TO THE EDITOR:
With the dramatic improvements in chronic myeloid leukemia (CML) survival and the transformation to blast phase becoming a rare occurrence following the introduction of tyrosine kinase inhibitors (TKIs),1,2 the clinical value of a diagnostic bone marrow aspirate/trephine (BMAT) has been questioned. It is an invasive test that is associated with rare but serious complications with potential logistic constraints that can delay prompt TKI commencement in resource-limited settings.3 This study reviewed the role of a diagnostic BMAT and calculated the discordance in classification and outcomes if a BMAT was excluded from the diagnostic workflow.
A retrospective review was conducted of patients who were treated at 2 tertiary hospitals in South Australia between 2008 and 2023. The BMAT workflow consisted of evaluating the aspirate morphology, conducting cytogenetics and flow cytometry, and obtaining a core trephine4 for histology and immunohistochemistry. CML staging, using an alternative workflow that incorporated complete blood examination, cytogenetics, and flow cytometry, was assessed for concordance with the BMAT. Blast and basophil percentages were determined as part of the complete blood examination for risk stratification. Cytogenetics were used to confirm the diagnosis and also to identified high-risk disease based on the detection of additional cytogenetic abnormalities (ACAs). Flow cytometry was used to quantify blasts and assign a lineage, relevant for identifying blast phase CML.
Key parameters that were evaluated included CML staging in bone marrow (BM) and peripheral blood (PB), marrow fibrosis on trephine, dysplasia, spleen size, European Treatment and Outcome Study (EUTOS) long-term survival (ELTS) score,5 cytogenetic analysis, BCR::ABL1 transcript type and value, molecular response, and clinical outcomes. The association between baseline variables and survival parameters was assessed using Kaplan-Meier estimates and log-rank tests. Disease staging was performed as per the World Health Organization (WHO) 2016, WHO 2022,6 and International Consensus Classification (ICC) 20227 diagnostic criteria. This study was conducted with local institutional review board ethical approval as per the Declaration of Helsinki.
Of the 476 cases identified for review, 322 had complete data available from the diagnostic BMAT. A number of cases were diagnosed at external institutions where the diagnostic BMAT was not conducted, whereas the remainder did not have a BMAT at diagnosis because of frailty, logistics, or the COVID-19 pandemic. Males accounted for 55% of cases (Figure 1A), and the median age at diagnosis was 54.6 years (range, 44.1-66.5 years).
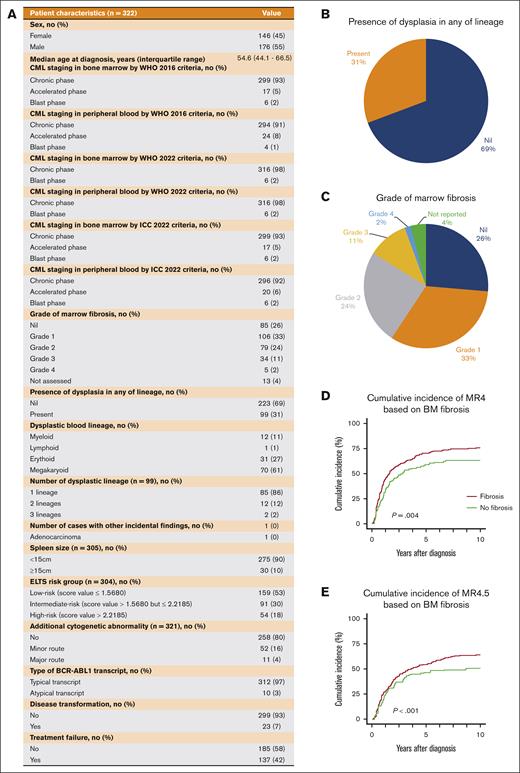
Patient characteristics and the distributions of marrow fibrosis and dysplasia. (A) Patient characteristics of the study cohort. (B) Pie chart showing the presence of dysplasia in assessed BM samples in any hematologic lineage. (C) Pie chart of the presence of BM fibrosis. (D) Cumulative incidence of MR4 based on BM fibrosis. (E) Cumulative incidence of MR4.5 based on BM fibrosis. ICC, International Consensus Classification.
Patient characteristics and the distributions of marrow fibrosis and dysplasia. (A) Patient characteristics of the study cohort. (B) Pie chart showing the presence of dysplasia in assessed BM samples in any hematologic lineage. (C) Pie chart of the presence of BM fibrosis. (D) Cumulative incidence of MR4 based on BM fibrosis. (E) Cumulative incidence of MR4.5 based on BM fibrosis. ICC, International Consensus Classification.
