Key Points
Patients receiving ibrutinib for R/R MCL in routine practice have inferior survival compared with trials.
Dose-limiting toxicities on ibrutinib were common, highlighting the need for monitoring and managing toxicities during treatment.
Visual Abstract
Ibrutinib was approved for relapsed/refractory (R/R) mantle cell lymphoma (MCL) based on high response rates in clinical trials, but it is unclear how effective ibrutinib is in the real-world setting. This study provides population-based response rates and survival estimates and characterization of prognostic indicators and adverse events (AEs) to ibrutinib for patients with R/R MCL. All patients diagnosed with MCL in Denmark from 2010 to 2022 were identified in the Danish Lymphoma Registry and screened for eligibility. Data were collected from health records. Patients receiving ibrutinib in second or later lines were included and followed from ibrutinib start until death or last follow-up. End points were overall response rate (ORR), progression-free survival (PFS), overall survival (OS), frequency of AEs, and AE-related discontinuation and dose reductions. In total, 146 patients were included (median age, 73 years); 90 (62%) received ibrutinib in second line. ORR was 56%, median PFS 5.8 months, and median OS 12.0 months. In Cox regressions, factors associated with inferior PFS were Ki67 of ≥50% (hazard ratio [HR], 2.34; 95% confidence interval [CI], 1.47-3.71), blastoid or pleomorphic subtype (HR, 3.00; 95% CI, 2.04-4.41), early relapses (HR, 1.65; 95% CI, 1.15-2.36), and refractory disease (HR, 1.57; 95% CI, 1.07-2.30). Three-year cumulative incidences of discontinuation and dose reductions owing to AEs were 19% and 22%, respectively. Median OS after ibrutinib discontinuation was 1.9 months. In conclusion, real-world outcomes after initiation of ibrutinib for R/R MCL were poorer than observed in clinical trials, and dose-limiting toxicities were common, emphasizing the need for more effective treatments and dose-optimization studies.
Introduction
Mantle cell lymphoma (MCL) is characterized by recurring relapses where remissions typically become shorter with each treatment intervention.1 Although some experience long-term remissions with a duration of >10 years after first-line therapy, disease progression is the cause of death for most patients with MCL.2-4 One of the preferred treatment options for relapsed/refractory (R/R) MCL is Bruton tyrosine kinase inhibitors (BTKis).5-7 Ibrutinib was the first BTKi granted accelerated approval by the US Food and Drug Administration and conditional marketing authorization by the European Medicines Agency for R/R MCL in 2013. The approvals were based on a single-arm phase 2 study where 111 patients with R/R MCL received ibrutinib with an overall response rate (ORR) of 68%, median progression-free survival (PFS) of 13.9 months, and median overall survival (OS) of 22.5 months.8,9 A later randomized phase 3 clinical trial supported the efficacy of ibrutinib for R/R MCL by demonstrating a median PFS of 14.6 months for ibrutinib vs 6.2 months for temsirolimus.10 However, in 2023, the US Food and Drug Administration approval of ibrutinib for R/R MCL was withdrawn owing to a lack of OS benefit in the confirmatory SHINE trial of older previously untreated patients with MCL randomized to first-line rituximab (R)-bendamustine (R-B) plus ibrutinib vs R-B plus placebo.11 Despite improved PFS in the ibrutinib arm (median of 80.6 months vs 52.9 months), OS was similar and more adverse events (AEs), including infections, were observed in the ibrutinib arm. Ibrutinib remains on the market for R/R MCL in Europe. The TRIANGLE trial, a randomized phase 3 study, renewed interest in first-line ibrutinib, demonstrating superior failure-free survival and OS when ibrutinib was added to induction and as maintenance for younger previously untreated patients with MCL, regardless of the use of autologous stem cell transplant.12,13
Uncertainties regarding the efficacy of ibrutinib for R/R MCL compared with conventional therapies, especially in older patients who would not necessarily fulfill the inclusion criteria for clinical trials, highlight the relevance of population-based studies of ibrutinib. This nationwide study investigated response rates, survival outcomes, AEs, and treatment adherence in patients with R/R MCL in a population-based setting. Second, the study explored survival after ibrutinib failure and associations between clinical and pathological features with response to ibrutinib.
Methods
Study design
This is a population-based cohort study. Patients were identified in the Danish Lymphoma Registry (LYFO) and screened for eligibility using health records. LYFO contains data on all adult patients with lymphoma in Denmark with high completeness and accuracy.14 Patients were included in the ibrutinib cohort if they were diagnosed with MCL between 2010 and 2022 and if they received ibrutinib in second or later treatment lines. Patients were excluded if they only received ibrutinib in clinical trials (supplemental Figure 1). To compare second-line (2L) ibrutinib with other therapies, patients with MCL receiving other 2L therapies were included (2L comparators). Comparators were excluded if 2L treatment was R monotherapy, radiotherapy monotherapy, chlorambucil monotherapy, or BTKis or if 2L treatment was in a clinical trial (supplemental Figure 2).
Data sources and variables
Characteristics from diagnosis and first-line therapies were collected from LYFO. Later lines of therapy and characteristics at relapses were collected from health records. Morphological MCL subtype and immunohistochemistry including Ki67 were collected from pathology reports for all biopsies before ibrutinib. Ki67 was measured according to routine practice by hematopathologists, for example, by visual approximation. A treatment line was considered treatment initiated owing to relapse, progression, or insufficient response. Best supportive care or watch and wait were not considered treatment lines, whereas radiotherapy as monotherapy was. Treatment responses were complete remission (CR), partial remission (PR), stable disease (SD), or progressive disease (PD) as assessed by computed tomography (CT) or positron emission tomography and CT (PET-CT) scans with or without bone marrow biopsy. Treatment responses were classified according to original imaging reports as assessed by radiologists, which as a rule followed the Lugano 2014 criteria.15 When imaging was not performed, clinical remission status was used. Refractory disease to the previous line was defined as an end-of-treatment response of SD or PD.
