Visual Abstract
TO THE EDITOR:
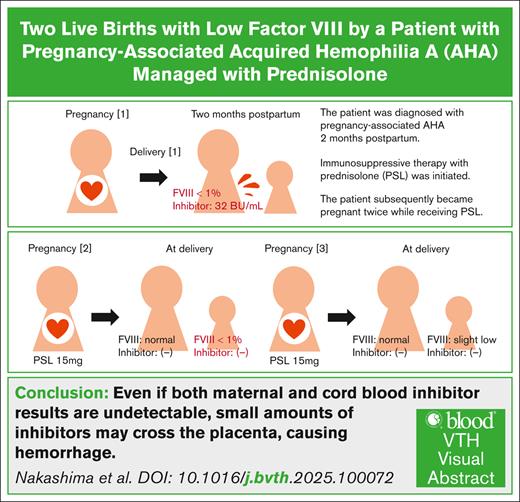
Acquired hemophilia A (AHA) is a rare autoimmune disorder caused by coagulation factor VIII inhibitors.1,2 About half of the patients with AHA have underlying conditions, such as autoimmune diseases, malignancies, medications, or pregnancy/delivery, whereas the other half are idiopathic.2,3 AHA has a bimodal distribution, affecting both older individuals and younger women, with pregnancy-associated patients accounting for ∼5% to 20%.2-6 There are few reports on pregnancies following pregnancy-associated AHA diagnosis.7-9 We report the case of a woman who successfully underwent 2 subsequent pregnancies while receiving prednisolone (PSL) for pregnancy-associated AHA. Although maternal and cord blood factor VIII inhibitors remained undetectable throughout pregnancies, the newborns exhibited a temporary severe factor VIII deficiency, indicating minimal placental inhibitor transfer. This case was previously described in part in Japanese in the Tokai Journal of Obstetrics and Gynecology.10
A 36-year-old Japanese woman, 2 months after cesarean birth, presented in March 20XX with complaints of extensive subcutaneous bleeding and pain, mainly in the lower extremities. Suspecting a hematological disorder, the patient was referred to our hospital. Based on physical examination and blood tests, including prolonged activated partial thromboplastin time (APTT), she was admitted with suspicion of AHA. The patient’s performance status was 2 due to pain. Extensive subcutaneous and intramuscular bleeding was observed, mainly in the lower extremities. Laboratory results are summarized in Table 1. APTT was 110.4 seconds, and the prothrombin time–international normalized ratio (PT-INR) was normal. The whole-body computed tomography revealed no obvious bleeding, except for subcutaneous and intramuscular hemorrhages in the extremities. Immunosuppressive therapy with PSL (40 mg [1 mg/kg]) was initiated for suspected AHA.
Two days later, AHA was diagnosed based on factor VIII inhibitor positivity (31.9 Bethesda unit [BU]/mL) and decreased factor VIII (<1.0%). To prevent compartment syndrome and severe menstruation, recombinant activated factor VII at 5 mg every 3 hours was initiated. Given the absence of other underlying conditions (Table 1), pregnancy-associated AHA was diagnosed. With treatment, pain and bleeding gradually improved. Recombinant activated factor VII administration was tapered over 6 days. The patient was discharged 16 days after admission with outpatient monitoring; when the inhibitor level decreased to 1.0 BU/mL, PSL tapering was initiated (Figure 1). The inhibitor remained undetectable (<0.5 BU/mL), but when the PSL dose was reduced to 7.5 mg, the inhibitor levels rose to a maximum of 1.5 BU/mL in June 20XX. Subsequently, the PSL dose was increased to 40 mg.
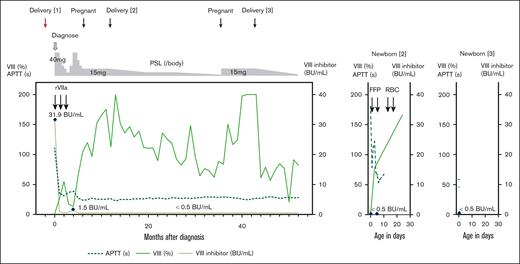
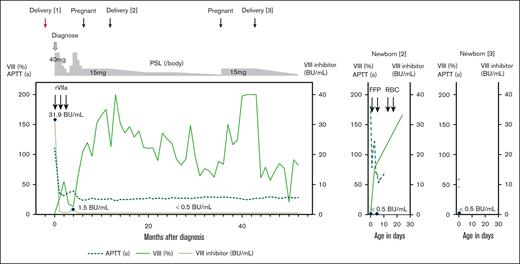
Two live births in a patient with pregnancy-associated AHA. Clinical course of a patient with pregnancy-associated AHA (left); the second (middle) and third newborns (right). FFP, fresh frozen plasma; RBC, red blood cell.
Two live births in a patient with pregnancy-associated AHA. Clinical course of a patient with pregnancy-associated AHA (left); the second (middle) and third newborns (right). FFP, fresh frozen plasma; RBC, red blood cell.
After the inhibitor levels became undetectable by August 20XX, the patient became pregnant while tapering PSL to 12.5 mg in October 20XX. The PSL dose was then increased to 15 mg and maintained throughout pregnancy to mitigate the risk of inhibitor formation. A cesarean birth was performed at 29 weeks and 2 days of gestation in March 20XX+1 because of fetal growth restriction and growth arrest, likely from placental dysfunction. Cause of fetal growth restriction and placental pathological findings are described in the supplemental Appendix.
