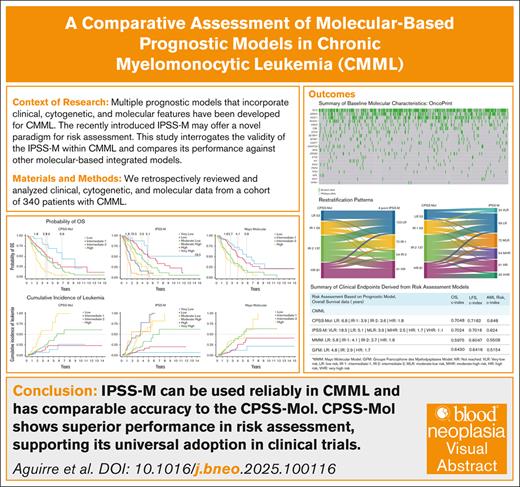
Key Points
IPSS-M can be used reliably in CMML to inform treatment decisions and has comparable accuracy to the CPSS-Mol in predicting OS and LFS.
CPSS-Mol shows superior performance in risk assessment for CMML, supporting its universal adoption in clinical trial design and enrollment.
Visual Abstract
Several prognostic systems integrating clinical, cytogenetic, and molecular parameters have been developed to estimate risk and inform treatment in chronic myelomonocytic leukemia (CMML). Recently, the molecular International Prognostic Scoring System (IPSS-M) was introduced for risk stratification in myelodysplastic syndromes (MDS), demonstrating improved prognostic accuracy over the mutation-agnostic Revised International Prognostic Scoring System (IPSS-R) and potentially offering a novel tool for risk assessment in this population. We aimed to assess whether the applicability of the IPSS-M extends to CMML while providing a comprehensive comparison of all major molecular-based integrated models. Baseline clinical and molecular data were collected from 340 patients with CMML. The most frequent mutations were TET2, SRSF2, ASXL1, RUNX1, and NRAS. The IPSS-M stratified patients into 6 risk categories, with median overall survival (OS) of 18.5, 5.1, 3.9, 2.65, 1.7, and 1.1 years, corresponding to very low to very high risk disease (P < .001). Additionally, the 4-year cumulative incidence of acute myeloid leukemia evolution was 4.2%, 12.1%, 19.4%, 25.9%, 32.8%, and 26.7%, respectively (P = .008). Both CMML-specific prognostic scoring system (CPSS)-Mol and IPSS-M improved OS discrimination compared to the Mayo molecular and Groupe Francophone des Myélodysplasies models. CPSS-Mol outperformed CPSS, and IPSS-M was superior to IPSS-R. CPSS-Mol demonstrated the highest prognostic accuracy for predicting leukemic evolution, establishing it as the superior overall model. Importantly, IPSS-M was reliably applicable in CMML and displayed prognostic accuracy comparable to CPSS-Mol. Furthermore, all models retained predictive validity in patients receiving frontline hypomethylating agent therapy, suggesting that using the IPSS-M is unlikely to adversely affect outcomes when guiding treatment decisions, particularly in community settings in which CMML is often grouped with MDS.
Introduction
Chronic myelomonocytic leukemia (CMML) is a dysfunctional marrow disorder of clonal hematopoiesis characterized by a distinctly heterogeneous natural history, resulting in fundamentally disparate clinical phenotypes (proliferative vs cytopenic) and outcomes largely dictated by differences in disease biology.1,2 Prognosis is generally poor, with median overall survival (mOS) estimates ranging from 20 to 40 months and high likelihood of leukemic transformation, but outcomes are largely influenced by the presence of high-risk traits.3-8 Treatment decisions in CMML are informed by risk, which is estimated based on clinical, laboratory, and cytogenetic features at presentation and further refined by genetic data.6-8
Multiple risk models integrating clinical, cytogenetic, and molecular data have been designed to better guide prognostic discrimination and predictive accuracy to best guide individualized treatment paradigms.6-8 More recently, the molecular IPSS (IPSS-M) model was introduced for risk stratification of patients with myelodysplastic syndromes (MDS), having demonstrated improved prognostic accuracy over its predecessor, the IPSS-R, and potentially representing a novel instrument for risk stratification in this patient population.9 This study sought to determine whether the applicability of the IPSS-M extends to patients with CMML and to provide a comprehensive and practical comparison between all major molecular-based integrated models.
Methods
Patient selection
We retrospectively reviewed and analyzed clinical, cytogenetic, and molecular data from 340 patients with a diagnosis of CMML who presented to the H. Lee Moffitt Cancer Center (Tampa, Florida) and had annotated data cataloged in the Moffitt Cancer Center CMML database. Laboratory values and prognostic scores were determined at the time of diagnosis or referral before treatment. Patients sequenced on targeted panels that did not include a minimum set of specific genes of interest critical for risk stratification using the models referenced in our study were excluded from all analyses. This study was approved by the H. Lee Moffitt Cancer Center Institutional Review Board.
Ethical statement
The authors are accountable for all aspects of the work, ensuring that any questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional review board, and individual consent for this retrospective analysis was waived.
Characterization of mutational profile
Patients underwent molecular profiling using a next-generation sequencing (NGS)-based platform performed on DNA extracted from peripheral blood or bone marrow mononuclear cells as part of a comprehensive evaluation conducted at the time of presentation. The NGS panels targeted genes across the following platforms: FoundationOne Heme, Genoptix Myeloid Molecular Profile, Genoptix NexCourse Complete, Illumina TruSight Myeloid-54, and Moffitt 98-gene Myeloid Action Panel. A minimum variant allele frequency of 5% was used to define single-nucleotide variants, and a cutoff of 10% was used for insertions or deletions. The reported mutations were evaluated by the investigators and referenced in the Catalogue Of Somatic Mutations In Cancer (COSMIC) database to ensure pathogenicity. TP53 multihit status/biallelic equivalence was determined by a variant allele frequency ≥50%, presence of ≥2 coexistent TP53 mutations, and/or presence of a TP53 mutation with concurrent chromosome 17/17p abnormalities.
Definition of CMML, clinical phenotypes, and disease stages
CMML was defined per World Health Organization (WHO) 2017 criteria as persistent absolute monocyte count (AMC) ≥1 × 109/L, with monocytes accounting for ≥10% of the white blood cell (WBC) differential, along with <20% blasts, presence of dysplasia in ≥1 myeloid lineage, and an acquired clonal abnormality. Two primary CMML phenotypes are recognized: dysplastic CMML (CMML-MDS) and myeloproliferative CMML (CMML-MPN). CMML-MDS is defined as WBC count <13 × 109/L and CMML-MPN as WBC count ≥13 × 109/L. Two CMML stages are recognized: СMΜԼ-1, defined as <4% blasts/promonocytes in peripheral blood and <9% blasts in bone marrow; and СΜML-2, defined as 5% to 19 % blasts/promonocytes in peripheral blood and 10% to 19% blasts in marrow (or Auer rods).
Definitions of survival
OS was measured from the time of diagnosis until death or censored at the time of last patient contact. Leukemia-free survival (LFS) was estimated from the time of diagnosis to transformation to acute myeloid leukemia (AML) or death or censored at the time of last patient contact, whichever occurred first.
Definitions of treatment response
Responses to frontline hypomethylating agents (HMAs) and ruxolitinib therapy were defined following the International Consortium 2015 MDS/MPN criteria by Savona et al.10 According to the best achieved response with frontline treatment, patients were categorized into responders (complete remission [CR], marrow response, partial remission, and hematologic improvement/clinical benefit) and nonresponders (stable disease and progressive disease).
Statistical methods
Categorical variables were compared using Fisher exact and χ2 tests. Quantitative data were summarized as median and range or interquartile range. We conducted correlative analyses between absolute CPSS-Mol, Mayo molecular model (MMM), and Groupe Francophone des Myélodysplasies (GFM) scores and the mean IPSS-M score, as well as outcome predictions on LFS, OS, and leukemic transformation. Time-to-event analyses were estimated using the Kaplan-Meier method, and groups were compared using the log-rank test. Model discrimination was evaluated using Harrell C concordance index (c-index). Statistical analyses were performed using IBM SPSS statistics version 27 and Stata 18.
Results
Patient demographics and baseline clinical characteristics
Table 1 illustrates the baseline patient demographics as well as clinical and molecular characteristics of the CMML cohort. Most patients were male (n = 235 [69%]), white (n = 303 [90%]), and presented with CMML-1 (n = 298 [88%]). The median age at diagnosis was 71 years. The cohort was almost equally distributed between primarily myelodysplastic (n = 173 [51%]) and myeloproliferative clinical phenotypes (n = 167 [49%]). Only a small proportion of patients (n = 55 [16%]) had an indolent disease course (ie, patients not requiring treatment within 3 years of presentation). Clinically, only 64 (19%) and 12 patients (4%) were transfusion dependent (TD) of red blood cells (RBCs; RBC-TD) and platelets (PLTs), respectively, with only a small proportion (n = 61 [18%]) presenting with splenomegaly. Similarly, most patients presented with good-risk cytogenetics based on CPSS (n = 261 [77%]) and IPSS classifications (n = 264 [78%]).
