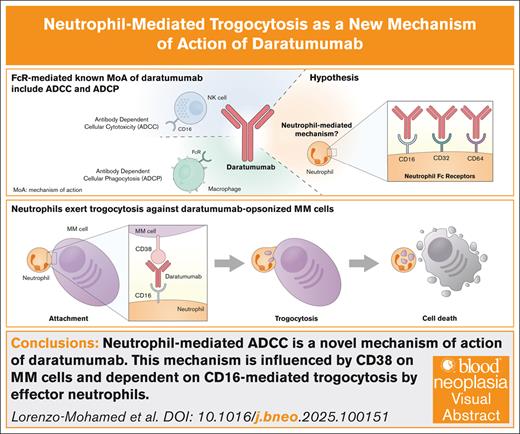
Visual Abstract
TO THE EDITOR:
Treatment of multiple myeloma (MM) has evolved markedly in the last decade, but mortality remains high, emphasizing the need for more effective therapies. Daratumumab is an anti-CD38 monoclonal antibody (mAb) approved for the treatment of patients with relapsed/refractory and newly diagnosed MM.1 Daratumumab acts by a combination of direct and immune-mediated effects, including complement-dependent cytotoxicity, antibody-dependent cellular cytotoxicity (ADCC), and antibody-dependent cellular phagocytosis (ADCP).2 This cellular component of daratumumab’s mechanism of action involves the activation of different surface molecules, namely Fc receptors, on various cell types such as NK cells and macrophages. Neutrophils, the most abundant leukocytes in human blood, also express Fc receptors, mainly FcγRI (CD64), FcγRIIA (CD32a), and FcγRIIIB (CD16b).3 It has been postulated that neutrophils may play a role in the mechanism of action of mAbs.4 In this context, researchers have discovered that neutrophils can engulf fragments of trastuzumab-opsonized breast cancer cell membranes by trogocytosis, resulting in cell death.5 Regarding myeloma and daratumumab treatment, trogocytosis by granulocytes and monocytes has been observed and associated with a reduction of CD38 expression on myeloma cells. Even though, this phenomenon does not appear to be a mechanism of resistance.6 Rather, based on clinical data, it has been suggested that trogocytosis may be a mechanism of action of daratumumab,7 although this remains to be proven. In this scenario, we sought to explore the potential role of neutrophils in daratumumab-mediated ADCC in the context of MM. Herein, we propose neutrophil-mediated trogocytosis as a new daratumumab-dependent cellular cytotoxicity mechanism, mediated by CD16b, and occurring both in neutrophils isolated from healthy donors and patients with MM.
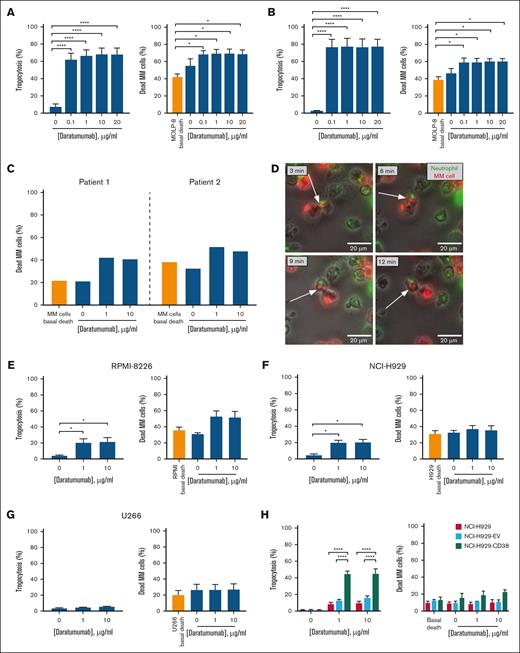
Results from trogocytosis and ADCC experiments show that neutrophils from healthy donors (n = 5) exert significant trogocytosis and cytotoxicity against the MOLP-8 cell line in the presence of daratumumab, with similar effects in the range of 0.1 to 20 μg/mL (Figure 1A). To explore the effects of lower doses, an additional set of similar experiments was performed (dose range: 0.001-10 μg/mL). The lowest dose (0.001 μg/mL) did not induce significant trogocytosis and the percentage of myeloma death was similar to that in the drug-free condition. Conversely, daratumumab (≥0.01 μg/mL) significantly increased trogocytosis and myeloma death (supplemental Figure 1). Therefore, daratumumab-induced trogocytosis and myeloma death are dose-dependent, although these effects are evident from relatively low doses (≥0.01 μg/mL). In addition, neutrophils derived from patients with MM also exerted significant trogocytosis and cytotoxicity on MOLP-8 in the presence of daratumumab (n = 5; Figure 1B). Moreover, these effects were similar to those found for healthy donors’ neutrophils when compared after data normalization (supplemental Figure 2). Additionally, daratumumab-mediated cytotoxic effect of neutrophils isolated from 2 other patients on autologous myeloma cells was observed (Figure 