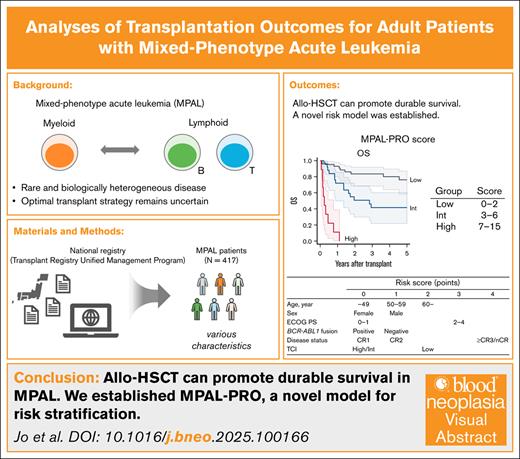
Key Points
Allo-HSCT can promote durable survival in MPAL, with outcomes comparable to AML and ALL, especially when performed in CR1.
We established the MPAL posttransplant prognostic score, a novel model stratifying outcomes after allo-HSCT using 6 pretransplant factors.
Visual Abstract
Mixed-phenotype acute leukemia (MPAL) is associated with poor prognosis. Although allogeneic hematopoietic stem cell transplantation (allo-HSCT) can achieve long-term survival, optimal transplantation strategies remain unclear. We analyzed a national registry of adult patients with MPAL who underwent allo-HSCT between 2008 and 2022 in Japan. Among 417 patients, median age at transplant was 44 years (interquartile range, 32-55); 61% were male, 20% were BCR::ABL1-positive, and 66% underwent transplant during the first complete remission (CR; CR1). At 5 years posttransplant, overall survival (OS) was 54%, disease-free survival was 49%, relapse was 28%, and nonrelapse mortality was 23%. Multivariate analysis identified older age (adjusted hazard ratio [aHR], 1.78 [95% confidence interval (CI), 1.11-2.84] for ages 50-59 years; aHR, 2.65 [95% CI, 1.54-4.55] for ≥60 years; both vs <40 years), male sex (aHR, 1.56; 95% CI, 1.07-2.27), Eastern Cooperative Oncology Group performance status scale ≥2 (aHR, 3.05; 95% CI, 1.72-5.40), BCR::ABL1 -negative status (aHR, 1.64; 95% CI, 1.02-2.64), advanced disease status (aHR, 1.642 [95% CI, 0.80-3.36] for second CR; aHR, 4.01 [95% CI, 2.61-6.15] for third or higher CR, or non-CR vs CR1), and low conditioning intensity (aHR, 2.49 [95% CI, 1.21-5.13] for low transplant conditioning intensity [TCI] vs high TCI) as adverse prognostic factors for OS. A propensity score–matched comparison with acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL) during the same period showed that MPAL did not have significantly worse prognosis than AML or ALL. These findings suggest that allo-HSCT offers long-term survival in MPAL, with outcomes not inferior to those of AML and ALL.
Introduction
Mixed-phenotype acute leukemia (MPAL) is a rare and biologically heterogeneous disease exhibiting characteristics of both myeloid and lymphoid lineages.1-4 Due to its rarity, optimal treatment strategy remains uncertain. As outcomes remain poor with either acute myeloid leukemia (AML)–directed or acute lymphoid leukemia (ALL)–directed multiagent chemotherapy alone, allogeneic hematopoietic stem cell transplantation (allo-HSCT) is generally adopted as a potentially curative approach.5-7
To our knowledge, the largest study to date, conducted by the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation (EBMT), retrospectively analyzed 519 patients with MPAL who underwent transplant during the first complete remission (CR; CR1) between 2000 and 2014.8 It reported a 3-year relapse incidence of 31.4%, nonrelapse mortality (NRM) of 22.1%, leukemia-free survival of 46.5%, and overall survival (OS) of 56.3%. Another major study by the Center for International Blood and Marrow Transplant Research (CIBMTR) analyzed 95 patients with MPAL who underwent transplant in CR between 1996 and 2012, showing similar results: a 3-year relapse rate 29%, NRM 15%, leukemia-free survival 56%, and OS 67%.9 However, these analyses were restricted to patients who received transplant in CR, and provided limited information into prognostic factors, including disease status at transplant, and that of BCR::ABL1 fusion.
Recent advances in transplant techniques are expected to improve posttransplant outcomes. However, the increasing proportion of older recipients has shifted both transplant success rates and complication patterns in AML or ALL, highlighting the need for updated MPAL data.10,11 Given the lack of MPAL-specific clinical data, transplant strategies for MPAL generally follow those for AML or ALL in clinical practice. Thus, further investigation is needed to characterize transplant outcomes, graft-versus-host disease (GVHD), and other complications specific to MPAL.
In this study, we aimed to identify prognostic factors for patients with MPAL undergoing allo-HSCT and establish risk prediction models using a Japanese nationwide registry. We also compared their posttransplant outcomes with those of AML and ALL to define MPAL-specific characteristics. Our findings provide valuable real-world evidence to support improved risk stratification and optimization of allo-HSCT strategies for this rare leukemia subtype.
Patients and methods
Patients
Data on adult patients (age ≥16 years) with de novo MPAL who underwent their first allo-HSCT between 2008 and 2022 were obtained through the Transplant Registry Unified Management Program sponsored by the Japanese Society for Transplantation and Cellular Therapy.12,13 MPAL was defined based on the World Health Organization fifth edition criteria for acute leukemia of mixed or ambiguous lineage.1 In accordance with the diagnostic criteria, leukemias that can be assigned to another well-defined entity, including therapy-related and myelodysplasia-related cases, were excluded. Patients without survival data or those who underwent haploidentical transplantation were excluded. The study was planned by the Adult ALL Working Group and the Adult AML Working Group of the Japanese Society for Transplantation and Cellular Therapy, approved by the data management committees of Transplant Registry Unified Management Program and by the institutional review board of Kyoto University Hospital, and was conducted in accordance with the Declaration of Helsinki.
