L.T., A.A., D.S., N.S., and S.G. analyzed the results; S.J.C. performed statistical analysis; M.T.B., A.A., and S.J.C. wrote the first draft of the manuscript; M.G., A.A., M. Gomes., L.T., and D.S. provided editorial comments for the manuscript; and A.A. participated in the editing of the manuscript and approved the final version.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Doran Mix, Division of Vascular Surgery, Department of Surgery, University of Rochester, Rochester, NY; email: doran_mix@urmc.rochester.edu; and Scott J. Cameron, Department of Cardiovascular Medicine, Section of Vascular Medicine, Heart Vascular and Thoracic Institute, Cleveland Clinic Foundation, Cleveland, OH; email: cameros3@ccf.org.
References
Author notes
M.T.B. and A.A. contributed equally to this study.
The generated and sequencing data will be made fully available by corresponding author Scott J. Cameron (cameross3@ccf.org) upon reasonable request after the completion of regulatory data use agreements.
The full-text version of this article contains a data supplement.

![Features of thrombi in patients with thrombotic stroke and VTE. (A) Time from symptom onset for acute thrombotic stroke (n = 13) and VTE (DVT and PE; n = 12) was reported as mean ± standard error of the mean (SEM). ∗P < .0001 (Mann-Whitney U test [MWU]). (B) Arterial thrombi (n = 13) and venous thrombi (DVT and PE, n = 12) by catheter and the concentration of protein in 7 mg of tissue were assessed. The venous thrombi were more heterogeneous than the arterial thrombi. Protein concentration was measured using the Bradford method and reported as the mean ± SEM. ∗P = .031 between groups by MWU.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodvth/2/1/10.1016_j.bvth.2024.100029/2/m_bvth_vth-2024-000218-gr1.jpeg?Expires=1769082417&Signature=2GfpepIOLBp0QfmfdoqTiUJICNwJIcX4ZpNizVfRSajIkttV9Kvpw1-5grJcTw7i1mXU5hSwlYqWHyPMKChzp1GCiCiCyQqoIwwvSRclZLYkjEPv71HdlyPhUxn-~AoAIvLVtlrOEOmI~4uXZEfYKsPCYxwKQ5YzNdXHcWvOk5X4djd-PcgVClOHz4xtUWhC6eVClUynuOXAgCqMJYaUJGBrP8HAdJG4G0aftW80QYAYPZ5FONauEwKNM3si5kN2YjbZyW04GXfawUSR8w8cr6t1WyNdc1dJps98G9s3rt2pYqUe~DU4ra1lgKu24fTMiHo~mN6A0PwJOUelTkXEqg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)