Based on the WHO 2016 criteria, 93% of patients were in chronic phase and 5% and 2% were in the accelerated and blast phases, respectively. We observed 2 cases with discordant CML staging between PB and BM in which PB blasts were 8% and 15% for the 2 respective cases and BM blasts were 21% and 45%, respectively. However, PB immunophenotyping confirmed the presence of lymphoblasts. Therefore, even if a BMAT had not been performed at diagnosis, the PB lymphoblasts would have prompted concern for lymphoid blast phase, necessitating further investigation with a BMAT. Consequently, cases of lymphoid blast phase would not have been missed because, according to the WHO6 and ICC 20227 classification, the presence of lymphoblasts would be indicative of lymphoid blast phase, implying no discordance in staging between PB and BM studies. This study demonstrated that blast phase CML would rarely be missed if the BMAT was omitted, irrespective of the staging classification.
BM dysplasia was observed in 31% of patients (Figure 1B). However, if present, a mild-to-moderate degree of dysplasia was typically reported. The severity of marrow reticulin fibrosis observed in the trephine samples was reported according to the WHO grading system (Figure 1C).8,9 Most patients had grade ≤1 reticulin fibrosis (59%), whereas only 2% had grade 4 reticulin. Unexpectedly, a single BM trephine sample demonstrated incidental BM infiltration by prostate adenocarcinoma, in addition to chronic phase CML.
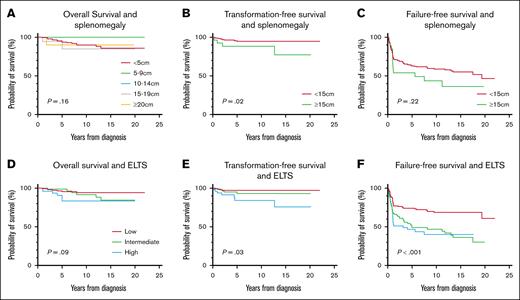
We analyzed the correlation between numerous diagnostic variables and survival outcomes in non–blast phase CML. This analysis was conducted to assess if any of these specific variables, particularly the quantification of marrow blasts, specifically influenced the outcomes. Only the ELTS score and splenomegaly emerged as significant predictors of transformation-free survival, and no significant difference was observed in the overall or failure-free survival (Figure 2). The presence of grade 3 to 4 BM fibrosis did not influence survival, but it did negatively impact the possibility of achieving MR4 and MR4.5 (Figure 1D-E). ACAs and the BCR::ABL1 transcript type did not significantly impact survival outcomes. However, we acknowledge that our ACA results were inconsistent with the literature.10-13 There are 3 possible explanations. First, our small sample size may be inadequate to confirm the negative impact of specific ACAs. Second, our population was not representative of high-risk ACAs because only 4% of our investigated population had a major route ACA at diagnosis. Furthermore, the 3q26.2 and 11q23 chromosomal rearrangements, well-documented rare adverse-risk ACAs,14,15 were not present in our cohort.11 Despite these results, we still recognize the prognostic adversity of major route ACAs at diagnosis based on much larger studies.
Survival outcomes vs splenomegaly and ELTS. (A) Overall survival and splenomegaly. (B) Transformation-free survival and splenomegaly. (C) Failure-free survival and splenomegaly. (D) Overall survival and ELTS score. (E) Transformation-free survival and ELTS score. (F) Failure-free survival and ELTS score.
Survival outcomes vs splenomegaly and ELTS. (A) Overall survival and splenomegaly. (B) Transformation-free survival and splenomegaly. (C) Failure-free survival and splenomegaly. (D) Overall survival and ELTS score. (E) Transformation-free survival and ELTS score. (F) Failure-free survival and ELTS score.