Study end points and definitions
The primary end point was OS after transplantation. Death regardless of the cause was considered an event. Secondary end points were disease-free survival (DFS), and cumulative incidences of relapse and NRM. Conditioning intensity was defined according to operational definitions of the National Marrow Donor Program/CIBMTR,14 and to the transplant conditioning intensity (TCI) score.15 Based on the TCI score, conditioning regimens were classified into low (1-2 points), intermediate (2.5-3.5 points), and high (4-6 points).15 Eastern Cooperative Oncology Group performance status (ECOG PS) scale at transplant was evaluated according to ECOG criteria.16 Hematopoietic cell transplantation–specific comorbidity index was determined according to the Seattle scale.17 HLA matching was assessed using allele data for the HLA-A, -B, -C, and -DRB1 loci in bone marrow and peripheral blood stem cell grafts, and by using serological data for the HLA-A, -B, and -DR loci in cord blood grafts.18,19 HLA mismatch was defined in the GVHD vector when recipient alleles or antigens were not shared by the donor, and was defined in the host-versus-graft direction when donor alleles were not shared by the recipient.
Cytogenetic risk for AML and MPAL was classified in accordance with criteria specified by the National Comprehensive Cancer Network Guidelines (Acute Myeloid Leukemia. Version 1.2016), as described elsewhere,20 and detailed in the supplemental Methods.
Propensity score matching
To compare clinical characteristics between MPAL and AML or ALL, data on adult patients with de novo AML or ALL who had undergone their first allo-HSCT during the same period were obtained using the same methodology as MPAL. To align with the diagnostic framework for MPAL, AML cases with inv(16), t(16;16), t(8;21), or t(15;17), as well as therapy-related or myelodysplasia-related AML, were excluded. For the comparison between MPAL and AML, patients with unevaluable cytogenetic risk were excluded, and for the MPAL vs ALL comparison, patients lacking BCR::ABL1 data were excluded. Propensity score matching was then performed to adjust for potential confounding factors (Table 1), as detailed in the supplemental Methods.
Patient characteristics
| . | Total N = 417 . |
|---|---|
| Age at transplant, y | 44 (32-55) |
| <40 | 168 (40.3) |
| 40-49 | 94 (22.5) |
| 50-59 | 91 (21.8) |
| ≥60 | 64 (15.3) |
| Sex | |
| Male | 253 (60.7) |
| Female | 164 (39.3) |
| ECOG PS | |
| 0-1 | 381 (91.4) |
| ≥2 | 34 (8.2) |
| HCT-CI | |
| 0-2 | 363 (87.1) |
| ≥3 | 48 (11.5) |
| CMV-Ab | |
| Negative | 70 (16.8) |
| Positive | 336 (80.6) |
| WBC at diagnosis, per 109/L | 9.50 (3.33-39.06) |
| Cytogenetics of MPAL | |
| BCR::ABL1 fusion | 82 (19.7) |
| KMT2A rearrangement | 9 (2.2) |
| Cytogenetic risk category (AML) | |
| Favorable | 0 (0.0) |
| Intermediate | 121 (29.0) |
| Poor | 112 (26.9) |
| Unevaluable | 184 (44.1) |
| Disease status at transplant | |
| CR1 | 276 (66.2) |
| CR2 | 27 (6.5) |
| CR3 or higher | 2 (0.5) |
| nCR | 98 (23.5) |
| Diagnosis to SCT, mo | 5.8 (4.6-7.8) |
| Sex mismatch | |
| Match | 211 (50.6) |
| Male to female | 88 (21.1) |
| Female to male | 103 (24.7) |
| ABO mismatch | |
| Matched | 198 (47.5) |
| Minor mismatch | 99 (23.7) |
| Major mismatch | 78 (18.7) |
| Major-minor mismatch | 39 (9.4) |
| HLA mismatch | |
| HLA-matched | 169 (40.5) |
| HLA-mismatched | 240 (57.6) |
| Conditioning | |
| MAC | 315 (75.5) |
| RIC | 100 (24.0) |
| Conditioning, TCI category | |
| High | 307 (73.6) |
| Intermediate | 92 (22.1) |
| Low | 15 (3.6) |
| TBI | |
| TBI no | 89 (21.3) |
| TBI yes | 326 (78.2) |
| Graft source | |
| Rel-BM | 26 (6.2) |
| Rel-PBSC | 87 (20.9) |
| UR-BM | 134 (32.1) |
| UR-PBSC | 23 (5.5) |
| UR-CB | 146 (35.0) |
| GVHD prophylaxis | |
| CyA-based | 113 (27.1) |
| Tac-based | 300 (71.9) |
| Transplant year | |
| 2008-2015 | 218 (52.3) |
| 2016-2022 | 199 (47.7) |
| . | Total N = 417 . |
|---|---|
| Age at transplant, y | 44 (32-55) |
| <40 | 168 (40.3) |
| 40-49 | 94 (22.5) |
| 50-59 | 91 (21.8) |
| ≥60 | 64 (15.3) |
| Sex | |
| Male | 253 (60.7) |
| Female | 164 (39.3) |
| ECOG PS | |
| 0-1 | 381 (91.4) |
| ≥2 | 34 (8.2) |
| HCT-CI | |
| 0-2 | 363 (87.1) |
| ≥3 | 48 (11.5) |
| CMV-Ab | |
| Negative | 70 (16.8) |
| Positive | 336 (80.6) |
| WBC at diagnosis, per 109/L | 9.50 (3.33-39.06) |
| Cytogenetics of MPAL | |
| BCR::ABL1 fusion | 82 (19.7) |
| KMT2A rearrangement | 9 (2.2) |
| Cytogenetic risk category (AML) | |
| Favorable | 0 (0.0) |
| Intermediate | 121 (29.0) |
| Poor | 112 (26.9) |
| Unevaluable | 184 (44.1) |
| Disease status at transplant | |
| CR1 | 276 (66.2) |
| CR2 | 27 (6.5) |
| CR3 or higher | 2 (0.5) |
| nCR | 98 (23.5) |
| Diagnosis to SCT, mo | 5.8 (4.6-7.8) |
| Sex mismatch | |
| Match | 211 (50.6) |
| Male to female | 88 (21.1) |
| Female to male | 103 (24.7) |
| ABO mismatch | |
| Matched | 198 (47.5) |
| Minor mismatch | 99 (23.7) |
| Major mismatch | 78 (18.7) |
| Major-minor mismatch | 39 (9.4) |
| HLA mismatch | |
| HLA-matched | 169 (40.5) |
| HLA-mismatched | 240 (57.6) |
| Conditioning | |
| MAC | 315 (75.5) |
| RIC | 100 (24.0) |
| Conditioning, TCI category | |
| High | 307 (73.6) |
| Intermediate | 92 (22.1) |
| Low | 15 (3.6) |
| TBI | |
| TBI no | 89 (21.3) |
| TBI yes | 326 (78.2) |
| Graft source | |
| Rel-BM | 26 (6.2) |
| Rel-PBSC | 87 (20.9) |
| UR-BM | 134 (32.1) |
| UR-PBSC | 23 (5.5) |
| UR-CB | 146 (35.0) |
| GVHD prophylaxis | |
| CyA-based | 113 (27.1) |
| Tac-based | 300 (71.9) |
| Transplant year | |
| 2008-2015 | 218 (52.3) |
| 2016-2022 | 199 (47.7) |
Continuous variables are presented as median (IQR); categorical variables as number (%).