Demographic and clinical characteristics of patients at baseline
| Characteristic . | All patients, N = 340 (100%) . | MDS-CMML, N = 173 (51%) . | MPN-CMML, N = 167 (49%) . | P value∗ . |
|---|---|---|---|---|
| Age at diagnosis, median (range) | 71 (17-88) | 71 (17-88) | 71 (38-86) | .478 |
| Sex, n (%) | .712 | |||
| Male | 235 (69.1) | 118 (68.2) | 117 (70.1) | |
| Female | 105 (30.9) | 55 (31.8) | 50 (29.9) | |
| Race, n (%) | .735 | |||
| White | 303 (89.9) | 152 (88.9) | 151 (91.0) | |
| Black | 14 (4.2) | 9 (5.3) | 5 (3.0) | |
| Hispanic | 11 (3.3) | 6 (3.5) | 5 (3.0) | |
| Other | 9 (2.7) | 4 (2.3) | 5 (3.0) | |
| WHO category, n (%) | .036 | |||
| CMML-1 | 298 (87.7) | 158 (91.3) | 140 (83.8) | |
| CMML-2 | 42 (12.3) | 15 (8.7) | 27 (16.2) | |
| FAB category, n (%) | <.001 | |||
| MDS-CMML | 173 (51) | 173 (100) | 0 (0.0) | |
| MPN-CMML | 167 (49) | 0 (0.0) | 167 (100) | |
| Indolent CMML, n (%) | .140 | |||
| Indolent CMML | 55 (16.2) | 33 (9.1) | 22 (13.2) | |
| Nonindolent CMML | 285 (83.8) | 140 (80.9) | 145 (86.8) | |
| Laboratory values, median (range) | ||||
| WBC, ×109/L | 12.6 (2.4-288.6) | 7.0 (2.4-12.8) | 25.1 (13-288.6) | <.001 |
| ANC, ×109/L | 6.1 (0.1-155.6) | 3.2 (0.1-9.8) | 13.5 (1.6-155.6) | <.001 |
| ALC, ×109/L | 2.2 (0.4-39.9) | 1.7 (0.4-4.7) | 3.3 (0.5-22.0) | <.001 |
| AMC, ×109/L | 2.4 (0.3-39.9) | 1.4 (0.3-6.4) | 4.2 (1.0-39.9) | <.001 |
| IMC, % | 1.0% (0%-37%) | 0.0% (0%-21%) | 3.0% (0%-37%) | <.001 |
| Circulating blasts, % | 0.0% (0%-12%) | 0.0% (0%-12%) | 0.0% (0%-14%) | <.001 |
| Hgb, g/dL | 11.1 (3.4-18.8) | 11.3 (5.7-18.8) | 11.0 (3.4-16.2) | .116 |
| Platelets, ×109/L | 102 (2-730) | 92 (5-712) | 108 (2-1945) | .170 |
| Serum iron, μg/dL | 85 (11-304) | 71 (38-86) | 86 (19-304) | .207 |
| Elevated LDH, n (%) | <.001 | |||
| Yes | 176 (53.0) | 68 (40.7) | 108 (65.5) | |
| No | 156 (47.0) | 99 (59.3) | 57 (34.5) | |
| Ferritin >1000 μg/L, n (%) | .260 | |||
| Yes | 51 (17.7) | 23 (15.2) | 28 (20.3) | |
| No | 238 (82.3) | 128 (84.8) | 110 (79.7) | |
| Circulating blasts, n (%) | <.001 | |||
| Yes | 87 (25.7) | 24 (13.9) | 63 (38.0) | |
| No | 252 (74.3) | 149 (86.1) | 103 (62.0) | |
| Cytopenias, n (%) | .040 | |||
| None | 99 (29.1) | 42 (24.3) | 57 (34.1) | |
| 1 line | 174 (51.2) | 59 (51.4) | 85 (50.9) | |
| Bicytopenia | 64 (18.8) | 39 (22.5) | 25 (15.0) | |
| Pancytopenia | 3 (0.9) | 3 (1.8) | 0 (0.0) | |
| Marrow blast burden, median (range) | 3 (0-16) | 2 (0-16) | 3 (0-17) | .115 |
| Marrow cellularity, median (range) | 80 (30-100) | 75 (30-100) | 90 (30-100) | .79 |
| RBC-TD, n (%) | .323 | |||
| Yes | 64 (18.8) | 29 (16.8) | 35 (21.0) | |
| No | 276 (81.2) | 144 (83.2) | 132 (79.0) | |
| PLT-TD, n (%) | .950 | |||
| Yes | 12 (3.5) | 6 (3.5) | 6 (3.6) | |
| No | 328 (96.5) | 167 (96.5) | 161 (96.4) | |
| Splenomegaly, n (%) | .010 | |||
| Yes | 61 (18.0) | 22 (12.7) | 39 (23.5) | |
| No | 278 (82.0) | 151 (87.3) | 127 (76.5) | |
| Cytogenetics | ||||
| CPSS karyotype risk, n (%) | .063 | |||
| Good | 261 (77.4) | 139 (80.8) | 122 (73.9) | |
| Intermediate | 37 (11.0) | 20 (11.6) | 17 (10.3) | |
| Poor | 39 (11.6) | 13 (7.6) | 26 (15.8) | |
| IPSS karyotype category, n (%) | .299 | |||
| Good | 264 (78.3) | 139 (80.8) | 125 (75.8) | |
| Intermediate | 53 (15.7) | 26 (15.1) | 27 (16.4) | |
| Poor | 20 (6.0) | 7 (4.1) | 13 (7.8) | |
| Risk assessment models | ||||
| IPSS-R, n (%) | .628 | |||
| Very Low | 95 (28.1) | 54 (31.2) | 41 (24.7) | |
| Low | 137 (40.4) | 67 (38.7) | 70 (42.2) | |
| Intermediate | 57 (16.8) | 30 (17.3) | 27 (16.3) | |
| High | 39 (11.5) | 17 (9.9) | 22 (13.3) | |
| Very High | 11 (3.2) | 5 (2.9) | 6 (3.5) | |
| IPSS-M, n (%) | .003 | |||
| Very Low | 24 (7.1) | 18 (10.4) | 6 (3.6) | |
| Low | 99 (29.1) | 60 (34.7) | 39 (23.4) | |
| Moderate Low | 72 (21.2) | 37 (21.4) | 35 (20.8) | |
| Moderate High | 54 (15.9) | 25 (14.4) | 29 (17.4) | |
| High | 61 (17.9) | 24 (13.9) | 37 (22.2) | |
| Very High | 30 (8.8) | 9 (5.2) | 21 (12.6) | |
| CPSS, n (%) | <.001 | |||
| Low | 118 (34.7) | 115 (66.5) | 3 (1.8) | |
| Intermediate-1 | 125 (36.8) | 36 (20.8) | 89 (53.3) | |
| Intermediate-2 | 80 (23.5) | 21 (12.1) | 59 (35.3) | |
| High | 17 (5.0) | 1 (0.6) | 16 (9.6) | |
| CPSS-Mol, n (%) | <.001 | |||
| Low | 53 (15.6) | 53 (30.6) | 0 (0.0) | |
| Intermediate-1 | 69 (20.3) | 48 (27.8) | 21 (12.6) | |
| Intermediate-2 | 137 (40.3) | 60 (34.7) | 77 (46.1) | |
| High | 81 (23.8) | 12 (6.9) | 69 (23.3) | |
| 4-point IPSS-M, n (%) | .001 | |||
| Low | 123 (36.2) | 78 (45.1) | 45 (26.9) | |
| Intermediate-1 | 72 (21.1) | 37 (21.4) | 35 (21.0) | |
| Intermeidate-2 | 54 (15.9) | 25 (14.4) | 29 (17.4) | |
| High | 91 (26.8) | 33 (19.1) | 58 (34.7) | |
| Mayo Molecular Model, n (%) | <.001 | |||
| Low | 35 (10.3) | 23 (13.4) | 12 (7.2) | |
| Intermediate-1 | 98 (28.9) | 66 (38.4) | 32 (19.2) | |
| Intermediate-2 | 109 (32.2) | 55 (31.9) | 54 (32.3) | |
| High | 97 (28.6) | 28 (16.3) | 69 (41.3) | |
| GFM model, n (%) | <.001 | |||
| Low | 146 (43.1) | 126 (73.3) | 20 (11.9) | |
| Intermediate | 124 (36.6) | 38 (22.1) | 86 (51.5) | |
| High | 69 (20.3) | 8 (4.6) | 61 (36.5) | |
| Clinical end points | ||||
| AML transformation, n (%) | .017 | |||
| Yes | 75 (22.1) | 29 (16.8) | 46 (27.5) | |
| No | 265 (77.9) | 144 (83.2) | 121 (72.5) | |
| AML at 1 year, n (%) | .065 | |||
| Yes | 29 (8.5) | 10 (5.8) | 19 (11.4) | |
| No | 311 (91.5) | 163 (94.2) | 148 (88.6) | |
| AML at 2 years, n (%) | .012 | |||
| Yes | 54 (15.9) | 19 (11.0) | 35 (21.0) | |
| No | 286 (84.1) | 154 (89.0) | 132 (79.0) | |
| AML at 4 years, n (%) | .014 | |||
| Yes | 69 (20.3) | 26 (15.0) | 43 (25.7) | |
| No | 271 (79.7) | 147 (85.0) | 124 (74.3) | |
| Allogeneic SCT, n (%) | 39 (11.5) | 14 (8.1) | 25 (15.0) | .047 |
| OS, median (range), y | 3.2 (2.7-3.7) | 4.1 (3.2-5.1) | 2.3 (1.9-2.7) | <.001 |