AEs were collected irrespective of causality with ibrutinib and retrospectively graded according to the Common Terminology Criteria for Adverse Events 5th version. Suspected causality was only recorded if explicitly mentioned in the health record by the treating physician. AEs were included if they were grade ≥3 AEs, serious AEs (SAEs), or any grade AEs causing dose reduction or discontinuation. MCL progressions were not considered AEs. Dose reductions, discontinuations, and causes were recorded. Data were collected by medical doctors with experience in hematology or lymphoma research in 2023 to 2024 using REDCap data capture tools.16
This study was approved by the Regional Ethical Committee in the Capital Region of Denmark (H-22053438) and registered in the Capital Region at Knowledge Center for Data reviews (P-2022-522).
End points
The primary end points were PFS and OS after ibrutinib initiation. Secondary end points were best ORR at any time after ibrutinib initiation, duration of response (DOR), cumulative incidences of AEs, discontinuations, dose reductions, and OS after discontinuation.
Statistical analysis
Patients in the ibrutinib cohort were followed from ibrutinib initiation until death or censoring at the last date of health record review. PFS was calculated from ibrutinib initiation to progression, relapse, or death, and OS to death. ORR was the proportion with the best response of CR, PR, or clinical remission. DOR was the time from the best response of CR, PR, or clinical remission to relapse or death. Survival estimates were calculated using the Kaplan-Meier method and compared using log-rank tests. Univariable and multivariable Cox regressions were performed to assess features associated with PFS, investigating age, sex, Ki67, blastoid or pleomorphic morphology, disease progression within 24 months (POD24) after first-line therapy, refractory disease, time since diagnosis, 2L vs later-line ibrutinib, and combination therapy vs monotherapy. Cumulative incidences of AEs of grade ≥3 or SAEs and AE-related discontinuations were estimated until discontinuation with death and relapse as competing events. For AE-related dose reductions, discontinuation, death, and relapse were competing events. Cumulative incidences were calculated using the Aalen-Johansen estimator. The mean number of AEs per patient was estimated using the Andersen-Gill Cox model.17
For comparison of survival outcomes between 2L ibrutinib patients and 2L comparators, inverse probability weighting (IPW) was applied to balance the following potential confounders: refractory disease, POD24 from first-line therapy, central nervous system (CNS) involvement, Ki67, blastoid or pleomorphic morphology, and calendar year of 2L treatment. PFS and OS were calculated from the time of 2L treatment initiation. Statistical analyses were performed using RStudio version 4.4.2.18
Results
Baseline characteristics
Of 918 patients with MCL, 146 were included in the ibrutinib cohort (supplemental Figure 1). Of these, 144 (99%) had cyclin D1 expression, and the remaining 2 had CD5 and SOX11 expression. The median age at ibrutinib initiation was 73 years (range, 28-94), and 69% were male. Median time from diagnosis to start of ibrutinib was 2.7 years (range, 0.1-11.4). A total of 42 patients (29%) were refractory to the previous treatment line, 81 patients (58%) had Ki67 of ≥50%, and 58 (40%) had blastoid or pleomorphic subtype at any time before ibrutinib initiation. The most common first-line therapies were R-B in 50 patients (36%) and R-MaxiCHOP and high-dose cytarabine in 45 patients (32%), and 35 patients (25%) received high-dose chemotherapy with autologous stem cell transplant (HDT-ASCT) (Table 1). Ibrutinib was used in 2L for 90 patients (62%), of whom 13 (14%) had CNS involvement compared with 5 (9%) among the later-line group. In the 2L ibrutinib group, 55 patients (61%) had POD24 from first-line therapy compared with 27 patients (48%) in the later-line group (supplemental Table 1). A total of 102 patients (70%) received ibrutinib monotherapy, whereas 19 patients (13%) received combination with R, 10 patients (7%) with other chemoimmunotherapy, 7 patients (5%) with intrathecal therapy or high-dose methotrexate, and 4 patients (3%) with lenalidomide (supplemental Table 2).