BM, bone marrow; CB, cord blood; CMV-Ab, cytomegalovirus antibody; CyA, cyclosporin A; HCT-CI, hematopoietic cell transplantation–specific comorbidity index; MAC, myeloablative conditioning; PBSC, peripheral blood stem cell; Rel, related; RIC, reduced-intensity conditioning; SCT, stem cell transplant; Tac, tacrolimus; TBI, total body irradiation; UR, unrelated; WBC, white blood cell.
Statistical analysis
Categorical variables were summarized as counts and percentages, and were compared between groups with Fisher exact test. Continuous variables were summarized as medians and interquartile ranges (IQRs), and were compared between groups with 2-tailed, unpaired Student’s t test. Probabilities of OS and DFS were estimated according to the Kaplan-Meier method, and compared among groups with the Cox proportional hazards model. Probabilities of relapse and NRM were estimated on the basis of cumulative incidence methods, and compared among groups with the Fine-Gray proportional hazards model,21 considering death without relapse as a competing event for relapse, relapse as a competing event for NRM. In the multivariate analysis, variables that showed statistical significance (P < .05) in univariate analysis for OS, DFS, relapse, or NRM were evaluated through stepwise inclusion and exclusion, and the final models were selected based on the lowest Bayesian Information Criterion value. A risk scoring system for MPAL was developed using a training-validation cohort approach based on OS, as detailed in the supplemental Methods.
All tests were 2-sided, and P values <.05 were considered statistically significant. All analyses were performed with Stata, version 18 (Stata Corp, College Station, TX).
This study was approved by the institutional review board and ethic committee of Kyoto University. Written informed consent was obtained from all participating patients.
Results
Patient characteristics
Of 435 adult patients with MPAL who underwent first allo-HSCT between 2008 and 2022, 18 were excluded due to haploidentical donor sources (supplemental Figure 1). As a result, 417 patients with a median age of 44 years (IQR, 32-55) were analyzed (Table 1; supplemental Table 1). Among them, 82 (19.7%) and 9 (2.2%) were positive for BCR::ABL1 fusion, and KMT2A rearrangement, respectively. Cytogenetic risk classification for AML included intermediate in 121 (29.0%), and poor risk in 112 (26.9%), respectively, whereas cytogenetic risk could not be evaluated in 184 (44.1%). Regarding remission status, 276 (66.2%) underwent transplant in CR1, 27 (6.5%) in the second CR (CR2), 2 (0.5%) in the third or higher CR (CR3), and 98 (23.5%) in non-CR (nCR). Conditioning regimens consisted of myeloablative conditioning in 315 (75.5%), and reduced-intensity conditioning in 100 (24.0%). Based on the TCI score, 307 patients (73.6%) were classified as high, 92 (22.1%) as intermediate, and 15 (3.6%) as low. A total of 326 patients (78.2%) underwent total body irradiation (TBI)–containing conditioning. Graft sources were: related bone marrow in 26 patients (6.2%), related peripheral blood stem cells in 87 (20.9%), unrelated bone marrow in 134 (32.1%), unrelated peripheral blood stem cells in 23 (5.5%), and unrelated cord blood in 146 (35.0%). For GVHD prophylaxis, cyclosporine-based regimens were used in 113 patients (27.1%), whereas tacrolimus-based regimens were used in 300 (71.9%). One hundred and ninety-nine patients (47.8%) underwent transplantation during the period from 2016 to 2022.
Posttransplant outcomes in MPAL
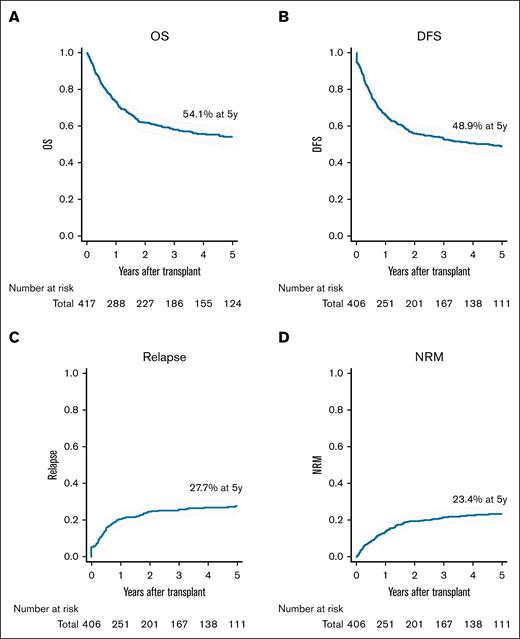
The median follow-up duration for survivors was 60.0 months (IQR, 32.6-97.0). The 5-year OS, DFS, cumulative incidence of relapse, and NRM were 54.1%, 48.9%, 27.7%, and 23.4%, respectively (Figure 1). At day 60 posttransplant, cumulative incidence of neutrophil and platelet engraftments were 94.4% and 80.6%, respectively (supplemental Figure 2A-B). Median times to neutrophil and platelet engraftment were 17 days (IQR, 14-21) and 30 days (IQR, 21-39), respectively.
Outcomes of patients with MPAL who underwent allo-HSCT. (A) OS. (B) DFS. (C) Cumulative incidence of relapse. (D) Cumulative incidence of NRM. Percentages in the figure represent data 5 years after transplantation.
Outcomes of patients with MPAL who underwent allo-HSCT. (A) OS. (B) DFS. (C) Cumulative incidence of relapse. (D) Cumulative incidence of NRM. Percentages in the figure represent data 5 years after transplantation.