| Characteristic . | All patients, N = 340 (100%) . | MDS-CMML, N = 173 (51%) . | MPN-CMML, N = 167 (49%) . | P value∗ . |
|---|---|---|---|---|
| Age at diagnosis, median (range) | 71 (17-88) | 71 (17-88) | 71 (38-86) | .478 |
| Sex, n (%) | .712 | |||
| Male | 235 (69.1) | 118 (68.2) | 117 (70.1) | |
| Female | 105 (30.9) | 55 (31.8) | 50 (29.9) | |
| Race, n (%) | .735 | |||
| White | 303 (89.9) | 152 (88.9) | 151 (91.0) | |
| Black | 14 (4.2) | 9 (5.3) | 5 (3.0) | |
| Hispanic | 11 (3.3) | 6 (3.5) | 5 (3.0) | |
| Other | 9 (2.7) | 4 (2.3) | 5 (3.0) | |
| WHO category, n (%) | .036 | |||
| CMML-1 | 298 (87.7) | 158 (91.3) | 140 (83.8) | |
| CMML-2 | 42 (12.3) | 15 (8.7) | 27 (16.2) | |
| FAB category, n (%) | <.001 | |||
| MDS-CMML | 173 (51) | 173 (100) | 0 (0.0) | |
| MPN-CMML | 167 (49) | 0 (0.0) | 167 (100) | |
| Indolent CMML, n (%) | .140 | |||
| Indolent CMML | 55 (16.2) | 33 (9.1) | 22 (13.2) | |
| Nonindolent CMML | 285 (83.8) | 140 (80.9) | 145 (86.8) | |
| Laboratory values, median (range) | ||||
| WBC, ×109/L | 12.6 (2.4-288.6) | 7.0 (2.4-12.8) | 25.1 (13-288.6) | <.001 |
| ANC, ×109/L | 6.1 (0.1-155.6) | 3.2 (0.1-9.8) | 13.5 (1.6-155.6) | <.001 |
| ALC, ×109/L | 2.2 (0.4-39.9) | 1.7 (0.4-4.7) | 3.3 (0.5-22.0) | <.001 |
| AMC, ×109/L | 2.4 (0.3-39.9) | 1.4 (0.3-6.4) | 4.2 (1.0-39.9) | <.001 |
| IMC, % | 1.0% (0%-37%) | 0.0% (0%-21%) | 3.0% (0%-37%) | <.001 |
| Circulating blasts, % | 0.0% (0%-12%) | 0.0% (0%-12%) | 0.0% (0%-14%) | <.001 |
| Hgb, g/dL | 11.1 (3.4-18.8) | 11.3 (5.7-18.8) | 11.0 (3.4-16.2) | .116 |
| Platelets, ×109/L | 102 (2-730) | 92 (5-712) | 108 (2-1945) | .170 |
| Serum iron, μg/dL | 85 (11-304) | 71 (38-86) | 86 (19-304) | .207 |
| Elevated LDH, n (%) | <.001 | |||
| Yes | 176 (53.0) | 68 (40.7) | 108 (65.5) | |
| No | 156 (47.0) | 99 (59.3) | 57 (34.5) | |
| Ferritin >1000 μg/L, n (%) | .260 | |||
| Yes | 51 (17.7) | 23 (15.2) | 28 (20.3) | |
| No | 238 (82.3) | 128 (84.8) | 110 (79.7) | |
| Circulating blasts, n (%) | <.001 | |||
| Yes | 87 (25.7) | 24 (13.9) | 63 (38.0) | |
| No | 252 (74.3) | 149 (86.1) | 103 (62.0) | |
| Cytopenias, n (%) | .040 | |||
| None | 99 (29.1) | 42 (24.3) | 57 (34.1) | |
| 1 line | 174 (51.2) | 59 (51.4) | 85 (50.9) | |
| Bicytopenia | 64 (18.8) | 39 (22.5) | 25 (15.0) | |
| Pancytopenia | 3 (0.9) | 3 (1.8) | 0 (0.0) | |
| Marrow blast burden, median (range) | 3 (0-16) | 2 (0-16) | 3 (0-17) | .115 |
| Marrow cellularity, median (range) | 80 (30-100) | 75 (30-100) | 90 (30-100) | .79 |
| RBC-TD, n (%) | .323 | |||
| Yes | 64 (18.8) | 29 (16.8) | 35 (21.0) | |
| No | 276 (81.2) | 144 (83.2) | 132 (79.0) | |
| PLT-TD, n (%) | .950 | |||
| Yes | 12 (3.5) | 6 (3.5) | 6 (3.6) | |
| No | 328 (96.5) | 167 (96.5) | 161 (96.4) | |
| Splenomegaly, n (%) | .010 | |||
| Yes | 61 (18.0) | 22 (12.7) | 39 (23.5) | |
| No | 278 (82.0) | 151 (87.3) | 127 (76.5) | |
| Cytogenetics | ||||
| CPSS karyotype risk, n (%) | .063 | |||
| Good | 261 (77.4) | 139 (80.8) | 122 (73.9) | |
| Intermediate | 37 (11.0) | 20 (11.6) | 17 (10.3) | |
| Poor | 39 (11.6) | 13 (7.6) | 26 (15.8) | |
| IPSS karyotype category, n (%) | .299 | |||
| Good | 264 (78.3) | 139 (80.8) | 125 (75.8) | |
| Intermediate | 53 (15.7) | 26 (15.1) | 27 (16.4) | |
| Poor | 20 (6.0) | 7 (4.1) | 13 (7.8) | |
| Risk assessment models | ||||
| IPSS-R, n (%) | .628 | |||
| Very Low | 95 (28.1) | 54 (31.2) | 41 (24.7) | |
| Low | 137 (40.4) | 67 (38.7) | 70 (42.2) | |
| Intermediate | 57 (16.8) | 30 (17.3) | 27 (16.3) | |
| High | 39 (11.5) | 17 (9.9) | 22 (13.3) | |
| Very High | 11 (3.2) | 5 (2.9) | 6 (3.5) | |
| IPSS-M, n (%) | .003 | |||
| Very Low | 24 (7.1) | 18 (10.4) | 6 (3.6) | |
| Low | 99 (29.1) | 60 (34.7) | 39 (23.4) | |
| Moderate Low | 72 (21.2) | 37 (21.4) | 35 (20.8) | |
| Moderate High | 54 (15.9) | 25 (14.4) | 29 (17.4) | |
| High | 61 (17.9) | 24 (13.9) | 37 (22.2) | |
| Very High | 30 (8.8) | 9 (5.2) | 21 (12.6) | |
| CPSS, n (%) | <.001 | |||
| Low | 118 (34.7) | 115 (66.5) | 3 (1.8) | |
| Intermediate-1 | 125 (36.8) | 36 (20.8) | 89 (53.3) | |
| Intermediate-2 | 80 (23.5) | 21 (12.1) | 59 (35.3) | |
| High | 17 (5.0) | 1 (0.6) | 16 (9.6) | |
| CPSS-Mol, n (%) | <.001 | |||
| Low | 53 (15.6) | 53 (30.6) | 0 (0.0) | |
| Intermediate-1 | 69 (20.3) | 48 (27.8) | 21 (12.6) | |
| Intermediate-2 | 137 (40.3) | 60 (34.7) | 77 (46.1) | |
| High | 81 (23.8) | 12 (6.9) | 69 (23.3) | |
| 4-point IPSS-M, n (%) | .001 | |||
| Low | 123 (36.2) | 78 (45.1) | 45 (26.9) | |
| Intermediate-1 | 72 (21.1) | 37 (21.4) | 35 (21.0) | |
| Intermeidate-2 | 54 (15.9) | 25 (14.4) | 29 (17.4) | |
| High | 91 (26.8) | 33 (19.1) | 58 (34.7) | |
| Mayo Molecular Model, n (%) | <.001 | |||
| Low | 35 (10.3) | 23 (13.4) | 12 (7.2) | |
| Intermediate-1 | 98 (28.9) | 66 (38.4) | 32 (19.2) | |
| Intermediate-2 | 109 (32.2) | 55 (31.9) | 54 (32.3) | |
| High | 97 (28.6) | 28 (16.3) | 69 (41.3) | |
| GFM model, n (%) | <.001 | |||
| Low | 146 (43.1) | 126 (73.3) | 20 (11.9) | |
| Intermediate | 124 (36.6) | 38 (22.1) | 86 (51.5) | |
| High | 69 (20.3) | 8 (4.6) | 61 (36.5) | |
| Clinical end points | ||||
| AML transformation, n (%) | .017 | |||
| Yes | 75 (22.1) | 29 (16.8) | 46 (27.5) | |
| No | 265 (77.9) | 144 (83.2) | 121 (72.5) | |
| AML at 1 year, n (%) | .065 | |||
| Yes | 29 (8.5) | 10 (5.8) | 19 (11.4) | |
| No | 311 (91.5) | 163 (94.2) | 148 (88.6) | |
| AML at 2 years, n (%) | .012 | |||
| Yes | 54 (15.9) | 19 (11.0) | 35 (21.0) | |
| No | 286 (84.1) | 154 (89.0) | 132 (79.0) | |
| AML at 4 years, n (%) | .014 | |||
| Yes | 69 (20.3) | 26 (15.0) | 43 (25.7) | |
| No | 271 (79.7) | 147 (85.0) | 124 (74.3) | |
| Allogeneic SCT, n (%) | 39 (11.5) | 14 (8.1) | 25 (15.0) | .047 |
| OS, median (range), y | 3.2 (2.7-3.7) | 4.1 (3.2-5.1) | 2.3 (1.9-2.7) | <.001 |
ALC, absolute lymphocyte count; ANC, absolute neutrophil count; Hgb, hemoglobin; IMC, immature myeloid cell; FAB, French-American-British; SCT, stem cell transplantation.