Clinical and pathological characteristics at the time of diagnosis of MCL and ibrutinib initiation
| Characteristics . | Time of diagnosis . | Time of ibrutinib start . |
|---|---|---|
| Total | 146 (100) | |
| Age, median (range), y | 70 (28-90) | 73 (28-94) |
| Sex, n (%) | ||
| Male | 101 (69.2) | |
| Female | 45 (30.8) | |
| Performance status, n (%) | ||
| 0-1 | 131 (89.7) | 65 (82.3) |
| 2 | 9 (6.2) | 7 (8.9) |
| 3-4 | 6 (4.1) | 7 (8.9) |
| Missing | 67 | |
| Stage, n (%) | ||
| I-II | 9 (6.2) | 19 (13.5) |
| III-IV | 136 (93.8) | 122 (86.5) |
| Missing | 1 | 5 |
| No. of extranodal sites, n (%) | ||
| 0 | 7 (4.8) | 22 (15.8) |
| 1-2 | 116 (79.5) | 86 (61.9) |
| ≥3 | 23 (15.8) | 29 (20.9) |
| Yes, not otherwise specified | 2 (1.4) | |
| Missing | 7 | |
| MIPI, n (%) | ||
| Low | 14 (9.6) | 4 (5.6) |
| Intermediate | 44 (30.1) | 27 (38.0) |
| High | 88 (60.3) | 40 (56.3) |
| Missing | 75 | |
| CNS involvement, n (%) | 1 (0.7) | 18 (12.3) |
| GI involvement, n (%) | 28 (21.4) | 33 (24.1) |
| Missing | 15 | 9 |
| Bone marrow involvement, n (%) | 128 (87.7) | 47 (39.8) |
| Missing | 28 | |
| Refractory to previous line, n (%) | 42 (28.8) | |
| Morphology, n (%) | ||
| Blastoid | 23 (15.8) | 23 (15.8) |
| Pleomorphic | 14 (9.6) | 13 (8.9) |
| Not blastoid or pleomorphic | 109 (74.7) | 77 (68.1) |
| Missing | 33 | |
| Blastoid or pleomorphic at any time∗, n (%) | 58 (39.7) | |
| Blastoid at any time | 38 (26.0) | |
| Pleomorphic at any time | 24 (16.4) | |
| Ki67 (highest tumor or bone marrow), n (%) | ||
| <30% | 37 (29.6) | 17 (19.8) |
| 30%-49% | 33 (26.4) | 15 (17.4) |
| 50%-100% | 55 (44.0) | 54 (62.8) |
| Missing | 21 | 60 |
| Ki67, highest ever before ibrutinib, n (%) | ||
| <30% | 29 (20.7) | |
| 30%-49% | 30 (21.4) | |
| 50%-100% | 81 (57.9) | |
| Missing | 6 | |
| Charlson comorbidity index†, n (%) | ||
| 0 | 91 (62.3) | |
| 1 | 20 (13.7) | |
| ≥2 | 35 (24.0) | |
| First-line therapy, type, n (%) | ||
| R-B ± other‡ | 50 (36.0) | |
| (R)-MaxiCHOP or alike§ + Hd cytarabine | 45 (32.4) | |
| (R)-CHOP or (R)-MaxiCHOP ± other|| | 26 (18.7) | |
| (R)-Hd cytarabine ± other | 5 (3.6) | |
| Ibrutinib containing | 3 (2.2) | |
| Other chemoimmunotherapy | 8 (5.8) | |
| Only radiotherapy or surgery | 2 (1.4) | |
| Missing | 7 | |
| HDT-ASCT consolidation in first-line therapy, n (%) | 35 (24.5) | |
| Rituximab maintenance after first-line therapy, n (%) | 58 (43.3) | |
| POD24 from first-line therapy, n (%) | 82 (56.2) | |
| Time since MCL diagnosis, median (range), y | 2.7 (0.1-11.4) | |
| Year of diagnosis/treatment, n (%) | ||
| 2010-2013 | 49 (33.6) | |
| 2014-2017 | 58 (39.7) | 51 (34.9) |
| 2018-2022 | 39 (26.7) | 89 (61.0) |
| 2023-2024 | 6 (4.1) | |
| Characteristics . | Time of diagnosis . | Time of ibrutinib start . |
|---|---|---|
| Total | 146 (100) | |
| Age, median (range), y | 70 (28-90) | 73 (28-94) |
| Sex, n (%) | ||
| Male | 101 (69.2) | |
| Female | 45 (30.8) | |
| Performance status, n (%) | ||
| 0-1 | 131 (89.7) | 65 (82.3) |
| 2 | 9 (6.2) | 7 (8.9) |
| 3-4 | 6 (4.1) | 7 (8.9) |
| Missing | 67 | |
| Stage, n (%) | ||
| I-II | 9 (6.2) | 19 (13.5) |
| III-IV | 136 (93.8) | 122 (86.5) |
| Missing | 1 | 5 |
| No. of extranodal sites, n (%) | ||
| 0 | 7 (4.8) | 22 (15.8) |
| 1-2 | 116 (79.5) | 86 (61.9) |
| ≥3 | 23 (15.8) | 29 (20.9) |
| Yes, not otherwise specified | 2 (1.4) | |
| Missing | 7 | |
| MIPI, n (%) | ||
| Low | 14 (9.6) | 4 (5.6) |
| Intermediate | 44 (30.1) | 27 (38.0) |
| High | 88 (60.3) | 40 (56.3) |
| Missing | 75 | |
| CNS involvement, n (%) | 1 (0.7) | 18 (12.3) |
| GI involvement, n (%) | 28 (21.4) | 33 (24.1) |
| Missing | 15 | 9 |
| Bone marrow involvement, n (%) | 128 (87.7) | 47 (39.8) |
| Missing | 28 | |
| Refractory to previous line, n (%) | 42 (28.8) | |
| Morphology, n (%) | ||
| Blastoid | 23 (15.8) | 23 (15.8) |
| Pleomorphic | 14 (9.6) | 13 (8.9) |
| Not blastoid or pleomorphic | 109 (74.7) | 77 (68.1) |
| Missing | 33 | |
| Blastoid or pleomorphic at any time∗, n (%) | 58 (39.7) | |
| Blastoid at any time | 38 (26.0) | |
| Pleomorphic at any time | 24 (16.4) | |
| Ki67 (highest tumor or bone marrow), n (%) | ||
| <30% | 37 (29.6) | 17 (19.8) |
| 30%-49% | 33 (26.4) | 15 (17.4) |
| 50%-100% | 55 (44.0) | 54 (62.8) |
| Missing | 21 | 60 |
| Ki67, highest ever before ibrutinib, n (%) | ||