1C; supplemental Figure 3). Considering the global immune suppression observed in patients with MM,8 the fact that this neutrophil-driven cytotoxic mechanism against myeloma cells functions similarly in both healthy individuals and myeloma patients could represent a new opportunity for treatment strategies in managing this disease. The trogocytosis mechanism was confirmed by live-cell microscopy in a coculture of DiO-labeled neutrophils from a donor with daratumumab-opsonized, DiI-labeled MOLP-8 cells (Figure 1D, supplemental Figure 4 and supplemental Videos 1 and 2 show 2 representative fields).
Analysis of trogocytosis and ADCC in cocultures of neutrophils and myeloma cells with different CD38 expression in presence of daratumumab. Neutrophils isolated from healthy donors or patients with MM were cocultured with the indicated MM cell lines or autologous MM cells in presence of daratumumab for the evaluation of trogocytosis and ADCC. For the analysis of trogocytosis by flow cytometry, MM cells were labeled with either PKH67 or PKH26 prior to coculture. (A) Cocultures of neutrophils derived from healthy donors (n = 5) and the MOLP-8 cell line for trogocytosis and ADCC evaluation. Left: after 4 hours of coculture, PKH67+CD138– events were assessed within the CD66b+ compartment by flow cytometry for trogocytosis analysis. Right: the percentage of dead MM cells (annexin V+, 7AAD+) within the CD38me+CD66b– compartment (CD38me: anti-CD38 multi-epitope) was assessed by flow cytometry for ADCC analysis. (B) Analysis of trogocytosis (left) and ADCC (right) in cocultures of neutrophils derived from patients with MM (n = 5) and the MOLP-8 cell line following the same methodology as in panel A. (C) Evaluation of daratumumab-mediated patient neutrophil cytotoxicity on autologous myeloma cells. Neutrophils and myeloma cells from 2 patients were isolated and subsequently cocultured in absence or presence of daratumumab for 4 hours. Cytotoxicity on myeloma cells was analyzed by flow cytometry as in panel A. (D) Representative images for trogocytosis by live-cell microscopy. DiO-labeled neutrophils (green) and DiI-labeled MOLP-8 cells (red) were cocultured (Effector:Target ratio of 3:1) in the presence of 1 μg/mL daratumumab. Time-lapse acquisition during the 4-hour coculture period, with images being captured every 3 minutes, was performed on a Nikon Eclipse TE-2000 microscope (Nikon, Tokyo, Japan). (E-G) Analysis of daratumumab-induced trogocytosis (left) and ADCC (right) in cocultures of neutrophils derived from healthy donors (n = 3) and RPMI-8226, NCI-H929 or U266 cell lines, respectively, following the same methodology as in panel A. (H) Analysis of trogocytosis (left) and ADCC (right) in cocultures of neutrophils derived from healthy donors (n = 3) and the indicated cell lines following the same methodology as in panel A but using PKH26 for cell labeling. In panels A,B,E-H, data represent the mean ± standard error of the mean (SEM). Means were compared using one-way analysis of variance and Tukey post hoc tests. ∗P < .05 and ∗∗∗∗P < .0001. Whenever “basal death” is indicated, it means the death of myeloma cells in basal culture conditions (without neutrophils and drug). The remaining conditions correspond to coculture of neutrophils and myeloma cells.