Statistically significant results (P < .05) are shown in bold.
Fisher exact test; Wilcoxon rank-sum test.
Clonal landscape and spectrum of mutations
We identified at least 914 driver point mutations involving up to 98 genes across 340 patients. We identified at least 1 gene mutation in 326 patients (96%) and ≥2 in 287 patients (84%). The median number of mutated genes was 3 (range, 0-7). The median duration of follow-up was 4.2 years (95% confidence interval [CI], 3.6-4.6).
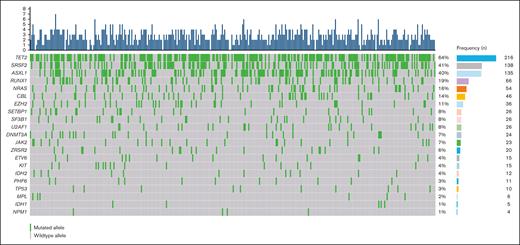
Figure 1 and Table 2 offer a comprehensive look at the clonal landscape and distribution of mutations of the entire patient cohort. The most prevalent mutations were TET2 (n = 216 [63%]), SRSF2 (n = 138 [41%]), ASXL1 (n = 135 [40%]), RUNX1 (n = 66 [19%]), and NRAS (n = 57 [16%]).
Baseline molecular characteristics. Oncoplot illustrating the frequency of mutated genes in 340 patients with CMML. The top panel depicts the number of mutated genes per index case (blue bars; range, 0-8). The central panel shows the type and number of mutated genes, as well as their relationships. Genes are sorted in order of prevalence. Green boxes represent mutated genes, and gray boxes represent wild-type alleles.
Baseline molecular characteristics. Oncoplot illustrating the frequency of mutated genes in 340 patients with CMML. The top panel depicts the number of mutated genes per index case (blue bars; range, 0-8). The central panel shows the type and number of mutated genes, as well as their relationships. Genes are sorted in order of prevalence. Green boxes represent mutated genes, and gray boxes represent wild-type alleles.
Clonal landscape and frequency of mutated genes
| Mutation . | All patients, N = 340 (100%) . | MDS-CMML, n = 173 (51%) . | MPN-CMML, n = 167 (49%) . | P value∗ . |
|---|---|---|---|---|
| DNA methylation, n (%) | ||||
| TET2 | 216 (63.5) | 122/173 (70.5) | 94/167 (56.3) | .006 |
| DNMT3A | 24 (7.1) | 10/173 (5.8) | 14/167 (8.4) | .349 |
| IDH1 | 5 (1.5) | 3/173 (1.7) | 2/167 (1.2) | .681 |
| IDH2 | 12 (3.5) | 8/173 (4.6) | 4/167 (2.4) | .265 |
| RNA splicing, n (%) | ||||
| SRSF2 | 138 (40.6) | 70/173 (40.4) | 68/167 (40.7) | .962 |
| SF3B1 | 26 (7.7) | 17/173 (9.8) | 9/167 (5.4) | .124 |
| U2AF1 | 26 (7.7) | 12/173 (6.9) | 14/167 (8.4) | .616 |
| ZRSR2 | 20 (5.9) | 15/173 (8.7) | 5/167 (3.0) | .026 |
| Chromatin modification, n (%) | ||||
| ASXL1 | 135 (39.7) | 43/173 (24.9) | 92/167 (55.1) | <.001 |
| EZH2 | 36 (10.6) | 13/173 (7.5) | 23/167 (13.8) | .061 |
| PHF6 | 11 (3.2) | 5/173 (2.9) | 6/167 (3.6) | .714 |
| Transcription, n (%) | ||||
| RUNX1 | 66 (19.4) | 29/173 (16.8) | 37/167 (22.2) | .209 |
| ETV6 | 15 (4.4) | 7/173 (4.0) | 8/167 (4.8) | .738 |
| RAS signaling, n (%) | ||||
| NRAS | 54 (15.9) | 20/173 (11.6) | 34/167 (20.4) | .026 |
| CBL | 46 (13.5) | 16/173 (9.2) | 30/167 (18.0) | .019 |
| Others, n (%) | ||||
| SETBP1 | 26 (7.7) | 6/173 (3.5) | 20/167 (12.0) | .003 |
| NPM1 | 4 (1.2) | 2/173 (1.2) | 2/167 (1.2) | .972 |
| Cytokine receptor/tyrosine kinase, n (%) | ||||
| JAK2 | 23 (6.8) | 9/173 (5.2) | 14/167 (8.4) | .243 |
| KIT | 15 (4.4) | 4/173 (2.3) | 11/167 (6.6) | .055 |
| MPL | 6 (1.8) | 4/173 (2.3) | 2/167 (1.2) | .435 |
| Checkpoint cycle, n (%) | ||||
| TP53 | 10 (2.9) | 4/173 (2.3) | 6/167 (3.6) | .485 |
| Mutation . | All patients, N = 340 (100%) . | MDS-CMML, n = 173 (51%) . | MPN-CMML, n = 167 (49%) . | P value∗ . |
|---|---|---|---|---|
| DNA methylation, n (%) | ||||
| TET2 | 216 (63.5) | 122/173 (70.5) | 94/167 (56.3) | .006 |
| DNMT3A | 24 (7.1) | 10/173 (5.8) | 14/167 (8.4) | .349 |
| IDH1 | 5 (1.5) | 3/173 (1.7) | 2/167 (1.2) | .681 |
| IDH2 | 12 (3.5) | 8/173 (4.6) | 4/167 (2.4) | .265 |
| RNA splicing, n (%) | ||||
| SRSF2 | 138 (40.6) | 70/173 (40.4) | 68/167 (40.7) | .962 |
| SF3B1 | 26 (7.7) | 17/173 (9.8) | 9/167 (5.4) | .124 |
| U2AF1 | 26 (7.7) | 12/173 (6.9) | 14/167 (8.4) | .616 |
| ZRSR2 | 20 (5.9) | 15/173 (8.7) | 5/167 (3.0) | .026 |
| Chromatin modification, n (%) | ||||
| ASXL1 | 135 (39.7) | 43/173 (24.9) | 92/167 (55.1) | <.001 |
| EZH2 | 36 (10.6) | 13/173 (7.5) | 23/167 (13.8) | .061 |
| PHF6 | 11 (3.2) | 5/173 (2.9) | 6/167 (3.6) | .714 |
| Transcription, n (%) | ||||
| RUNX1 | 66 (19.4) | 29/173 (16.8) | 37/167 (22.2) | .209 |
| ETV6 | 15 (4.4) | 7/173 (4.0) | 8/167 (4.8) | .738 |
| RAS signaling, n (%) | ||||
| NRAS | 54 (15.9) | 20/173 (11.6) | 34/167 (20.4) | .026 |
| CBL | 46 (13.5) | 16/173 (9.2) | 30/167 (18.0) | .019 |
| Others, n (%) | ||||
| SETBP1 | 26 (7.7) | 6/173 (3.5) | 20/167 (12.0) | .003 |
| NPM1 | 4 (1.2) | 2/173 (1.2) | 2/167 (1.2) | .972 |
| Cytokine receptor/tyrosine kinase, n (%) | ||||
| JAK2 | 23 (6.8) | 9/173 (5.2) | 14/167 (8.4) | .243 |
| KIT | 15 (4.4) | 4/173 (2.3) | 11/167 (6.6) | .055 |
| MPL | 6 (1.8) | 4/173 (2.3) | 2/167 (1.2) | .435 |
| Checkpoint cycle, n (%) | ||||
| TP53 | 10 (2.9) | 4/173 (2.3) | 6/167 (3.6) | .485 |
Statistically significant results (P < .05) are shown in bold.