| <30% | 29 (20.7) | |
| 30%-49% | 30 (21.4) | |
| 50%-100% | 81 (57.9) | |
| Missing | 6 | |
| Charlson comorbidity index†, n (%) | ||
| 0 | 91 (62.3) | |
| 1 | 20 (13.7) | |
| ≥2 | 35 (24.0) | |
| First-line therapy, type, n (%) | ||
| R-B ± other‡ | 50 (36.0) | |
| (R)-MaxiCHOP or alike§ + Hd cytarabine | 45 (32.4) | |
| (R)-CHOP or (R)-MaxiCHOP ± other|| | 26 (18.7) | |
| (R)-Hd cytarabine ± other | 5 (3.6) | |
| Ibrutinib containing | 3 (2.2) | |
| Other chemoimmunotherapy | 8 (5.8) | |
| Only radiotherapy or surgery | 2 (1.4) | |
| Missing | 7 | |
| HDT-ASCT consolidation in first-line therapy, n (%) | 35 (24.5) | |
| Rituximab maintenance after first-line therapy, n (%) | 58 (43.3) | |
| POD24 from first-line therapy, n (%) | 82 (56.2) | |
| Time since MCL diagnosis, median (range), y | 2.7 (0.1-11.4) | |
| Year of diagnosis/treatment, n (%) | ||
| 2010-2013 | 49 (33.6) | |
| 2014-2017 | 58 (39.7) | 51 (34.9) |
| 2018-2022 | 39 (26.7) | 89 (61.0) |
| 2023-2024 | 6 (4.1) | |
GI, gastrointestinal; Hd, high-dose; MIPI, MCL international prognostic index.
At any time before ibrutinib, included 38 blastoid and 20 pleomorphic.
Identified in the period from ten years until six months before first MCL diagnosis.
Six received R-B + other.
MaxiCHOP (dose-intensified CHOP) or CHOP (cyclophosphamide, doxorubicin, vincristine, prednisolone) or CHOEP (cyclophosphamide, doxorubicin, vincristine, etoposide, prednisolone) or CEOP (cyclophosphamide, etoposide, vincristine, prednisolone).
Two received (R)-CHOP + other.
For comparison with 2L ibrutinib patients, 85 patients with MCL (2L comparators) were included, of whom most (74%) received 2L R-B (supplemental Table 3).
Treatment responses
Median follow-up in the ibrutinib cohort (reverse Kaplan-Meier) was 4.0 years (95% confidence interval [CI], 3.0-7.2). During this period, treatment responses of 142 patients (97%) were assessed using PET-CT with bone marrow biopsy (6%), PET-CT without bone marrow biopsy (40%), CT (24%), bone marrow biopsy only (1%), or clinical assessment only (29%). Among evaluable patients, ORR was 56% (22% CR, 22% PR, and 12% clinical remission) (Table 2). Median DOR was 14.3 months (95% CI, 7.7-20.7) (supplemental Figure 3). ORRs were similar in patients who received previous HDT-ASCT and those who did not (ORR 56% vs 54%).
Primary and secondary outcomes after ibrutinib initiation, including treatment responses, DOR, discontinuations, dose reductions, median PFS, and OS
| Outcome . | Result . |
|---|---|
| Median PFS (95% CI), mo | 5.8 (4.5-10.1) |
| Median OS (95% CI), mo | 12.0 (7.7-19.0) |
| Best response, n (%) | |
| CR | 31 (21.8) |
| PR | 31 (21.8) |
| Clinical remission | 17 (12.0) |
| SD | 8 (5.6) |
| No clinical response | 14 (9.9) |
| PD | 41 (28.9) |
| Missing | 4 |
| ORR, % | 55.6 |
| Median DOR (95% CI) , mo | 14.3 (7.7-20.7) |
| Discontinuations, n (%) | 113 (77.4) |
| Discontinuation reason, n (%) | |
| Therapy-related toxicity | 28 (19.2) |
| Progression/no response | 70 (47.9) |
| Other reasons | 15 (10.3) |
| Median OS after ibrutinib discontinuation (95% CI), mo | 1.9 (1.2-5.4) |
| Discontinuation owing to insufficient response or progression | 1.3 (0.8-2.6) |
| Discontinuation owing to therapy-related toxicity or other reasons | 11.2 (1.7-20.3) |
| Any relapse or progression on ibrutinib, n (%) | 110 (75.3) |
| Outcome . | Result . |
|---|---|
| Median PFS (95% CI), mo | 5.8 (4.5-10.1) |
| Median OS (95% CI), mo | 12.0 (7.7-19.0) |
| Best response, n (%) | |
| CR | 31 (21.8) |
| PR | 31 (21.8) |
| Clinical remission | 17 (12.0) |
| SD | 8 (5.6) |
| No clinical response | 14 (9.9) |
| PD | 41 (28.9) |
| Missing | 4 |
| ORR, % | 55.6 |
| Median DOR (95% CI) , mo | 14.3 (7.7-20.7) |
| Discontinuations, n (%) | 113 (77.4) |
| Discontinuation reason, n (%) | |
| Therapy-related toxicity | 28 (19.2) |
| Progression/no response | 70 (47.9) |
| Other reasons | 15 (10.3) |
| Median OS after ibrutinib discontinuation (95% CI), mo | 1.9 (1.2-5.4) |
| Discontinuation owing to insufficient response or progression | 1.3 (0.8-2.6) |
| Discontinuation owing to therapy-related toxicity or other reasons | 11.2 (1.7-20.3) |
| Any relapse or progression on ibrutinib, n (%) | 110 (75.3) |
PD, progressive disease; SD, stable disease.