There are specific high-risk ACAs that are associated with high transformative potential and TKI resistance. One such lesion is 3q26.2 (identified in <1% of cases), and it is almost ubiquitously associated with eventual disease transformation and should be considered as an indication for early allogeneic transplantation.14 If a diagnostic BMAT is not performed, PB cytogenetics will be required and the success of obtaining sufficient metaphases for cytogenetic analysis from PB is dependent on the amount of circulating myeloid progenitors.16 However, PB may only yield limited metaphases with suboptimal quality. PB karyotyping can detect structural abnormalities,17,18 whereas the reported success of PB karyotyping in CML was 53%.19 However, to truly determine the relative sensitivity of PB and BM karyotyping, paired PB and BM samples are required, which were not available in this study. Despite this, the diagnostic karyotype and the presence of ACAs do not tend to alter the frontline management for newly diagnosed chronic phase CML. However, if ACA identification will alter management, aid TKI selection, or influence future therapy, then a diagnostic BMAT should be performed. In the future, improvements in genomic techniques may replace conventional karyotyping and improve the yield and accuracy of the PB karyotype.16
In conclusion, this study demonstrated that the diagnostic BMAT rarely contributes additional information when the PB assessment is complete. A complete PB assessment includes flow cytometry (for blast quantification and lineage identification), cytogenetics, and baseline BCR::ABL1 quantification and transcript determination. Fewer than 1% of the cases studied here would have had therapy altered based on the diagnostic BMAT, and in this study, it was only because of an unexpected finding of a second malignancy in the study sample. Limiting the diagnostic BMAT to specific circumstances would reduce the need for an invasive procedure, which is associated with rare but significant complications, and also conserve resources in resource-limited regions. However, a limitation of a conservative BMAT approach is missing the rare case of blast phase CML (not present in our cohort) that is only evident on the BMAT. We have focused on the risk of missing cases of de novo blast phase CML because this represents a missed opportunity to proceed along an allograft pathway at the most favorable time. We have not focused on the risk of missing a case of accelerated-phase disease as this does not have the same consequences nor does the treatment differ from high-risk ELTS CML, essentially TKI monotherapy with an overall, favorable outlook.
If a BMAT is not performed at diagnosis, we would recommend that all other key investigations are conducted on the PB. Although we have shown that there is limited added benefit to a BMAT in most chronic-phase CML diagnoses, we have outlined specific criteria for when a BMAT is recommended to minimize the risk of missing high-risk CML based on experience, observation, and some limited data. Our suggested recommended criteria in chronic-phase CML are as follows:
≥10% PB blasts
Presence of PB lymphoblasts detected by morphology or flow cytometry
Presence of an extramedullary proliferation of blasts (ie, chloroma)
Presence of high-risk ACAs in Philadelphia chromosome-positive cells (such as 3q26.2)
High ELTS score
Splenomegaly (≥15 cm below the left costal margin)
Inability to perform PB cytogenetic analysis
However, in patients for whom ensuring a complete karyotype could alter the management, a diagnostic BMAT may still have sufficient value to outweigh any logistic or cost concerns. Furthermore, in patients with treatment failure, a BMAT remains important to assess progression because transformation to blast phase has poorer outcomes than de novo blast phase. In this study, we have not addressed the future introduction of genomic analysis and more novel cytogenetic techniques, which is an important area of ongoing investigation. This will further customize the diagnostic investigative process. It is also important to emphasize the value of collecting and biobanking diagnostic BM samples in the academic setting, which is a key driver of ongoing research and development. These findings contribute to ongoing discussions within the clinical community regarding the benefits, costs, and risks of a diagnostic BMAT in CML.
Contribution: N.F.T.F., T.P.H., and N.S. designed the study; N.F.T.F. and N.S. collected and analyzed the data and wrote the manuscript; N.S. revised the manuscript; and all authors reviewed the manuscript.
Conflict-of-interest disclosure: N.S. reports receiving honoraria from Novartis and Takeda and travel support from Janssen. D.M.R. reports receiving research funding and honoraria from Novartis and Bristol Myers Squibb and honoraria from Takeda. D.T.Y. reports receiving research funding from Novartis and Bristol Myers Squibb; and receiving honoraria from Pfizer, Amgen, Novartis, and Bristol Myers Squibb. T.P.H. reports serving as a member of advisory boards and receiving honoraria from Novartis, Takeda, Terns, Enliven, and Ascentage Pharma. N.F.T.F. declares no competing financial interests.
Correspondence: Naranie Shanmuganathan, Department of Haematology, Royal Adelaide Hospital and SA Pathology, Port Rd, Adelaide, SA 5000, Australia; email: naranie.shanmuganathan@sa.gov.au.
References
Author notes
Deidentified participant data that were collected for the study can be made available to researchers once appropriate ethical approval and a signed data access agreement are obtained. Contact the corresponding author for discussion, Naranie Shanmuganathan (naranie.shanmuganathan@sa.gov.au)