Χ2 test.
Clinically, TET2 (P = .006) and ZRSR2 mutations (P = .026) were associated with a predominantly dysplastic CMML phenotype, whereas ASXL1 (P < .001), SETBP1 (P = .003), CBL (P = .019), and NRAS (P = .026) were associated with a proliferative clinical phenotype.
All 340 patients were stratified using IPSS-M and all other molecularly informed and mutation-agnostic models. Using the IPSS-M schema, patients were classified as very low (VL; n = 24 [7%]), low (n = 99 [29%]), moderate low (ML; n = 72 [21%]), moderate high (MH; n = 54 [16%]), high (n = 61 [18%]), and very high (VH) risk (n = 30 [9%]).
Impact of somatic mutations on Overall Survival
In univariate analysis, the following mutations were significantly associated with shortened OS: TP53, 0.8 vs 3.3 years (95% CI, 0.1-1.1 vs 2.9-3.9; P < .001); ASXL1, 2.4 vs 3.9 years (95% CI, 1.9-2.9 vs 3.3-4.9; P < .001); EZH2, 1.9 vs 3.5 years (95% CI, 1.3-2.7 vs 2.9-4.0; P = .0041); RUNX1, 1.9 vs 3.6 years (95% CI, 1.7-2.9 vs 3.0-4.4; P = .0051); DNMT3A, 2.0 vs 3.3 years (95% CI, 1.1-3.4 vs 2.9-3.9; P = .0481); NRAS, 2.5 vs 3.4 years (95% CI, 1.8-3.1 vs 2.9-4.0; P = .0495); and NPM1, 1.0 vs 3.2 years (95% CI, 0.68 to not reached vs 2.7-3.8; P = .0008). In contrast, TET2 mutations were associated with improved survival at 3.7 years vs 2.6 years (95% CI, 3.0-4.4 vs 1.8-3.0; P = .0065).
Survival outcomes according to risk model
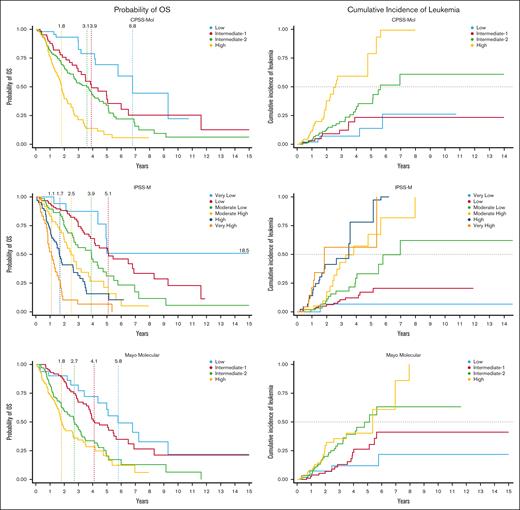
The IPSS-M identified 6 risk categories with corresponding mOS of 18.5, 5.1, 3.9, 2.5, 1.7, and 1.1 years for the very low, low, moderate low, moderate high, high, and very high risk groups, respectively (P < .001). Compared to those with very low risk CMML, the risk of death increased by a factor of 1.6, 2.7, 4.4, 6.3, and 13.1 for the low risk, moderate low, moderate high, high, and very high risk groups, respectively. Conversely, the median LFS was 5.0, 5.1, 3.4, 2.0, 1.4, and 0.9 years for the very low, low, moderate low, moderate high, high, and very high risk groups, respectively (P < .001). The 6 groups showed different cumulative incidence of leukemic transformation, with a 4-year cumulative incidence of evolution to AML of 4.2% for very low (n = 1 [of 24]), 12.1% for low (n = 12 [of 99]), 19.4% for moderate low (n = 14 [of 72]), 25.9% for moderate high (n = 14 [of 54]), 32.8% for high (n = 20 [of 61]), and 26.7% for very high risk categories (n = 8 [of 30]), respectively (P = .008).
We subsequently estimated mOS and LFS for the study cohort using all other previously described molecular-based risk stratification models: CPSS-Mol, MMM, and GFM (Figure 2).
Estimated OS and cumulative incidence of leukemic transformation according to risk model. (A) Kaplan-Meier curves for OS according to IPSS-M, CPSS-Mol, and MMM risk categories. (B) Kaplan-Meier failure curves depicting cumulative incidence of leukemic transformation according to IPSS-M, CPSS-Mol, and MMM risk categories.
Estimated OS and cumulative incidence of leukemic transformation according to risk model. (A) Kaplan-Meier curves for OS according to IPSS-M, CPSS-Mol, and MMM risk categories. (B) Kaplan-Meier failure curves depicting cumulative incidence of leukemic transformation according to IPSS-M, CPSS-Mol, and MMM risk categories.
CPSS-Mol, which integrates cytogenetic risk, presence/absence of ASXL1, NRAS, RUNX1, and SETBP1 mutations, burden of bone marrow blasts (<5% or ≥5%), WBC count (<13 × 109/L or ≥13 × 109/L), and RBC transfusion dependence, successfully identified 4 risk groups with different OS estimates: 6.8 years for low risk; 3.9 for intermediate-1 (IR1); 3.6 years for IR2; and 1.8 years for high risk disease (P < .001). LFS was 6.8 years, 4.4 years, 2.8 years, and 1.4 years for low risk to high risk categories, respectively (P < .001).
The MMM (which considers baseline hemoglobin, AMC, circulating immature myeloid cells, PLT values, and the presence of ASXL1 mutation) identified 4 risk groups, with an mOS of 5.8 years for those with low risk disease, 4.1 years for IR1, 2.7 years for IR2, and 1.8 years for high risk CMML (P < .001). Median LFS estimates were 5.8, 3.9, 2.0, and 1.6 years for low risk, IR1, IR2, and high risk groups, respectively (P < .001).
The GFM model (which is based on age, hemoglobin [Hgb], WBC and PLT counts, and ASXL1 mutation) identified 3 groups with different OS estimates: 4.8 years for low risk, 2.9 years for intermediate-risk, and 1.7 years for high risk CMML (P < .001). LFS was 4.4 years for low risk, 2.7 years for intermediate-risk, and 1.5 years for high risk CMML (P < .001).
Both CPSS-Mol and IPSS-M were equivalent in terms of predictive OS accuracy and showed improved OS discrimination over the MMM and GFM models, as demonstrated by a 10- and 6-percentage point increase in c-index, respectively (c-index, 0.70 vs 0.60 and 0.64). Compared to the classical models, the CPSS-Mol outperformed the CPSS by 2-percentage points (c-index, 0.70 vs 0.68), as did the IPSS-M relative to the IPSS-R (5% increase; 0.70 vs 0.65).
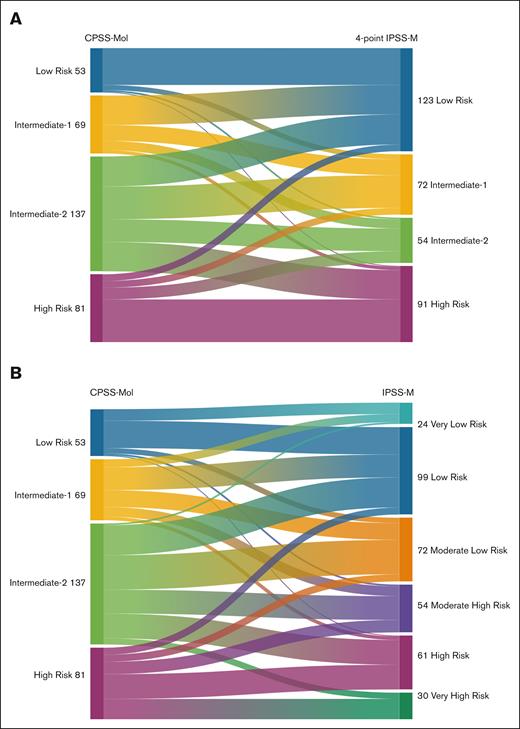
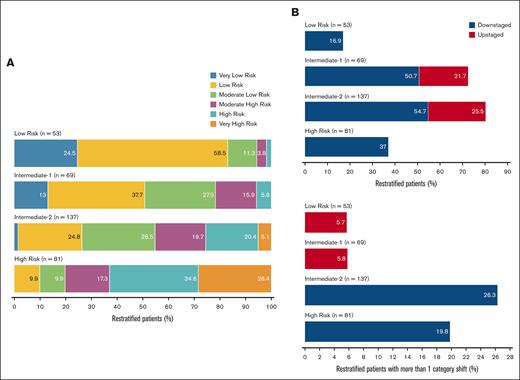
A 4-to-4 mapping between the CPSS-Mol and IPSS-M risk subgroups (obtained by merging VL/low into a single low risk category and high/VH risk into a single high risk category) resulted in the restratification of 199 patients (58%). Seventeen percent of low risk CPSS-Mol patients were upstaged, with 6% shifting to IR2/high risk strata in IPSS-M. Notably, 73% of CPSS-Mol IR1 patients shifted categories: 51% (n = 35) were downstaged in IPSS-M compared to 22% who were upstaged (the majority to IR2). Similarly, 80% of patients classified as IR2 in CPSS-Mol shifted: 55% (n = 75) were downstaged (26% to low risk), whereas 26% were upstaged (n = 35). Furthermore, 37% (n = 30) of high risk patients were downstaged in IPSS-M; 20% (n = 16) to IR1 and low risk strata and 17% to IR2. Alluvial diagrams illustrating the restratification patterns from CPSS-Mol into IPSS-M and 4-point IPSS-M models are depicted in Figure 3. Figure 4 illustrates the distribution of restratified patients in each CPSS-Mol category, including the proportion of patients with >1 shift in IPSS-M risk.