Cumulative incidences of grade 2 to 4 and 3 to 4 aGVHD at day 100 were 42.1% and 12.8%, respectively. Cumulative incidences of chronic GVHD (cGVHD) and extensive cGVHD at 5 years were 36.4% and 15.8% (supplemental Figure 2C-F). Among 186 deaths, the main causes of death included primary disease in 105 patients (56.5%), infections in 27 (14.5%), and GVHDs in 12 (6.5%; supplemental Table 2).
Prognostic factors for posttransplant outcomes in MPAL
To evaluate impacts of clinical variables on posttransplant outcomes, univariate analyses were performed (supplemental Table 3). Greater age, poor ECOG PS, higher white blood cell counts at diagnosis, BCR::ABL1 negativity, advanced disease status at transplant, and a longer duration from diagnosis to transplantation appeared to be potential risk factors for inferior OS and DFS. Additionally, low conditioning intensity (TCI low), use of a non-TBI conditioning regimen, HLA-mismatched transplantation, graft sources other than related bone marrow, and transplantation performed between 2008 and 2015 were potential adverse prognostic factors for OS and DFS. No significant association was observed between immunophenotypic subclassification and posttransplant outcomes, although a substantial proportion of patients lacked complete immunophenotypic data (supplemental Tables 1 and 3).
Next, multivariate analyses were conducted by considering variables that were significant in the univariate analyses for OS, DFS, relapse, or NRM (Table 2). The following variables were identified as independent adverse prognostic factors for OS; greater age (adjusted hazard ratio [aHR], 1.778 [95% confidence interval (CI), 1.114-2.837; P = .016] for ages 50-59 years; aHR, 2.649 [95% CI, 1.542-4.550; P < .001] for ≥60 years; both vs <40 years), male sex (aHR, 1.562; 95% CI, 1.072-2.274; P = .020 vs female), ECOG PS 2 to 4 (aHR, 3.049; 95% CI, 1.721-5.403; P < .001 vs 0-1), BCR::ABL1-negative (aHR, 1.639; 95% CI, 1.019-2.636; P = .042 vs positive), advanced disease status at transplant (aHR, 1.642 [95% CI, 0.802-3.362; P = .175] for CR2; aHR, 4.008 [95% CI, 2.614-6.146; P < .001] for CR3 or higher, or nCR; both vs CR1), and low TCI (aHR, 2.493; 95% CI, 1.212-5.129; P = .013; supplemental Figure 3A-F). In contrast, white blood cell counts at diagnosis, HLA mismatch, graft source, TBI, TBI dose, and transplant year did not remain as significant prognostic factors. Similarly, for DFS, greater age, poor ECOG PS, BCR::ABL1 negativity, advanced disease status at transplant, and low TCI were adverse prognostic factors. Greater age and male sex were significantly associated with an increased risk of NRM, whereas advanced disease status at transplant was significantly associated with an increased risk of relapse. Although not statistically significant, BCR::ABL1 negativity showed a trend toward increased risk of relapse. Poor ECOG PS also showed a trend toward increasing both relapse and NRM. Low TCI was linked to a tendency toward increased relapse risk.
Multivariate analyses for transplant outcomes
| . | OS . | DFS . | Relapse . | NRM . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR . | 95% CI . | P value . | HR . | (95% CI) . | P value . | HR . | (95% CI) . | P value . | HR . | (95% CI) . | P value . | |
| Age at transplant, y | ||||||||||||
| 40-49 vs <40 | 0.986 | (0.573-1.699) | .961 | 0.872 | (0.520-1.463) | .604 | 0.656 | (0.317-1.356) | .255 | 1.251 | (0.532-2.943) | .608 |
| 50-59 vs <40 | 1.778 | (1.114-2.837) | .016∗ | 1.486 | (0.957-2.306) | .078 | 0.894 | (0.500-1.596) | .704 | 2.501 | (1.201-5.205) | .014∗ |
| ≥60 vs <40 | 2.649 | (1.542-4.550) | <.001∗ | 1.838 | (1.082-3.122) | .024∗ | 0.491 | (0.190-1.265) | .141 | 3.513 | (1.621-7.610) | .001∗ |
| Sex, male vs female | 1.562 | (1.072-2.274) | .020∗ | 1.532 | (1.062-2.208) | .022 | 1.085 | (0.669-1.759) | .740 | 2.030 | (1.124-3.668) | .019∗ |
| ECOG PS, ≥2 vs 0-1 | 3.049 | (1.721-5.403) | <.001∗ | 3.023 | (1.709-5.347) | <.001 | 1.663 | (0.757-3.654) | .206 | 1.754 | (0.605-5.079) | .301 |
| Cytogenetics of MPAL, BCR::ABL1-neg vs -pos | 1.639 | (1.019-2.636) | .042∗ | 1.641 | (1.046-2.574) | .031∗ | 1.384 | (0.741-2.586) | .308 | 1.651 | (0.854-3.193) | .136 |
| Disease status at transplant | ||||||||||||
| CR2 vs CR1 | 1.642 | (0.802-3.362) | .175 | 1.458 | (0.743-2.861) | .273 | 1.119 | (0.429-2.921) | .818 | 1.703 | (0.764-3.796) | .193 |
| CR3 or higher/nCR vs CR1 | 4.008 | (2.614-6.146) | <.001∗ | 3.644 | (2.426-5.475) | <.001∗ | 4.006 | (2.382-6.736) | <.001∗ | 1.027 | (0.493-2.140) | .943 |
| Conditioning | ||||||||||||