Analysis of trogocytosis and ADCC in cocultures of neutrophils and myeloma cells with different CD38 expression in presence of daratumumab. Neutrophils isolated from healthy donors or patients with MM were cocultured with the indicated MM cell lines or autologous MM cells in presence of daratumumab for the evaluation of trogocytosis and ADCC. For the analysis of trogocytosis by flow cytometry, MM cells were labeled with either PKH67 or PKH26 prior to coculture. (A) Cocultures of neutrophils derived from healthy donors (n = 5) and the MOLP-8 cell line for trogocytosis and ADCC evaluation. Left: after 4 hours of coculture, PKH67+CD138– events were assessed within the CD66b+ compartment by flow cytometry for trogocytosis analysis. Right: the percentage of dead MM cells (annexin V+, 7AAD+) within the CD38me+CD66b– compartment (CD38me: anti-CD38 multi-epitope) was assessed by flow cytometry for ADCC analysis. (B) Analysis of trogocytosis (left) and ADCC (right) in cocultures of neutrophils derived from patients with MM (n = 5) and the MOLP-8 cell line following the same methodology as in panel A. (C) Evaluation of daratumumab-mediated patient neutrophil cytotoxicity on autologous myeloma cells. Neutrophils and myeloma cells from 2 patients were isolated and subsequently cocultured in absence or presence of daratumumab for 4 hours. Cytotoxicity on myeloma cells was analyzed by flow cytometry as in panel A. (D) Representative images for trogocytosis by live-cell microscopy. DiO-labeled neutrophils (green) and DiI-labeled MOLP-8 cells (red) were cocultured (Effector:Target ratio of 3:1) in the presence of 1 μg/mL daratumumab. Time-lapse acquisition during the 4-hour coculture period, with images being captured every 3 minutes, was performed on a Nikon Eclipse TE-2000 microscope (Nikon, Tokyo, Japan). (E-G) Analysis of daratumumab-induced trogocytosis (left) and ADCC (right) in cocultures of neutrophils derived from healthy donors (n = 3) and RPMI-8226, NCI-H929 or U266 cell lines, respectively, following the same methodology as in panel A. (H) Analysis of trogocytosis (left) and ADCC (right) in cocultures of neutrophils derived from healthy donors (n = 3) and the indicated cell lines following the same methodology as in panel A but using PKH26 for cell labeling. In panels A,B,E-H, data represent the mean ± standard error of the mean (SEM). Means were compared using one-way analysis of variance and Tukey post hoc tests. ∗P < .05 and ∗∗∗∗P < .0001. Whenever “basal death” is indicated, it means the death of myeloma cells in basal culture conditions (without neutrophils and drug). The remaining conditions correspond to coculture of neutrophils and myeloma cells.
Following these results, we carried out similar experiments with healthy donors’ neutrophils using other MM cell lines with lower CD38 surface expression, as compared to MOLP-8 (supplemental Figure 5). In both RPMI-8226 and NCI-H929 cell lines, the percentage of trogocytosis was significantly higher in the presence of daratumumab (either at 1 or 10 μg/mL) than in the untreated control, with no differences between doses; however, these results were not translated into significantly higher cytotoxic effects in treated cocultures, although higher mean values were obtained for RPMI-8226 (Figure 1E,F). In the case of the U266 cell line, with almost negligible CD38 levels, no significant values were obtained in the presence of daratumumab for neither trogocytosis nor cytotoxic effects (Figure 1G). These results suggest a possible relationship between the level of CD38 expression and neutrophil-mediated daratumumab effects. To further explore the influence of CD38 levels on neutrophil-mediated trogocytosis and ADCC, we performed the same type of experiments in the NCI-H929 cell line with stable overexpression of CD38 (NCI-H929-CD38) previously generated in our laboratory by lentiviral transduction (supplemental Figure 6). NCI-H929-CD38 cells achieved significantly higher levels of trogocytosis in the presence of daratumumab as compared to the parental NCI-H929 cell line and NCI-H929 cells transduced with the empty vector (NCI-H929-EV), however, cell death percentage did not reach statistical significance (Figure 1H). Thus, we conclude that the level of expression of CD38 seems to play an important role in the mechanism of neutrophil-mediated trogocytosis exerted by daratumumab; nevertheless, it appears that this mechanism alone may not be the exclusive factor contributing to cell death, or that a threshold level of trogocytosis needs to be reached to trigger cell death.