Disease risk restratification patterns. Alluvial diagrams illustrating patient restratification patterns from CPSS-Mol in 4-point IPSS-M (A) and IPSS-M (B) models.
Disease risk restratification patterns. Alluvial diagrams illustrating patient restratification patterns from CPSS-Mol in 4-point IPSS-M (A) and IPSS-M (B) models.
Distribution of restratified patients in each CPSS-Mol category. (A) Stacked bar plots showing restratification patterns of patients from CPSS-Mol to IPSS-M risk categories. (B) Stacked bar plots depicting the distribution of restratified patients from CPSS-Mol into IPSS-M based on risk stratum (top) and proportion of patients with >1 category shift from CPSS-Mol in IPSS-M (bottom).
Distribution of restratified patients in each CPSS-Mol category. (A) Stacked bar plots showing restratification patterns of patients from CPSS-Mol to IPSS-M risk categories. (B) Stacked bar plots depicting the distribution of restratified patients from CPSS-Mol into IPSS-M based on risk stratum (top) and proportion of patients with >1 category shift from CPSS-Mol in IPSS-M (bottom).
The restratification of patients from CPSS-Mol intermediate-risk categories led to significant survival differences in IPSS-M, with mOS estimates as low as 1.5 years and as high as 5.1 years for those who shifted categories (compared to 3.9 years for IR1 and 3.6 years for IR2). The impact on survival estimates resulting from restratifying intermediate-risk patients was significant for those restratified from IR1 (P = .022) and those from IR2 categories (P < .001). There were no significant differences in OS estimates between IPSS-M and CPSS-Mol for the low risk and high risk categories (P = .930 and .135, respectively).
Regarding LFS, CPSS-Mol demonstrated superior prognostic performance compared to the IPSS-M, MMM, and GFM (c-index, 0.72 vs 0.70 vs 0.60 vs 0.64). CPSS-Mol surpassed CPSS, as evidenced by a 3% increase in c-index (0.72 vs 0.69), as did IPSS-M compared to IPSS-R (5%; 0.70 vs 0.65).
Risk of leukemic transformation
Figure 2 illustrates the cumulative incidence of leukemic transformation based on risk category across the different molecular-based models and adjusting for allogeneic stem cell transplantation. A total of 75 patients (22.1%) experienced transformation to secondary AML: 29 patients developed AML by 1 year (8.5%), 54 by 2 years (15.9%), and 69 by 4 years (20.3%). There was significant segregation between IPSS-M risk groups and risk of AML transformation (P < .001), as well as between CPSS-Mol groups (P < .001), MMM (P = .0004), and GMF groups (P = .0087). CPSS-Mol showed the highest prognostic accuracy of leukemic evolution among the molecular-based models (c-index, 0.65), followed by IPSS-M (c-index, 0.62), MMM (c-index, 0.55), and GFM (c-index, 0.51).
Risk model accuracy in treated patients
We subsequently analyzed the prognostic and predictive value of IPSS-M in patients who received frontline HMAs (n = 130) or ruxolitinib monotherapy (n = 29) and those who underwent transplant (n = 39). The mOS for the HMA-treated cohort was 2.6 years (95% CI, 2.0-3.1). The median number of administered HMA cycles was 6 (interquartile range, 3-12). IPSS-M showed significant segregation between risk strata (P < .001), as did CPSS-Mol (P = .0001), GFM (P = .001), and MMM (P = .0157). The c-index for OS was 0.68 for CPSS-Mol, 0.62 for IPSS-M, 0.60 for GFM, and 0.56 for MMM.
Nonetheless, risk group allocation did not predict response to frontline HMA therapy in either IPSS-M (P = .654) or CPSS-Mol (P = .229). Notably, there was association between best response to frontline HMA therapy and risk group assignment in both MMM (P = .029) and GFM models (P = .016).
We then proceeded to analyze frontline responses to HMA therapy based on CMML phenotype. For dysplastic CMML (CMML-MDS), risk group assignment did not predict treatment response in any of the molecular-based models: CPSS-Mol (P = .633), IPSS-M (P = .519), GFM (P = .164), and MMM (P = .255). Prediction ability, however, was different when the models were applied to the subset of patients with proliferative CMML (CMML-MPN). Risk group allocation failed to predict treatment responses to frontline HMA in either CPSS-Mol (P = .479) or IPSS-M (P = .292). However, there was an apparent association between response to frontline HMA therapy in CMML-MPN and risk group allocation in both GFM (P = .047) and MMM (P = .034).
For the subset of patients receiving frontline ruxolitinib, the mOS was 3.7 years (95% CI, 1.3-4.9). IPSS-M showed significantly segregated risk groups (P = .0484), along with CPSS-Mol (P = .052), MMM (P = .0047), and GFM (P = .0017). IPSS-M did not retain its prognostic accuracy (c-index, 0.551), nor did CPSS-Mol (c-index. 0.596). In contrast, both MMM and GFM demonstrated higher c-indices in patients treated with frontline ruxolitinib than those receiving frontline HMA therapy, with MMM showing a c-index of 0.611 and GFM a c-index of 0.729. Notably, risk group allocation did not predict response to frontline ruxolitinib therapy in any of the molecular-based models: CPSS-Mol (P = .521), IPSS-M (P = .729), MMM (P = .407), and GFM (P = .754).
For the subset of patients who went to transplant, the mOS was 5.0 years (95% CI, 3.3 to not reached). IPSS-M groups did not correlate with survival (P = .989); nor did CPSS-Mol (P = .666), MMM (P = .143), or GFM (P = .499). All models lost their prognostic accuracy, as reflected by a drop in c-index: IPSS-M (0.51), CPSS-Mol (0.49), MMM (0.38), and GFM (0.47).
Analysis based on CMML phenotype
We conducted a subgroup analysis to characterize significant differences between dysplastic and proliferative CMML phenotypes. Patient demographics and intergroup differences are shown in detail in Table 1. A total of 173 patients (51%) were identified as having CMML-MDS, whereas 167 patients (48%) were classified with CMML-MPN. Refer to the supplemental Appendix for additional information.
Model performance based on CMML phenotype
Table 3 depicts granular outcomes data for each of the models based on phenotype. Model performance showed remarkable differences across all clinical end points (ie, OS, LFS, and risk of AML transformation) based on disease phenotype, with CPSS-Mol, IPSS-M, MMM, and GFM models showing much more refined prognostic accuracy in CMML-MDS than CMML-MPN.