| TCI intermediate vs high | 1.067 | (0.652-1.744) | .796 | 1.160 | (0.733-1.836) | .526 | 0.856 | (0.381-1.926) | .707 | 1.305 | (0.695-2.448) | .408 |
| TCI low vs high | 2.493 | (1.212-5.129) | .013∗ | 2.587 | (1.190-5.622) | .016∗ | 2.233 | (0.648-7.693) | .203 | 1.434 | (0.371-5.535) | .601 |
| . | OS . | DFS . | Relapse . | NRM . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR . | 95% CI . | P value . | HR . | (95% CI) . | P value . | HR . | (95% CI) . | P value . | HR . | (95% CI) . | P value . | |
| Age at transplant, y | ||||||||||||
| 40-49 vs <40 | 0.986 | (0.573-1.699) | .961 | 0.872 | (0.520-1.463) | .604 | 0.656 | (0.317-1.356) | .255 | 1.251 | (0.532-2.943) | .608 |
| 50-59 vs <40 | 1.778 | (1.114-2.837) | .016∗ | 1.486 | (0.957-2.306) | .078 | 0.894 | (0.500-1.596) | .704 | 2.501 | (1.201-5.205) | .014∗ |
| ≥60 vs <40 | 2.649 | (1.542-4.550) | <.001∗ | 1.838 | (1.082-3.122) | .024∗ | 0.491 | (0.190-1.265) | .141 | 3.513 | (1.621-7.610) | .001∗ |
| Sex, male vs female | 1.562 | (1.072-2.274) | .020∗ | 1.532 | (1.062-2.208) | .022 | 1.085 | (0.669-1.759) | .740 | 2.030 | (1.124-3.668) | .019∗ |
| ECOG PS, ≥2 vs 0-1 | 3.049 | (1.721-5.403) | <.001∗ | 3.023 | (1.709-5.347) | <.001 | 1.663 | (0.757-3.654) | .206 | 1.754 | (0.605-5.079) | .301 |
| Cytogenetics of MPAL, BCR::ABL1-neg vs -pos | 1.639 | (1.019-2.636) | .042∗ | 1.641 | (1.046-2.574) | .031∗ | 1.384 | (0.741-2.586) | .308 | 1.651 | (0.854-3.193) | .136 |
| Disease status at transplant | ||||||||||||
| CR2 vs CR1 | 1.642 | (0.802-3.362) | .175 | 1.458 | (0.743-2.861) | .273 | 1.119 | (0.429-2.921) | .818 | 1.703 | (0.764-3.796) | .193 |
| CR3 or higher/nCR vs CR1 | 4.008 | (2.614-6.146) | <.001∗ | 3.644 | (2.426-5.475) | <.001∗ | 4.006 | (2.382-6.736) | <.001∗ | 1.027 | (0.493-2.140) | .943 |
| Conditioning | ||||||||||||
| TCI intermediate vs high | 1.067 | (0.652-1.744) | .796 | 1.160 | (0.733-1.836) | .526 | 0.856 | (0.381-1.926) | .707 | 1.305 | (0.695-2.448) | .408 |
| TCI low vs high | 2.493 | (1.212-5.129) | .013∗ | 2.587 | (1.190-5.622) | .016∗ | 2.233 | (0.648-7.693) | .203 | 1.434 | (0.371-5.535) | .601 |
neg, negative; pos, positive.
Development of a risk scoring system for MPAL: MPAL-PRO
To weight the prognostic impact of each clinical factor clearly, a risk scoring system for use in clinical settings was developed. Based on the multivariate analysis using the training cohort, we defined an MPAL posttransplant prognostic score (MPAL-PRO) system, including 6 variables with independent prognostic impact on OS: age at transplant, patient sex, ECOG PS at transplant, BCR::ABL1 fusion, disease status at transplant, and TCI category (supplemental Table 4). One point each was assigned for age of 50 to 59 years, male sex, negative for BCR::ABL1 fusion, and CR2 at transplant; 2 points for age ≥60 years, and low TCI; 3 points for ECOG PS 2 to 4; 4 points for CR3 or higher, or nCR at transplant. MPAL-PRO risk categories were determined by combining the scores of these 6 factors. According to this score, patients were divided into the following 3 risk groups: low (score 0-2), intermediate (score 3-6), and high (score 7-15; Table 3).
Prognostic scoring system for MPAL
| . | Risk score (points) . | ||||
|---|---|---|---|---|---|
| 0 . | 1 . | 2 . | 3 . | 4 . | |
| Age, y | <50 | 50-59 | ≥60 | ||
| Sex | Female | Male | |||
| ECOG PS | 0-1 | 2-4 | |||
| BCR::ABL1 fusion | Positive | Negative | |||
| Disease status at transplant | CR1 | CR2 | CR3 or higher/nCR | ||
| Conditioning intensity, TCI | High/Int | Low | |||
| . | Risk score (points) . | ||||
|---|---|---|---|---|---|
| 0 . | 1 . | 2 . | 3 . | 4 . | |
| Age, y | <50 | 50-59 | ≥60 | ||
| Sex | Female | Male | |||
| ECOG PS | 0-1 | 2-4 | |||
| BCR::ABL1 fusion | Positive | Negative | |||
| Disease status at transplant | CR1 | CR2 | CR3 or higher/nCR | ||
| Conditioning intensity, TCI | High/Int | Low | |||
According to this score, patients were divided into the following 3 risk groups: low (score 0-2), intermediate (score 3-6), and high (score 7-15).
Int, intermediate.
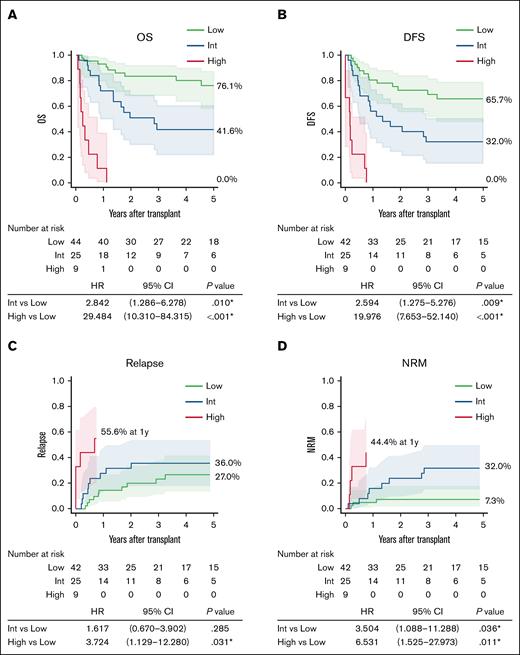
The prognostic impact of the MPAL-PRO was evaluated in the validation cohort. Risk groups defined by the MPAL-PRO had significantly different 5-year OS (76.1%, 41.6%, and 0.0%), and DFS (65.7%, 32.0%, and 0.0%; Figure 2A-B). The intermediate group had significantly higher NRM (HR, 3.504; 95% CI, 1.088-11.288; P = .036), and a trend toward higher relapse rate than the low group (HR, 1.617; 95% CI, 0.670-3.902; P = .285). The high group had significantly higher relapse (HR, 3.724; 95% CI, 1.129-12.280; P = .031) and NRM (HR, 6.531; 95% CI, 1.525-27.973; P = .011) than the low group (Figure 2C-D).