The newborn, weighing 667 g (standard deviation, −3.7), exhibited a markedly prolonged APTT (>180 seconds) and decreased factor VIII level (<1.0%) at delivery, whereas factor VIII inhibitors were undetectable in both maternal peripheral and cord blood (Table 1). Intermittent fresh frozen plasma transfusions were performed. The newborn’s factor VIII levels normalized to 77.9% on day 3 and 165.9% on day 24 (Figure 1). The newborn experienced anemia requiring red blood cell transfusions on days 14 to 15 without obvious bleeding. The patient's PSL dose was gradually tapered after delivery without relapse.
In March 20XX+3, while on 2.5 mg of PSL, the patient became pregnant again. The previous treatment (PSL 15 mg) was followed, and low-dose aspirin was given to prevent placental dysfunction. The patient delivered a live newborn weighing 2318 g by cesarean birth at 37 weeks and 2 days of gestation in October 20XX+3. Both maternal peripheral and cord blood were undetectable for factor VIII inhibitors, and the cord blood showed slightly decreased factor VIII (45.9%) and mildly prolonged APTT (58.3 seconds) (Table 1). The newborn was discharged 6 days after birth without bleeding or anemia (Figure 1). The patient’s PSL dose was tapered after her third delivery. At the time of writing, she showed no decrease in factor VIII, inhibitor detection, or signs of other diseases for over 1 year.
Severe uterine bleeding can occur if factor VIII inhibitors develop during pregnancy.11 However, factor VIII inhibitors are rarely detected during pregnancy and are more commonly identified after childbirth, typically within 1 to 4 months after delivery.5,12 In this case, disease onset occurred 2 months after childbirth, confirming pregnancy-associated AHA after excluding other underlying conditions. Although >60% of patients with pregnancy-associated AHA resolve spontaneously and recurrence in subsequent pregnancy is rare,1,7 in this case, the patient’s inhibitor levels rose during PSL tapering, requiring intensified immunosuppressive therapy.
Factor VIII inhibitors are mainly immunoglobulin G, raising concerns regarding their placental transfer, resulting in severe fetal hemorrhage.13 Ries et al reported a newborn with intracranial hemorrhage at 5 days of age, whose mother was diagnosed with postpartum AHA.14 Kotani et al described a patient diagnosed with AHA following persistent postpartum hemorrhage, whose newborn had difficulty controlling bleeding after routine blood sampling.15 Lulla et al reported a newborn requiring intubation for epistaxis and hematemesis, whose mother was diagnosed with AHA after postpartum hemorrhage.16 These patients were not diagnosed with AHA or treated at delivery, but VIII inhibitors were detected in their newborns’ peripheral blood. Subsequent pregnancies with negative newborn inhibitors and normal factor VIII levels have also been reported.7-9
In our patient, PSL was maintained at 15 mg throughout subsequent pregnancies after AHA diagnosis to minimize the risk of inhibitor formation. Factor VIII inhibitors remained undetectable in both maternal peripheral and cord blood throughout subsequent pregnancies. Nevertheless, the second newborn's prolonged APTT and marked reduction in factor VIII suggested a possibility about minimal placental inhibitor transfer. Factors V and VIII typically mature in the fetus earlier than other coagulation factors,17 and fibrinogen and PT-INR levels were within expected ranges, considering the gestational age and birth weight,17 suggesting a specific decrease in factor VIII. The second newborn presented with anemia requiring red blood cell transfusion; however, there were no obvious signs of bleeding and anemia of prematurity was diagnosed. Other differential diagnoses, such as immature coagulation factor production due to inadequate liver function, congenital hemophilia A, von Willebrand disease, and antiphospholipid antibody syndrome were excluded based on clinical history and spontaneous factor VIII recovery. Although an optimal PSL dose during pregnancy remains unclear, maintaining an elevated dose appears necessary to prevent inhibitor formation and placental transfer.
Emicizumab, a bispecific monoclonal antibody mimicking factor VIII, has shown promising results for bleeding control in patients with AHA in clinical trials: AGEHA18 and GTH-AHA-EMI.19 However, pregnant women were excluded from both trials.18,19 One primary concern was the potential placental transfer of emicizumab, which carries a risk of thrombosis. In fact, emicizumab has been detected in cord blood when used during pregnancy in a patient with congenital hemophilia A with factor VIII inhibitors.20
This report shows that, with appropriate management, patients with pregnancy-associated AHA can achieve successful live births without significant complications. However, to our knowledge, this is the first report of a marked decrease in newborn factor VIII levels and prolongation of APTT, despite both maternal and cord blood inhibitor results being undetectable due to PSL, indicating that small amounts of inhibitors may cross the placenta, leading to hemorrhage. Careful attention should be paid to optimize maternal and newborn’s outcomes.
Acknowledgments: The authors thank the patient for providing consent for the publication of this case report. They thank Tadashi Matsushita (Nagoya University) for consulting with this patient.
Contribution: T.N. prepared and wrote the manuscript; T.N., N.M., A.K., T. Kikuchi, T. Kanamori, S.K., and A.I. were responsible for clinical management for hematology; S.T. was responsible for obstetrics and gynecology and the management of pregnancy and delivery; Y.W. was responsible for the care of children born to the patients; and all authors contributed to the article and approved the submitted version.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Takahiro Nakashima, Department of Hematology and Oncology, Nagoya City University Graduate School of Medical Sciences, 1 Kawasumi, Mizuho-cho, Mizuho-ku, Nagoya 467-8601, Japan; email: t_naka@med.nagoya-cu.ac.jp.
References
Author notes
Original data are available on request from the corresponding author, Takahiro Nakashima (t_naka@med.nagoya-cu.ac.jp).
The full-text version of this article contains a data supplement.