Summary of clinical end points derived from risk assessment models according to CMML phenotype
| Risk assessment based on CMML phenotype, survival data (years) . | OS, c-index . | LFS, c-index . | AML risk, c-index . |
|---|---|---|---|
| MDS-CMML | |||
| CPSS-Mol: low risk: 6.8 | IR-1: 5.05 | IR-2: 2.87 | high risk: 1.9 | 0.7128 | 0.7067 | 0.666 |
| IPSS-M: VLR: NR | low risk: 6.5 | MLR: 3.49 | MHR: 2.87 | high risk: 2.96 | VHR: 1.37 | 0.7022 | 0.6918 | 0.6831 |
| MMM: low risk: 6.8 | IR-1: 5.44 | IR-2: 3.17 | high risk: 1.9 | 0.6347 | 0.6337 | 0.5714 |
| GFM: low risk: 5.05 | IR: 3.1 | high risk: 1.53 | 0.5828 | 0.5696 | 0.4293 |
| MPN-CMML | |||
| CPSS-Mol: low risk: None | IR-1: 2.88 | IR-2: 3.82 | high risk: 1.71 | 0.612 | 0.6474 | 0.5898 |
| IPSS-M: VLR: 4.37 | low risk: 3.74 | MLR: 3.94 | MHR: 2.16 | high risk: 1.33 | VHR: 1.1 | 0.6706 | 0.6774 | 0.5499 |
| MMM: low risk: 4.37 | IR-1: 3.74 | IR-2: 1.79 | high risk: 1.81 | 0.4874 | 0.4971 | 0.4987 |
| GFM: low risk: 3.94 | IR: 2.61 | high risk: 1.71 | 0.5696 | 0.5683 | 0.4752 |
| Risk assessment based on CMML phenotype, survival data (years) . | OS, c-index . | LFS, c-index . | AML risk, c-index . |
|---|---|---|---|
| MDS-CMML | |||
| CPSS-Mol: low risk: 6.8 | IR-1: 5.05 | IR-2: 2.87 | high risk: 1.9 | 0.7128 | 0.7067 | 0.666 |
| IPSS-M: VLR: NR | low risk: 6.5 | MLR: 3.49 | MHR: 2.87 | high risk: 2.96 | VHR: 1.37 | 0.7022 | 0.6918 | 0.6831 |
| MMM: low risk: 6.8 | IR-1: 5.44 | IR-2: 3.17 | high risk: 1.9 | 0.6347 | 0.6337 | 0.5714 |
| GFM: low risk: 5.05 | IR: 3.1 | high risk: 1.53 | 0.5828 | 0.5696 | 0.4293 |
| MPN-CMML | |||
| CPSS-Mol: low risk: None | IR-1: 2.88 | IR-2: 3.82 | high risk: 1.71 | 0.612 | 0.6474 | 0.5898 |
| IPSS-M: VLR: 4.37 | low risk: 3.74 | MLR: 3.94 | MHR: 2.16 | high risk: 1.33 | VHR: 1.1 | 0.6706 | 0.6774 | 0.5499 |
| MMM: low risk: 4.37 | IR-1: 3.74 | IR-2: 1.79 | high risk: 1.81 | 0.4874 | 0.4971 | 0.4987 |
| GFM: low risk: 3.94 | IR: 2.61 | high risk: 1.71 | 0.5696 | 0.5683 | 0.4752 |
MMM: Mayo Molecular Model. GFM: Groupe Francophone des Myelodysplasies Model. NR: not reached. VLR: very-low risk; IR-1: intermediate-1; IR-2: intermediate-2; MLR: moderate-low risk; MHR: moderare-high risk; VHR: very-high risk.
Statistically significant results are shown in bold.
In the CMML-MDS cohort, CPSS-Mol and IPSS-M demonstrated comparable efficacy in predicting OS and LFS, with CPSS-Mol showing slight superiority over IPSS-M (c-index for OS, 0.713 vs 0.702 [Δ, 1%]; LFS, 0.707 vs 0.692 [Δ, 1%]). Conversely, IPSS-M was more effective in predicting the risk of AML transformation (c-index, 0.683 vs 0.667; Δ, 2%). Both models significantly outperformed the MMM across all metrics, including OS (c-index, 0.635; Δ, 7%-8%), LFS (c-index, 0.634; Δ, 6%-7%), and risk of AML evolution (c-index, 0.571; Δ, 10%-11%). Furthermore, all models surpassed the GFM across all end points: OS (c-index, 0.583; Δ, 5%-13%), LFS (c-index, 0.569; Δ, 7%-14%), and risk of AML transformation (c-index, 0.429; Δ, 14%-25%).
Notably, in the subset of patients with CMML-MPN, the predictive accuracy of all models significantly diminished, with the IPSS-M demonstrating the best performance, followed by CPSS-Mol, GFM, and MMM. Specifically, the c-indices for OS was ranked as follows: IPSS-M (0.671), CPSS-Mol (0.612), GFM (0.569), and MMM (0.487), corresponding to differences of 6%, 10%, and 18%, respectively, compared to IPSS-M as the reference. For LFS, the c-indices were 0.677 for IPSS-M, 0.647 for CPSS-Mol, 0.568 for GFM, and 0.497 for MMM, showing differences of 3%, 11%, and 18%, respectively. In contrast, for the risk of AML transformation, CPSS-Mol led with a c-index of 0.589, followed closely by IPSS-M (0.549), whereas MMM and GFM trailed at 0.499 and 0.475, leading to differences of 4%, 9%, and 11% when CPSS-Mol served as the reference model.
IPSS-M and the indolent CMML phenotype
Patients in the lower IPSS-M risk groups were less likely to require treatment within 3 years of presentation (a term that has been previously referred to by the authors as “indolent CMML”; P < .001).11 A total of 55 indolent CMML cases were segregated as follows from VL to VH risk categories: VLR, 37.5% (n = 9 [of 24]); low risk, 29.3% (n = 29 [of 99]); MLR, 15.3% (n = 11 [of 72]); MHR, 5.6% (n = 3 [of 54]); high risk, 4.9% (n = 3 [of 61]); and VHR, 0% (n = 0 [of 30]).
Discussion
CMML is a biologically complex disease, whose clinical behavior is defined and dictated by a highly heterogeneous process involving serial acquisition of genetic mutations involving epigenetic regulators, splicing modulators, and cytokine signaling molecules, which ultimately confer a survival advantage and develops into dysfunctional clonogenesis.1,2,12-19 As functional disorders of hematopoiesis driven by clonal pathobiological mechanisms, knowledge of cytogenetic architecture and mutational profiling remains essential in an attempt to characterize and predict its clinical behavior and risk of leukemic transformation, so as to effectively guide and individualize therapy in hopes of optimizing clinical outcomes and minimizing risks of toxicity.
In concordance with the literature, the most prevalent mutations in the CMML cohort were TET2, SRSF2, ASXL1, RUNX1, and NRAS.1,2TET2 and ZRSR2 mutations were significantly associated with dysplastic CMML, whereas ASXL1, SETBP1, CBL, and NRAS were commonly seen in proliferative disease. ASXL1, RUNX1, EZH2, TP53, DNMT3A, NRAS, and NPM1 mutations were significantly associated with shortened survival. Conversely, harboring a TET2 clone was associated with improved outcomes.
Treatment with epigenetic modulators (ie, HMAs) remains a standard of care for transplant-ineligible patients with high risk disease because they have shown ability to delay leukemic transformation compared to placebo and prolong survival in retrospective studies.20-22 Similarly, use of cytoreductive therapies seems to be associated with improved outcomes in a subgroup of patients with hyperproliferative features by mitigating end organ damage.23 However, epigenetic modulation has not proven to effectively prevent leukemic evolution or alter the clonal burden of disease even among responders.24,25 For these reasons, allogeneic stem cell transplantation offers the only potential option for achieving a cure and remains the treatment of choice for clinically fit individuals with high risk disease.26,27 Therefore, adequate characterization of disease risk is crucial in clinical decision-making and improving outcomes.
The 3 major molecular-based models most commonly used for prognostication purposes and clinical decision-making in CMML are CPSS-Mol, the MMM, and the GFM model. The IPSS-M represents a novel prognostic model now in mainstream use for clinical decision-making in MDS but not specifically in CMML.9 Furthermore, the IPSS-M discovery cohort included a total of 272 patients with CMML (accounting for 9.5% of the entire study population), but clinical outcomes were analyzed in aggregate after compounding them with all other MDS cases and related entities.9 Survival estimates were not specifically defined for this subgroup of patients. By comparison, the CPSS-Mol discovery cohort comprised 214 patients with CMML exclusively and was validated in a secondary cohort of 260 patients.6 The MMM discovery cohort comprised 466 patients,7 whereas GFM analyzed a total of 312 patients with CMML.8
In this study, we tested the prognostic value of the IPSS-M as applied exclusively to patients with CMML and offered a comprehensive and practical comparison of its prognostic accuracy with that of other major risk models that integrate molecular data into their stratification paradigms.
Of the 4 molecular-based models compared in this study, both CPSS-Mol and IPSS-M were shown to have equivalent accuracy in prognosticating OS estimates, with the former showing slight superiority in establishing risk groups that more accurately predicted LFS (c-index, 0.72 vs 0.70). Both models were notably superior to their mutation-agnostic predecessors (CPSS and IPSS-R) and outperformed both MMM and GFM model across both clinical end points. Notably, CPSS-Mol was also the most accurate model in predicting the risk of leukemic evolution, followed by IPSS-M, MMM, and GFM. These hierarchical differences in AML prognostication accuracy between models are likely explained by the inclusion of biologically relevant somatic mutations other than ASXL1 into the CPSS-Mol and IPSS-M risk stratification algorithms (in contrast to both MMM and GFM, in which their impact on disease biology remains uncaptured).28-31 Similarly, differences between IPSS-M and CPSS-Mol are likely secondary to the lack of consideration for RBC-TD and high WBC count in the former, factors which are known to carry significant adverse connotations in CMML (particularly the latter, which is used to distinguish between dysplastic and proliferative phenotypes).3-6,11 CPSS-Mol also places more value in the prognostic relevance of SETBP1 mutations than the IPSS-M (which considers it a lower weighted variable or “residual gene”).6,9,31
All molecular-based models were able to successfully identify and catalog HMA-treated patients into discretely defined risk groups with different survival estimates, with CPSS-Mol showing the highest prognostic accuracy for OS and outperforming IPSS-M by 6%, GFM by 8%, and MMM by 12%. However, this was not the case for patients receiving frontline ruxolitinib therapy, in which both the MMM and, especially, the GFM model showed notable prognostic accuracy.