Transplantation outcomes based on prognostic categories in the validation cohort. (A) OS. (B) DFS. (C) Cumulative incidence of relapse. (D) Cumulative incidence of NRM. Percentages in the figure represent data 5 years after transplantation, unless otherwise specified. Int, intermediate.
Transplantation outcomes based on prognostic categories in the validation cohort. (A) OS. (B) DFS. (C) Cumulative incidence of relapse. (D) Cumulative incidence of NRM. Percentages in the figure represent data 5 years after transplantation, unless otherwise specified. Int, intermediate.
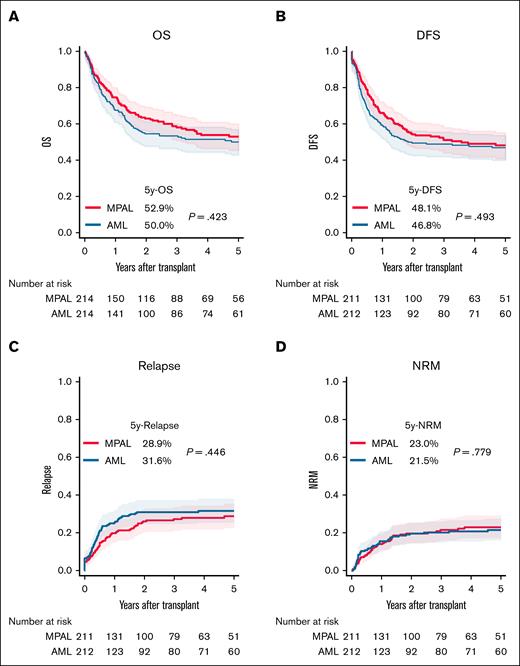
Comparison of posttransplant outcomes with AML
In a matched analysis, 214 patients each with MPAL and AML were compared (supplemental Table 5; supplemental Figure 4A). OS and DFS were similar between patients with MPAL and those with AML (Figure 3A-B). Although not statistically significant, MPAL showed a trend toward a lower cumulative relapse and a comparable NRM compared with AML (Figure 3C-D). Grade 2 to 4 acute GVHD (aGVHD) was significantly higher in patients with MPAL (48.4% vs 34.6% at 100 days; P = .003), whereas grade 3 to 4 aGVHD was not significantly different (13.8% vs 8.9%; P = .114). Similarly, overall cGVHD at 5 years was more frequent in MPAL (39.6% vs 30.9%; P = .037), but extensive cGVHD incidence was comparable (18.2% vs 15.3%; P = .369; supplemental Figure 5A-D).
Comparison of transplantation outcomes between patients with MPAL and those with AML after propensity score matching. (A) OS. (B) DFS. (C) Cumulative incidence of relapse. (D) Cumulative incidence of NRM.
Comparison of transplantation outcomes between patients with MPAL and those with AML after propensity score matching. (A) OS. (B) DFS. (C) Cumulative incidence of relapse. (D) Cumulative incidence of NRM.
Then, a propensity score–matched comparison was conducted for each disease status at transplant. In CR1, MPAL showed significantly better 5-year OS than AML (64.5% vs 54.7%; P = .049). Although DFS (60.1% vs 50.6%; P = .106) and relapse rate (16.6% vs 22.4%; P = .192) also favored MPAL, the differences were not statistically significant (supplemental Figure 6A-D; supplemental Table 6). Grade 2 to 4 aGVHD at 100 days (51.8% vs 34.3%; P = .008) and overall cGVHD at 5 years (45.4% vs 35.2%; P = .047) were more frequent in MPAL, but no significant differences were observed in grade 3 to 4 aGVHD or extensive cGVHD (supplemental Figure 6E-H).
In CR2, despite the limited number of patients, MPAL tended to show higher relapse and slightly inferior OS and DFS compared with AML, with no significant differences in GVHD (supplemental Figure 7A-H; supplemental Table 7).
In CR3 or higher, or nCR, MPAL and AML showed no significant differences in OS, DFS, relapse, or NRM (supplemental Figure 8A-D; supplemental Table 8). Although not statistically significant, both aGVHD and cGVHD tended to be more frequent in MPAL (supplemental Figure 8E-H). These findings suggest better outcomes in CR1, a trend toward worse outcomes in CR2, and similar outcomes in CR3 or higher, or nCR for MPAL compared with AML. Although significance varied by GVHD type and severity, patients with MPAL tended to have a higher GVHD risk than AML.
Comparison of posttransplant outcomes with ALL
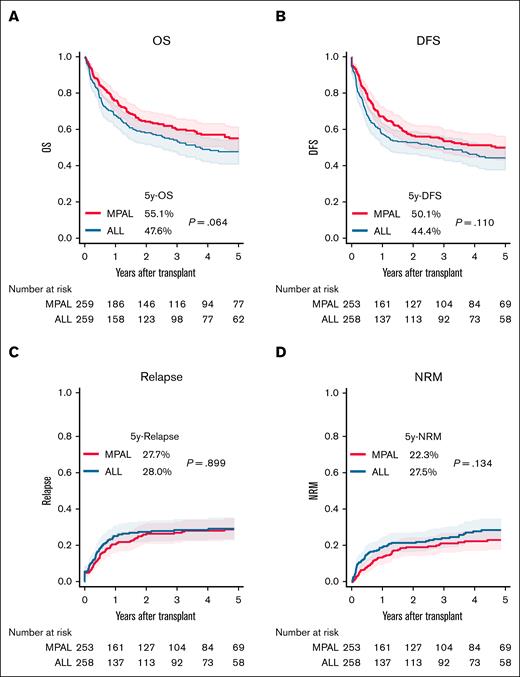
In a matched analysis, 259 patients each with MPAL and ALL were compared (supplemental Table 9; supplemental Figure 4B). Although not statistically significant, MPAL tended to show lower NRM and more favorable OS and DFS than ALL (Figure 4). Although cGVHD was significantly more frequent in MPAL (39.3% vs 31.1%; P = .045), no significant differences were observed in aGVHD or extensive cGVHD (supplemental Figure 9A-D).