Survival
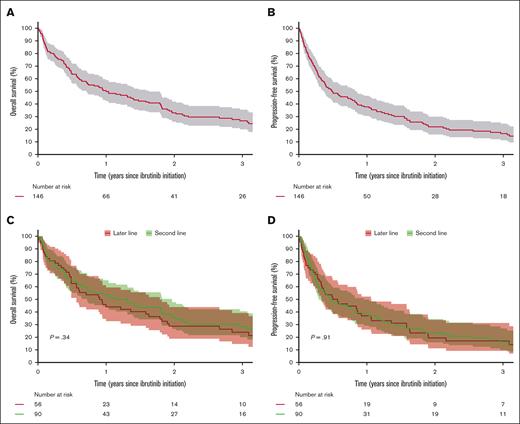
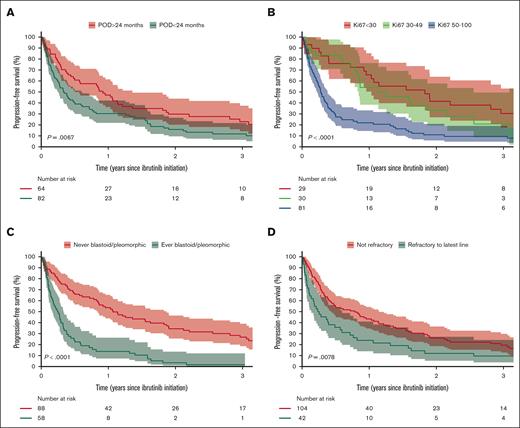
During follow-up, 113 patients (77%) died, of whom 74 (65%) died from MCL progression. A total of 110 patients (75%) relapsed or progressed after initiation of ibrutinib, of whom 65 (59%) received subsequent therapies (supplemental Table 4). Median OS from ibrutinib initiation was 12.0 months (95% CI, 8.5-19.5), and 3-year OS was 26% (95% CI, 20-36) (Figure 1A). Median PFS was 5.8 months (95% CI, 4.5-10.1), and 3-year PFS was 17% (95% CI, 11-24) (Figure 1B). Similar PFS and OS were observed between patients receiving ibrutinib in 2L and later lines, with median PFS of 5.6 months (95% CI, 3.5-11.5) for 2L and 6.4 months (95% CI, 4.0-12.8) for later lines and median OS of 13.7 months (95% CI, 8.6-23.6) for 2L vs 10.9 months (95% CI, 6.8-21.5) for later lines (Figure 1C-D). The age- and sex-adjusted hazard ratio (HR) for PFS between 2L vs later-line was 0.97 (95% CI, 0.75-1.26). There was no difference in PFS between patients receiving ibrutinib in combination with other therapies and monotherapy, with age- and sex-adjusted HR of 1.01 (95% CI, 0.68-1.50). Patients with Ki67 of ≥50% had worse survival than those with Ki67 of <30%, with median PFS of 3.3 months (95% CI, 2.7-4.6) vs 21.9 months (95% CI, 12.4-37.9) and median OS of 6.8 months (95% CI, 5.3-10.9) vs 31.9 months (95% CI, 21.8-66.3). The corresponding age- and sex-adjusted HR for PFS was 2.34 (95% CI, 1.47-3.71). There was no difference in PFS between patients with Ki67 of <30% and 30% to 49%, with age- and sex-adjusted HR of 1.11 (95% CI, 0.62-1.99) (Figure 2B). Patients with blastoid or pleomorphic MCL had inferior survival compared with those without with median PFS of 12.8 months (95% CI, 10.1-22.4) vs 3.0 months (95% CI, 2.0-4.0) and median OS of 5.7 months (95% CI, 4.5-8.7) vs 23.6 months (95% CI, 16.3-37.1) (Figure 2C; supplemental Figure 4C). The corresponding age- and sex-adjusted HR for PFS was 3.00 (95% CI, 2.04-4.41). PFS increased with increasing time from diagnosis to ibrutinib initiation with age- and sex-adjusted HR of 0.88 (95% CI, 0.82-0.95) per year (supplemental Table 5). Patients with refractory disease or POD24 from first-line therapy also had worse PFS and OS than those without (Figure 2A,D; supplemental Figure 4A,D). PFS was similar between patients receiving HDT-ASCT in first line and those who did not (supplemental Figure 5).
Survival from the time of first ibrutinib initiation. (A) OS, (B) PFS, (C) OS stratified by 2L vs later-line ibrutinib use, and (D) PFS stratified by 2L vs later-line ibrutinib use.
Survival from the time of first ibrutinib initiation. (A) OS, (B) PFS, (C) OS stratified by 2L vs later-line ibrutinib use, and (D) PFS stratified by 2L vs later-line ibrutinib use.
PFS stratification. (A) POD24 from first-line therapy, (B) Ki67 categories (highest ever before ibrutinib), (C) morphological subtypes (blastoid or pleomorphic vs other), and (D) refractory disease to the latest line of therapy before ibrutinib.
PFS stratification. (A) POD24 from first-line therapy, (B) Ki67 categories (highest ever before ibrutinib), (C) morphological subtypes (blastoid or pleomorphic vs other), and (D) refractory disease to the latest line of therapy before ibrutinib.