Regarding treatment, neither CPSS-Mol nor IPSS-M was able to accurately predict responses to frontline HMA therapy, regardless of risk stratum allocation. This assumption held its validity even after post hoc analysis looking at patients with CMML with a primarily dysplastic phenotype, which gives further credence to the complex relationship between treatment response to HMA and outcomes. Similar findings were observed in those who received frontline ruxolitinib therapy. This reflects current knowledge postulating that HMA-based therapy does not carry disease-modifying properties in CMML.24,25,32 It is important to note, however, that risk group assignment in both GFM and MMM appeared to correlate with treatment response to frontline HMA therapy, particularly among patients with CMML-MPN.
The disparity in prognostic accuracy among the models may stem from the differing emphasis on risk stratification elements, with GFM and MMM prioritizing peripheral blood counts, such as AMC, circulating immature myeloid cells, PLTs, and hemoglobin, over the clonal architecture, which is emphasized more in IPSS-M and CPSS-Mol. Because these peripheral blood counts are more susceptible to changes induced by HMA and ruxolitinib therapies, their variability could influence outcomes more than the relatively stable clonal architecture, which remains largely unaffected by these treatments.
To directly compare and estimate the impact of risk restratification, we compared the 2 models with the highest prognostic accuracy: CPSS-Mol and IPSS-M. A total of 199 patients (58.5% of the study cohort) underwent group restratification from CPSS-Mol into IPSS-M: 140 patients (41.2%) were downstaged compared to 59 patients (17.3%) who were upstaged. Overall, IPSS-M was more likely to downstage patients than CPSS-Mol.
It is important to note that we estimated and used median (rather than absolute) IPSS-M risk scores to account for nonessential, nonannotated molecular data. One of the most appealing and pragmatic features of the IPSS-M model is its relative flexibility to account for some missing genomic data by providing an average risk score without compromising its validity (as well as prognostic accuracy to some extent). This approach has been validated by the GenoMed4All investigators and the Moffitt group independently.33,34
Potential study limitations include the retrospective nature of our study, highlighting the need for prospective validation of these important findings. Additionally, there were missing data on TP53 copy-neutral loss of heterozigosity (CN-LOH), which is still not routinely screened in general clinical practice. Our CMML database also did not capture cases with MLL-PTD; however, MLL-PTD remains exceedingly rare in CMML, with 1 study reporting no instances in 500 CMML samples analyzed using whole-exome sequencing and fluorescence in situ hybridization.35 Another limitation pertains to the definition of CMML, which was based on the older WHO 2017 criteria, with data collection and patient identification preceding the release of the new International Consensus Classification (ICC) and 2022 WHO classifications. This may have led to the omission of a subset of patients, previously identified as having oligomonocytosis per WHO 2017, who could now be reclassified as CMML and thus were not captured in our analysis. However, it is worth noting that a recent comprehensive analysis by Komrokji et al presented at the 66th American Society of Hematology Annual Meeting found that among 1044 patients with reclassified CMML according to the WHO/ICC 2022 criteria, who were previously classified as having oligomonocytosis (AMC 0.5-1), oligomonocytic CMML molecularly resembled MDS and also shared overlapping clinical features and outcomes with it.36
Conclusions
CPSS-Mol proved to be superior at predicting outcomes in patients with CMML across all examined clinical end points: OS, LFS, and risk of AML transformation. This has significant implications for its universal adoption in CMML clinical trial enrollment and design, because it is a more precise, practical tool that requires fewer data points and is easy to use. Consequently, it should be preferred over the IPSS-M in this context. However, IPSS-M showed comparable prognostic accuracy for OS and LFS, making it a reliable tool for prognosticating outcomes in CMML. Both CPSS-Mol and IPSS-M demonstrated superior prognostic accuracy over both the MMM and GFM models. These observations held true in CMML-MDS, in which CPSS-Mol outperformed IPSS-M in predicting OS and LFS but not the risk of AML transformation. All models were less accurate in patients with CMML-MPN, with IPSS-M outperforming CPSS-Mol, whereas both MMM and GFM proved unreliable. All models effectively identified various prognostic risk groups among HMA-treated patients but showed reduced prognostic accuracy for OS, with CPSS-Mol outperforming IPSS-M, GFM (by 8%), and MMM (by 12%). CPSS-Mol showing the highest prognostic accuracy for OS followed by IPSS-M, GFM, and MMM. In ruxolitinib-treated patients, the GFM model was particularly effective in stratifying patients and outperformed all other models; however, no models could predict responses to ruxolitinib. Notably, a correlation was found between risk group assignment and OS with HMAs in both the MMM and, particularly, the GFM model. Despite refinements in risk stratification offered by the newer molecular models, there is still a substantial unmet need for a more effective risk assessment tool to improve risk stratification, specifically for patients with CMML-MPN.
Authorship
Contribution: L.E.A. and R.S.K. contributed to conception and design and wrote the manuscript; R.S.K. and N.A.A. provided administrative support; R.S.K., L.E.A., D.S., J.E.L., K.S., E.P., and A.K. contributed to provision of study materials or patients; N.A.A. and L.E.A. collected and assembled data; R.S.K., L.E.A., and N.A.A. analyzed and interpreted data; and all authors provided final approval of manuscript.
Conflict-of-interest disclosure: D.S. reports research funding from Syntrix Pharmaceuticals and Aprea; membership on an entity's board of directors or advisory committees for Nemucore, Novartis, Intellia, Syndax, Kite, Shattuck Labs, Bristol Myers Squibb, Aprea, Agios, and AbbVie; patents and royalties with Lixte (LB-100); consultancy fees from Magenta, Novartis, and Takeda; and speakers bureau fees from Incyte and Bristol Myers Squibb. A.K. reports research support from Protagonist, GlaxoSmithKline–Sierra Oncology, Prelude Pharmaceuticals, Bristol Myers Squibb, and MorphoSys; and consultancy fees, honoraria, and speakers bureau fees from Imago Biosciences, Incyte, Blueprint Medicines Corporation, Novartis, AbbVie, GlaxoSmithKline–Sierra Oncology, Bristol Myers Squibb, PharmaEssentia, and CTI Biopharma. K.S. reports consultancy fees from Mablytics, Pfizer, Curis, BerGenBio, Arog, Gilead Sciences, Inc, Novartis, Astellas, and Bristol Myers Squibb; membership on an entity's board of directors or advisory committees for Mablytics, Pfizer, Curis, BerGenBio, Arog, Gilead Sciences, Inc, Novartis, Astellas, and Bristol Myers Squibb; honoraria from Mablytics, Curis, BerGenBio, Arog, Gilead Sciences, Inc, Novartis, Astellas, and Bristol Myers Squibb; and research funding from Pfizer, Syntrix Pharmaceuticals, and Incyte. J.E.L. reports consultancy fees from Novartis, Jasper Therapeutics, Dedham Group, Boxer Capital, Dava Oncology, Astellas, Agios/Servio, Jazz, BerGenBio, Millenium Pharma/Takeda, ElevateBio Management, Daiichi Sankyo, AbbVie, and Servier; and research funding from Syntrix Pharmaceuticals and Celgene/Bristol Myers Squibb. E.P. reports research funding from Incyte, Kura, Syntrix Pharmaceuticals, and Bristol Myers Squibb; and honoraria from Stemline, Taiho, and Blueprint. R.S.K. reports honoraria from Jazz, Servio, CTI Biopharma, PharmaEssentia, Bristol Myers Squibb, Novartis, AbbVie, Geron, and Taiho; membership on an entity's board of directors or advisory committees for Jazz, Servio, CTI Biopharma, Bristol Myers Squibb, Novartis, AbbVie, Geron, and Taiho; and speakers bureau fees from Jazz, Servio, CTI Biopharma, and PharmaEssentia. The remaining authors declare no competing financial interests.
Correspondence: Luis E. Aguirre, Dana-Farber Cancer Institute, 450 Brookline Ave, Boston, MA 02215; email: luis_aguirre@hsph.harvard.edu; and Rami S. Komrokji, Moffitt Cancer Center, Magnolia Campus, 12902 USF Magnolia Dr, Tampa, FL 33612; email: rami.komrokji@moffitt.org.
References
Author notes
Parts of this manuscript were presented in preliminary form as 2 separate abstracts at the 2023 American Society of Clinical Oncology Annual Meeting; 2-6 June 2023 in Chicago, IL, where it was awarded a 2023 Conquer Cancer Merit Award and at the 2023 European Hematology Association Congress; 8-11 June 2023 in Frankfurt Germany.
Data are available on request from the senior author, Rami S. Komrokji (rami.komrokji@moffitt.org). The data are not publicly available owing to privacy or ethical restrictions.
The full-text version of this article contains a data supplement.