Comparison of transplantation outcomes between patients with MPAL and those with ALL after propensity score matching. (A) OS. (B) DFS. (C) Cumulative incidence of relapse. (D) Cumulative incidence of NRM.
Comparison of transplantation outcomes between patients with MPAL and those with ALL after propensity score matching. (A) OS. (B) DFS. (C) Cumulative incidence of relapse. (D) Cumulative incidence of NRM.
In CR1, posttransplant outcomes were similar (supplemental Figure 10A-D; supplemental Table 10), and GVHD incidence was not significantly different (supplemental Figure 10E-H).
In CR2, OS and DFS were not significantly different, though cGVHD and extensive cGVHD tended to be more frequent in MPAL (supplemental Figure 11A-H; supplemental Table 11).
In CR3 or higher, or nCR, no significant differences were found between MPAL and ALL in OS, DFS, relapse, or NRM (supplemental Figure 12A-D; supplemental Table 12). MPAL showed a trend toward higher grade 3 to 4 aGVHD and extensive cGVHD, whereas grade 2 to 4 aGVHD and overall cGVHD were similar between groups (supplemental Figure 12E-H). These findings suggest that MPAL has a posttransplant prognosis similar to that of ALL, irrespective of disease status at transplant with a trend toward higher risk of cGVHD.
Discussion
Using the Japanese nationwide registry, this study analyzed posttransplant outcomes of adult patients with MPAL who underwent allo-HSCT. There were 3 major findings: (1) among 417 patients, 5-year OS and DFS were 54.1% and 48.9%, respectively, along with a cumulative incidence of relapse of 27.7% and NRM of 23.4%; (2) a novel risk stratification system incorporating adverse prognostic factors, including greater age, male sex, poorer ECOG PS at transplantation, BCR::ABL1 negativity, advanced disease status at transplant, and low TCI, was developed to predict prognosis after allo-HSCT in patients with MPAL; (3) propensity score–matched analysis comparing MPAL with AML and ALL showed that OS and DFS in MPAL were not inferior to those in AML or ALL.
First, we evaluated outcomes of allo-HSCT in the MPAL cohort. Despite 23.5% of patients receiving transplant in nCR, OS, DFS, relapse, and NRM in our study were similar to those reported by the CIBMTR and EBMT groups.8,9 Prognostic factor analysis identified greater age, male sex, poorer ECOG PS at transplantation, BCR::ABL1 negativity, advanced disease status at transplant, and low TCI as independent adverse prognostic factors for OS. Older age was a well-established prognostic factor.8 Notably, although OS and DFS did not differ significantly between patients <40 years and those aged 40 to 49 years, outcomes were significantly worse in patients aged ≥50 years, mainly due to increased NRM. Male sex was also associated with increased NRM and reduced OS, which aligns with findings in AML and ALL, and may be attributed to higher comorbidities and reduced organ reserves in male patients.22,23
In this study, BCR::ABL1 negativity was associated with poor prognosis after transplantation in patients with MPAL. Historically, BCR::ABL1 fusion was regarded as a poor prognostic factor in ALL.24,25 However, with the advent of tyrosine kinase inhibitors (TKIs), improved pretransplant disease control has turned BCR::ABL1 positivity into a favorable prognostic marker.11,23 Our findings suggest a similar trend might apply to MPAL. Although, in our cohort, all patients with MPAL with BCR-ABL1 fusion positive received TKI therapy before transplant, our registry did not capture information on posttransplant TKI maintenance therapy or minimal residual disease. Further studies are needed to assess how minimal residual disease status and TKI maintenance affect outcomes in MPAL.
Disease status at transplant was also a critical prognostic factor. The EBMT report (n = 519), which included only CR1 patients, did not provide data on disease status influence. The CIBMTR report (n = 95) found that CR2 (n = 17) was not inferior to CR1 (n = 78) in univariate analysis. However, our multivariate analysis showed significantly worse OS for CR3 or higher, or nCR compared with CR1 (HR, 4.008; 95% CI, 2.614-6.146) and a trend toward inferior OS for CR2, though not statistically significant (HR, 1.642; 95% CI, 0.802-3.362). Given the small number of CR2 patients (n = 27) and the lack of data on patients who did not undergo transplant in CR1 in our study, conclusions regarding impact of disease status should be interpreted with caution. Nonetheless, given the poor prognosis with chemotherapy alone and the unfavorable outcomes associated with transplant beyond CR1, performing transplant during CR1 appears a reasonable strategy.
In this study, although the conventional myeloablative conditioning/reduced-intensity conditioning classification did not reveal a significant impact on outcomes, the more refined TCI score successfully demonstrated the prognostic relevance of conditioning intensity in MPAL. Stratification by TCI into high, intermediate, and low groups showed comparable outcomes between the high and the intermediate groups, whereas the low group had significantly worse OS and DFS, even after multivariate adjustment, and a trend toward increased relapse. Although optimal intensity remains unclear due to the small number of patients who were TCI low in this study, our findings and previous reports suggesting inferior outcomes with reduced intensity,8 indicate that maintaining conditioning intensity may be important for improving outcomes in MPAL.
Other previously reported adverse prognostic factors include non-TBI conditioning, and earlier transplant eras.8 In our study, although these factors appeared to be associated with worse outcomes in univariate analysis, they were not significant in multivariate analysis. Similarly, lower TBI doses showed a trend toward increased NRM in univariate analysis, but not after multivariate adjustment. These findings suggest that after adjusting for other patient characteristics, these factors may not influence prognosis. Given the significant association between TCI score and outcomes, the prognostic impact of TBI dose in MPAL should be interpreted in the context of its combination with other chemotherapeutic agents.
To translate findings in the multivariate analysis into clinical practice, we developed a novel risk stratification system, MPAL-PRO, which stratified patients into 3 distinct prognostic groups for OS. According to MPAL-PRO, patients in the favorable-risk group demonstrated promising posttransplant OS and PFS, supporting benefits of allo-HSCT. In contrast, the high-risk group, characterized by multiple adverse factors, had frequent relapse, and increased NRM, making long-term survival difficult even with transplant. For this population, allo-HSCT might not be an optimal treatment choice. Future studies should explore novel therapeutic approaches for this high-risk group, including molecularly targeted therapies and cellular therapies. Also, as the current model does not incorporate molecular data, it requires further refinement through the inclusion of MPAL-specific molecular information.