Crude comparison without IPW showed inferior PFS and OS in 2L ibrutinib patients compared with 2L comparators (supplemental Figure 6A-B). When comparing 2L ibrutinib with other 2L therapies after IPW, median PFS in 2L ibrutinib patients was 9.2 months (95% CI, 5.3-15.4) compared with 11.9 months (95% CI, 8.4-21.7) in 2L comparators (supplemental Figure 6C). In a sensitivity analysis, the exclusion of patients with CNS involvement at the time of 2L therapy did not change the results of crude or IPW analyses (data not shown).
Discontinuation
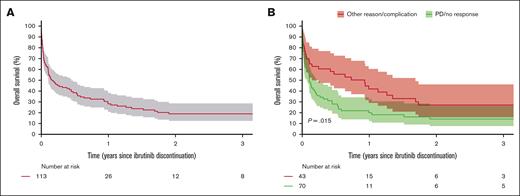
Median duration of ibrutinib treatment was 4.8 months (95% CI, 4.4-6.8), and 113 patients (77%) discontinued therapy. Discontinuation was caused by progression, relapse, or insufficient response in 70 patients (62%), AEs in 28 patients (25%), or other reasons (13%). Patients who discontinued ibrutinib owing to AEs were older with a median age of 77 years (interquartile range, 68-82) compared with 72 years (interquartile range, 68-72) for patients who did not. Median OS after discontinuation was 1.3 months (95% CI, 0.8-2.6) for discontinuation owing to progression, relapse, or insufficient treatment response and 11.2 months (95% CI, 1.7-20.3) for discontinuation owing to toxicities or other reasons (Figure 3). Patients who received active antilymphoma therapies after progression on ibrutinib showed superior OS compared with those who only received best supportive care (supplemental Figure 7).
OS after ibrutinib discontinuation. A) All cause discontinuation and B) stratified by reason for discontinuation (PD/no response versus other causes).
OS after ibrutinib discontinuation. A) All cause discontinuation and B) stratified by reason for discontinuation (PD/no response versus other causes).
AEs
In total, 99 patients (68%) experienced ≥1 AE, resulting in a total of 283 AEs, of which 209 (76%) were grade ≥3, occurring in 78 patients (53%). The 2-year cumulative incidence of AEs of grade ≥3 or SAE was 60% (95% CI, 52-68) (supplemental Figure 8). For 11 patients, death was assessed as related to the AE, of whom 6 also had progression at death. The AE-related deaths included 8 infections, 1 case of infection and acute myeloid leukemia, 1 case of gastrointestinal bleeding and concurrent infection, and 1 case of respiratory insufficiency.
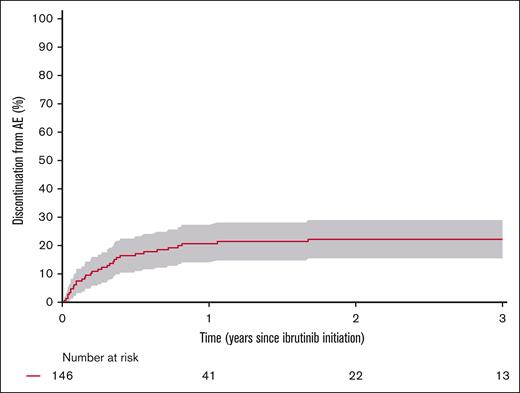
The most common AEs were infections with 121 events in 58 patients (40%), with pneumonia being the most frequent. Twenty-three patients (16%) had ≥1 microbiologically verified opportunistic infection, of whom 4 (3%) had Aspergillus, 5 (3%) Candida, 6 (4%) Enterobacter or Enterococcus species, 5 (3%) Pseudomonas, and 9 (6%) others. Bleeding was observed in 17 patients (12%), with 6 patients (4%) experiencing skin bleeding, 6 (4%) gastrointestinal bleeding, and 3 (2%) CNS bleeding. Atrial fibrillation was observed in 14 patients (10%), whereas 3 patients (2%) experienced heart failure and 6 (4%) syncope (supplemental Table 6). Dose reduction owing to AE was decided for 34 patients (23%). The 3-year cumulative incidence of dose reduction owing to AE was 22% (95% CI, 15-29) (supplemental Figure 9). The cumulative incidences of discontinuation owing to AE were 15% (95% CI, 10-21) at 1 year, 18% (95% CI, 13-26) at 2 years, and 19% (95% CI, 13-26) at 3 years (Figure 4). Using the univariate Andersen-Gill Cox model for recurrent AEs, the mean number of AEs for each patient at 4 years after ibrutinib initiation was 2.3 (95% CI, 1.9-2.7) (supplemental Figure 10).
Cumulative incidence of discontinuation owing to AEs from the time of ibrutinib initiation with death and relapse after ibrutinib initiation as competing events.
Cumulative incidence of discontinuation owing to AEs from the time of ibrutinib initiation with death and relapse after ibrutinib initiation as competing events.