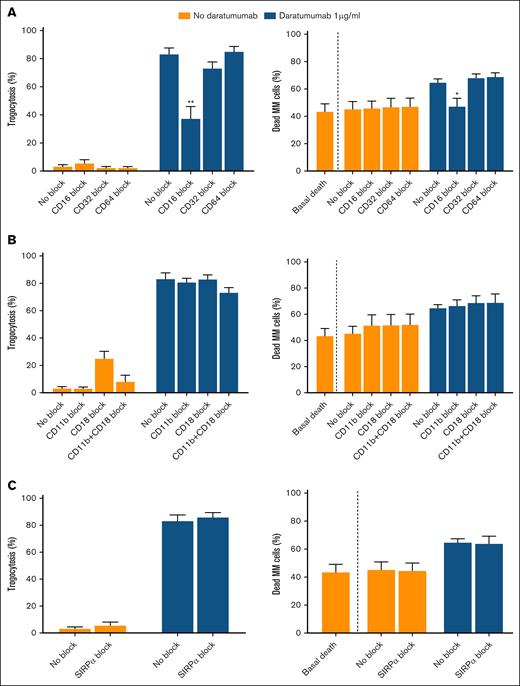
To further investigate the factors implicated in these processes, we performed the same type of studies in cocultures of healthy donors’ neutrophils and MOLP-8, while blocking surface molecules involved in neutrophil functionality. In this way, based on their function, 3 functionally different groups of molecules were blocked: Fc receptors CD16, CD32, and CD64; integrin subunits CD18 and CD11b, separately and in combination; and Signal Regulatory Protein Alpha (SIRPα), involved in the don’t-eat-me signal. Results show that CD16 blockade reduces daratumumab-mediated trogocytosis and completely abolishes neutrophil-mediated cell death; however, no effect was observed when blocking CD32 or CD64 (Figure 2A). The individual role of these membrane receptors in neutrophil-mediated trogocytosis and cytotoxicity is under discussion. Regarding CD16, some authors propose that CD16b, the main isoform expressed by neutrophils, is the primary driver of neutrophil-mediated trogocytosis and ADCC. For example, Nakagawa et al9 showed that CD16b is the main effector in neutrophil-mediated ADCC against rituximab-opsonized lymphoma cells. Golay et al10 confirmed that CD16b predominantly mediates trogocytosis in antibody-opsonized CLL B cells, with CD32a playing a role only in CD16b-null neutrophils. However, Treffers et al11 suggested that CD16b may inhibit these mechanisms and identified CD32a as the primary driver of neutrophil-mediated ADCC against breast cancer cells in the presence of trastuzumab, whereas van Rees et al12 proposed CD64 as the main receptor responsible for ADCC in B-cell lymphoma cells treated with rituximab. Here, we have shown that CD16 blockade reduced both trogocytosis and ADCC, whereas CD32 or CD64 blockade did not reduce these effects. Because we confirmed that neutrophils predominantly express CD16b (supplemental Figure 7), our results collectively suggest that CD16b may be involved in neutrophil-mediated trogocytosis and subsequent myeloma cell death in the presence of daratumumab. These discrepancies among studies may be attributed to both the target cell and the type of antibody, depending on the characteristics of each antibody Fc region, which determines its binding to Fc receptors. In fact, it has been shown that the participation of individual Fc receptors in ADCC is influenced by the density of the target antigen and the antibody isotype.13 In line with the latter, IgG1 isotype, which is characteristic of most therapeutic antibodies including daratumumab, is able to bind to all FcγRs, although each FcγR is capable of initiating a particular response in neutrophils.14
Effect of blocking neutrophil surface molecules on the mechanisms of trogocytosis and ADCC. Trogocytosis (left) and ADCC (right) of neutrophils derived from healthy donors (n = 6) on the MOLP-8 myeloma cell line. (A) Effect of Fc receptor (CD16, CD32, and CD64) blockade on trogocytosis (left) and ADCC (right). (B) Effect of CD11b and/or CD18 integrin blockade on trogocytosis (left) and ADCC (right). (C) Effect of the don’t-eat-me signal molecule Signal Regulatory Protein Alpha (SIRPα) blockade on trogocytosis (left) and ADCC (right). Data represent mean ± SEM from 6 individual experiments. Each blocking condition was compared to the unblocked control by unpaired t test. ∗P < .05 and ∗∗P < .01. Whenever “basal death” is indicated, it means the death of MOLP-8 cells in basal culture conditions (without neutrophils and drug). The remaining conditions correspond to coculture of neutrophils and myeloma cells.