Propensity score–matched comparisons revealed that posttransplant survival in MPAL was not inferior to that in AML or ALL. Before matching, MPAL patients had a higher proportion of poor-risk cytogenetics than AML patients. After matching, more poor-risk patients were included in the AML cohort to match MPAL, which may have contributed to inferior transplant outcomes in AML.22 These findings suggest that prognostic factors other than cytogenetic risk may have a greater impact on posttransplant outcomes in MPAL than in AML. Actually, poor cytogenetic risk was not significantly associated with OS or DFS in MPAL, unlike in AML (data not shown).
Regarding GVHDs, MPAL showed a tendency for higher cumulative incidence of grade 2 to 4 aGVHD and cGVHD compared with AML, particularly in CR1. Compared with ALL, MPAL also had higher cGVHD incidence, when all disease statuses were included. Although differences vary by disease status at transplant and diagnosis, these findings suggest that MPAL carries a higher GVHD risk than AML or ALL, consistent with previous reports.8,9 Given the retrospective nature of this study, we lack data on posttransplant GVHD prophylaxis adjustments. However, as MPAL is considered a high-risk leukemia, adjustments in GVHD prophylaxis to enhance the graft-versus-leukemia effect may have been implemented. Although the increased GVHD incidence in MPAL did not significantly raise NRM, careful GVHD management remains essential for patients with MPAL.
Although the study’s strengths include analysis of real-world data, several limitations must be acknowledged. First, this was a retrospective multicenter, registry study with varying protocols; therefore, patient backgrounds could not be fully adjusted despite multivariate analysis. Second, this real-world registry study has inherent limitations in data availability. Molecular marker data were largely missing, as relevant tests were not covered by the national health insurance. Given the substantial number of patients lacking genetic or immunophenotypic subclassification, further studies are needed to clarify the impact of these biologic subsets on transplant outcomes in MPAL. Third, a large proportion of patients in this MPAL cohort had unevaluable cytogenetics. This may reflect a diagnostic tendency, as leukemias with well-defined cytogenetic abnormalities are excluded from the diagnosis of MPAL, leading to an overrepresentation of patients without definitive cytogenetic findings. Indeed, previous large international studies also reported a high rate of unavailable cytogenetics in MPAL.8 Fourth, although propensity score matching balanced baseline characteristics in AML and ALL comparisons, residual confounding may persist. In our cohort, haploidentical transplants were performed in only 18 of 435 patients (<5%), mostly at a few specialized centers. Due to potential selection bias, these patients were excluded from the present analysis. As the use of post-transplant cyclophosphamide (PTCy)-based transplants increases globally, future studies will be needed to evaluate outcomes in patients with MPAL receiving PTCy-based transplant. In this study, therapy-related and myelodysplasia-related cases, generally associated with poor prognosis, were excluded from both the AML and MPAL cohorts in accordance with the diagnostic criteria for MPAL. Therefore, posttransplant outcomes of therapy-related and myelodysplasia-related leukemias with mixed immunophenotypes were not evaluated in this analysis.
In conclusion, our study suggests that allo-HSCT can offer long-term survival benefits in MPAL, with outcomes similar to those in AML and ALL. We have developed a novel risk stratification system, MPAL-PRO, which may assist treatment decision-making for this patient population. The clinical utility of the MPAL-PRO therefore merits further validation.
Acknowledgments
The authors thank all physicians and data managers at centers who contributed valuable data on transplantation to the Japanese Society Transplantation and Cellular Therapy.
This work was supported, in part, by Japan Society for the Promotion of Science Grants-in-Aid for Scientific Research (JSPS) KAKENHI (grant 24K19198 [T.J.]); the Program for Development of Next-generation Leading Scientists with Global Insight, sponsored by the Ministry of Education, Culture, Sports, Science and Technology, Japan, the Lotte Foundation, and the Japan Health & Research Institute Research Grant (Y. Arai).
Authorship
Contribution: T.J. and Y. Arai designed the study; T.J. and Y. Arai performed the statistical analysis; T.J., T.K., S.M., S.K., M.Y., and Y. Arai interpreted the data; T.J. and Y. Arai wrote the manuscript; N.D., N.U., M.T., T.N., T.F., M.S., Y.Kanda, S.T., Y.H., Y. Katayama, and S.Y. provided the patient data; and K.K., T.F., M.O., and Y. Atsuta collected patient data; and all authors reviewed the manuscript and provided critical feedback.
Conflicts-of-interest disclosure: S.M. has received consultancy fees from Johnson & Johnson; and research funding Hayashikane Sangyo. N.U. has received consultancy fees from AbbVie and Novartis;: and honoraria from Astellas. M.S. has received consultancy fees from Kyowa Kirin, Asahi Kasei, Takeda, AbbVie, and Daiichi Sankyo; and honoraria from Kyowa Kirin, Chugai, Pfizer, Astellas, Nippon Shinyaku, Ono Pharmaceuticals, Merck Sharp & Dohme LLC, Bristol Myers Squibb, Asahi Kasei, Novartis, Eisai, Otsuka, Sumitomo-Dainippon, Sanofi, Takeda, Mundipharma, AbbVie, CSL Behring, SymbBio, Janssen, AstraZeneca, Daiichi Sankyo, Amgen, Novo Nordisk, and Nippon Kayaku. Y.K. has received consultancy fees from Bristol Myers Squibb, Novartis, Chugai,; Pfizer, Sanofi, Janssen, Asahi Kasei, Merck Sharp & Dohme LLC, Kyowa Kirin, Takeda, and Meiji Seika; honoraria from Celgene; and research funding from Chugai, Kyowa Kirin, Sumitomo-Dainippon, and Eisai. The remaining authors declare no competing financial interests.
Correspondence: Tomoyasu Jo, Department of Hematology, Graduate School of Medicine, Kyoto University, 54 Shogoin Kawahara-cho, Sakyo-ku, Kyoto 606-8507, Japan; email: tjoh@kuhp.kyoto-u.ac.jp.
References
Author notes
Data used in this study are not publicly available, because releasing these data would exceed the scope of patient consent for research use in the registry.
The full-text version of this article contains a data supplement.