Discussion
This study evaluated treatment responses, survival, and treatment adherence in patients with R/R MCL treated with ibrutinib in a nationwide population-based setting. We demonstrated an ORR of 56% compared with ORRs of 63% to 78% from clinical trials of ibrutinib monotherapy for R/R MCL.8,10,19 The survival estimates observed in the present study were also inferior to those observed in clinical trials with a median PFS of 6 months compared with 11 to 15 months in trials. Similarly, median OS was lower with 12 months than 20 to 27 months in trials.8,10,19-22 After the approval of ibrutinib, studies sought to improve the efficacy by combining ibrutinib with other therapies. In a single-arm phase 2 trial from a single center in the United States, patients with R/R MCL were treated with ibrutinib-R in combination. The study showed an ORR of 88%, indicating that this combination could lead to higher remission rates than ibrutinib monotherapy.23,24 Addition of lenalidomide to ibrutinib-R was investigated for R/R MCL in the Nordic countries in a single-arm phase 2 trial with an ORR of 76%.25 In the present study, 30% received ibrutinib in combination, but there was no difference in PFS between combination and monotherapy. Differences in treatment responses and survival between these trials and the present study may be explained by differences in patient populations. Patients included in the trials were younger with a median age of 67 to 69 years compared with 73 years in the present study, and blastoid morphology was observed in only 9% to 15% in the trials compared with our finding of 26%.8,10,21,23,25 In line with previous studies of ibrutinib, blastoid or pleomorphic morphology was associated with inferior survival in this study.22,26,27 Compared with most other studies, the proportion of patients with Ki67 of ≥30% was also higher (79% compared with 51%-68%), which likely contributes to the survival outcomes observed here.5,26-28 In line with the present study, the ibrutinib-R trial showed that Ki67 of ≥50% was associated with inferior PFS.23,24 Similarly, other observational studies found inferior PFS for those with Ki67 of ≥30%.28,29 In this study, 18 patients (12%) had CNS involvement, which could contribute to inferior survival, given that this was an exclusion criterion in trials. More patients with high-risk features in this study could also reflect that ibrutinib was chosen over other therapies for high-risk patients. This was supported by an increase in survival in patients receiving 2L ibrutinib after applying IPW to weigh high-risk features that may affect treatment allocation. After taking these high-risk features into account, PFS and OS were similar in patients receiving 2L ibrutinib compared with other 2L therapies.
In a pooled analysis of the ibrutinib monotherapy trials for R/R MCL, 2L ibrutinib was associated with superior survival compared with later-line use, which could not be confirmed in this study.20 Similar survival for patients treated with ibrutinib in second and later lines in this study is consistent with results in an observational study from the United States.30 Receiving ibrutinib in later lines could reflect more indolent disease or fitter patients, given that they have survived long enough to experience >1 relapse. The disparities in survival outcomes between clinical trials and the present real-world population-based study highlight that results from highly selected trial patients are less generalizable to patients with MCL treated in routine practice. Previous studies have shown that the efficacy found in trials does not necessarily translate to real-world effectiveness in a heterogeneous population, given that many patients with cancer are excluded from trials owing to age, comorbidities, performance status, frailty, or other reasons.31 One Danish study reported that 19% to 29% of patients with diffuse large B-cell lymphoma were not eligible for clinical trials and that these ineligible patients had inferior survival compared with the trial-eligible patients.32 Similarly, Baggio et al33 showed that, of 44 patients with R/R MCL treated with ibrutinib in Australia, 41% were not eligible for ibrutinib trials and had inferior survival.
Although this study shows major differences in response and survival between trials and real-world outcomes, the findings are not in line with other observational studies. McCulloch et al26 reported survival outcomes for 211 patients (median age, 73 years) after 2L ibrutinib for MCL at 38 centers in the United Kingdom. The study reported ORR of 69% and median PFS and OS of 17.8 months and 23.9 months, respectively, which are notably higher than our estimates. Other smaller studies from the United Kingdom, the Czech Republic, Spain, Italy, and the United States also showed superior treatment response and survival compared with this study with ORR of 62% to 78%, median PFS of 10 to 22 months, and median OS of 19 to 38 months.27,29,30,34-36 Similarly, median DOR in this study (14 months) was short compared with 17 to 46 months observed across trials and other observational studies.8,10,21,24,29,36 However, the study by Baggio et al33 found an ORR of 46% and median PFS and OS of 14 months and 16 months, respectively, which are closer to our estimates.