Effect of blocking neutrophil surface molecules on the mechanisms of trogocytosis and ADCC. Trogocytosis (left) and ADCC (right) of neutrophils derived from healthy donors (n = 6) on the MOLP-8 myeloma cell line. (A) Effect of Fc receptor (CD16, CD32, and CD64) blockade on trogocytosis (left) and ADCC (right). (B) Effect of CD11b and/or CD18 integrin blockade on trogocytosis (left) and ADCC (right). (C) Effect of the don’t-eat-me signal molecule Signal Regulatory Protein Alpha (SIRPα) blockade on trogocytosis (left) and ADCC (right). Data represent mean ± SEM from 6 individual experiments. Each blocking condition was compared to the unblocked control by unpaired t test. ∗P < .05 and ∗∗P < .01. Whenever “basal death” is indicated, it means the death of MOLP-8 cells in basal culture conditions (without neutrophils and drug). The remaining conditions correspond to coculture of neutrophils and myeloma cells.
Results on blocking CD11b/CD18 subunits indicate that this integrin does not seem to play a role in neutrophil-mediated daratumumab myeloma cell killing (Figure 2B), unlike previous studies with other mAbs in solid cancer models such as breast,5 or melanoma,15 among others. In addition, other investigators have determined that the neutrophil-mediated and trogocytosis-related process of tumor cell death against trastuzumab-opsonized breast cancer cells is enhanced by blocking the CD47-SIRPα checkpoint.5 Although MOLP-8 cells express CD47 (supplemental Figure 8), in our case SIRPα blockade did not augment either trogocytosis or myeloma cell death (Figure 2C). We speculate that trogocytosis is already very potent without any blockade, so that the maximum level of cell death achievable through this mechanism has already been reached in the MOLP-8 cell line.
In brief, we propose neutrophil-mediated ADCC as a novel mechanism of action of daratumumab, partially influenced by CD38 expression levels on myeloma cells, and dependent on CD16b mediated trogocytosis by effector neutrophils. Our results provide rationale for further studying MM cell–neutrophil dynamics and open the door to potentially enhancing this interaction through drug combinations.
Acknowledgments: The authors thank the Pharmacy Department of the Hospital Universitario de Salamanca (Salamanca, Spain) for providing daratumumab; the Centro de Hemoterapia y Hemodonación de Castilla y León (Valladolid, Spain) for providing blood samples; the Hospital Universitario de Salamanca, especially Verónica González and Beatriz Rey, for their help in providing blood and bone marrow samples; and all patients and donors.
This work was funded by Instituto de Salud Carlos III, cofunded by the European Union (ERDF, “A way to make Europe”) through the project PI18/01600; and Instituto de Salud Carlos III, cofunded by the European Union (PI21/01508, PI22/00877). M.L.-M. and M.G.-R. are supported by fellowships cofinanced by the Consejería de Educación (Junta de Castilla y León) and the European Social Fund+. B.C. is supported by a fellowship from the Ministerio de Ciencia, Innovación y Universidades (FPU22/04397).
Contribution: M.L.-M., C.V.-B., and T.P. designed the experiments; M.L.-M., C.V.-B., A.D.-T., L.G.-M., M.M.-S., and L.S.-S. carried out the experiments; M.L.-M., C.V.-B., A.D.-T., M.G.-R., B.C., J.S.-R., M.G., and T.P. analyzed and interpreted the data; M.L.-M., C.V.-B., M.G., and T.P. wrote and reviewed the manuscript; and all authors have approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Teresa Paíno, Centro de Investigación del Cáncer, Laboratorio 12, Campus Miguel de Unamuno s/n, 37007 Salamanca, Spain; email: tpaino@usal.es.
References
Author notes
M.L.-M. and C.V.-B. contributed equally to this study.
Original data are available upon reasonable request from the author, Mauro Lorenzo-Mohamed (lorenzomohamed.mauro@usal.es).
The full-text version of this article contains a data supplement.