Apart from differences in high-risk features among the studies, conflicting findings could reflect different practices and availability of ibrutinib and postibrutinib therapies between countries and study periods. Previous studies only evaluated a small proportion of patients treated at selected centers, which could cause the selection of low-risk patients. In contrast, this study was nationwide with equal and free-of-charge health care access for all citizens and included the entire patient population treated with ibrutinib for R/R MCL. Inferior survival in the present study compared with previous reports could also reflect differences in the median duration of ibrutinib of only 4.8 months in the present study compared with 7.8 to 16 months in previous studies.10,21,24,28-30 In this study, cessation of ibrutinib was associated with a poor median OS of 1.9 months, mainly driven by progression, in line with previous studies reporting post-BTKi median OS of 2.5 to 13 months.36-41 In our study period, treatment options in this setting were limited, few patients received bispecific antibodies, and none received noncovalent BTKi or chimeric antigen receptor T-cell therapy, which have all shown promising efficacy in the postcovalent BTKi setting.42-44
Retrospective evaluation of ibrutinib is relevant for not only effectiveness but also toxicities. In this study, infections occurred in 40% compared with grade ≥3 infections in 22% to 29% across trials, and opportunistic infections occurred in 16%, which has previously been raised as potential complications of ibrutinib.8,11,24,25 Infections could also be attributable to the MCL or previous chemoimmunotherapy. Atrial fibrillation occurred in 10% in this study compared with grade ≥3 atrial fibrillation in 2% to 8% in most trials.8,10,11,20,25 However, the trial of ibrutinib-R reported grade 3 atrial fibrillation in 12% of patients with R/R MCL and 22% of older previously untreated patients with MCL.23 The higher risk in this study than in most trials might be explained by older patients or the high incidence of infections. Heart failure occurred in 2% in this study with no cases in trials, although heart failure has previously been linked to ibrutinib.45,46 The cardiac events highlight the importance of cardiac assessments before and during ibrutinib, particularly in older patients, although it was reassuring that we did not find any grade 5 cardiac AEs. Bleedings occurred slightly more frequently in this study (12%) than in the trials (5%-10%).8,10,11,20,23,24 Discontinuation owing to AEs occurred in 19% of patients in this study compared with 6% to 10% in trials, with dose reductions at 22% vs 4%.10,23,25 However, in the ibrutinib-R trial of older previously untreated patients with MCL, AE-related discontinuation occurred in 42%.47 These results suggest that older patients are less likely to tolerate ibrutinib, supported by the higher median age among those discontinuing owing to AEs in the present study. Observational studies from the United Kingdom and Italy reported lower rates than this study of AE-related discontinuation (5% and 12%) and dose reduction (10% and 12%).26,35 In contrast, our findings align with a US observational study showing AE-related discontinuation for 21% and dose reduction for 16%.30 Differences in treatment adherence between the studies could also represent differences in the availability of postibrutinib therapies between countries and time periods. Fewer available treatment options could increase willingness to manage toxicities without cessation. The AEs in this study could also result from preexisting comorbidities, for which data were not available. These findings highlight the need for real-world evaluations of toxicities in studies with long follow-up. When ibrutinib is implemented in first-line treatment for younger patients with MCL based on the TRIANGLE trial, similar studies of effectiveness and treatment adherence should be performed when follow-up is sufficient.12 This is also relevant for other BTKis to be able to select the optimal treatment for the individual patient, considering different toxicity profiles of BTKis.42,48,49
This study is, to our knowledge, the first to evaluate the effectiveness, survival, and AEs of ibrutinib for R/R MCL in a nationwide setting. Main strengths are the population-based design of a complete representative patient population, limiting selection bias, high-quality registry data, detailed information from health records, and long follow-up. The study was limited by a lack of data on TP53 alterations, which holds strong prognostic importance for MCL, given that it was only analyzed in a minority of included patients.50 Another limitation was the lack of central verification of Ki67, often measured by visual approximation, which is subject to interobserver variability.51 Comparison of 2L ibrutinib to other 2L therapies should be interpreted cautiously owing to the observational design. Treatment responses were only assessed clinically in some patients, limiting comparisons with trials. Finally, AEs were not systematically collected with prospective assessment and grading as in clinical trials, low-grade AEs were only included if they caused dose changes owing to a lack of registration in routine care, and causality with ibrutinib remains uncertain.
In conclusion, this study showed meaningful activity of ibrutinib for R/R MCL, but survival and response duration were limited and inferior to trial findings. There was no difference in survival between 2L ibrutinib and other 2L therapies. Ibrutinib failure was associated with poor survival, and there was no benefit from administering ibrutinib in 2L compared with later lines. Factors associated with inferior survival were Ki67 of ≥50%, blastoid or pleomorphic subtype, POD24, and refractory disease. Patients with these high-risk features should be considered for enrollment in clinical trials if possible. Finally, AE-related discontinuation occurred more frequently than in trials, perhaps indicating an older and more fragile population, emphasizing careful consideration and management of toxicities during ibrutinib, especially infections, bleedings, and cardiovascular toxicities.
Acknowledgments
The authors thank the clinical staff who collected and entered data for the Danish National Lymphoma Registry and the Danish Clinical Quality Program for the infrastructure of the Danish clinical quality registers.
This study was supported by Janssen Cilag as an investigator-initiated study; funding was paid to the institution (Rigshospitalet). The study was also supported by the Danish Cancer Society (grant numbers R378-A22530 and R381-A23024), the William Demant Foundation, and the Danish Lymphoma Group.
Janssen Cilag and its employees had no access to data or any role in the data management or analysis.
Authorship
Contribution: T.T., S.H., T.C.E.-G., and K.G. contributed to the conception and design of the study; T.T., I.C., A.L.A.-M., E.B.D., S.R., M. Johansen, M.R.C., T.S.L., J.H.C., R.S.P., M.F., C.B.P., L.M.H., T.C.E.-G., P.B., and K.G. contributed to the acquisition of data; T.T., I.C., A.L.A.-M., E.B.D., S.R., and M. Johansen directly accessed and collected health record data; T.T. managed and coordinated the responsibilities, verified the data, performed statistical analyses, and drafted the manuscript; M.R.S. contributed to statistical analyses and interpretation of data; M. Jerkeman contributed to the interpretation of the data and revision of the manuscript; and all authors interpreted the results, revised the manuscript, and read and approved the final manuscript.
Conflict-of-interest disclosure: T.T. received research support from Janssen as funding paid to the institution; and travel support from Immedica Pharma. A.L.A.-M. received research funding from Genentech. P.B. was on the advisory board of Roche, AbbVie, Bristol Myers Squibb, and SERB. T.S.L. received research support from Genentech; was on the advisory board of Roche, Bristol Myers Squibb, and Gilead; and received travel support from Gilead and Roche. M.R.C. was on the advisory board of AbbVie, AstraZeneca, Genmab, Gilead, Incyte, Janssen, and Roche; and received travel support from AbbVie, AstraZeneca, Genmab, Janssen, Pfizer, and Roche. M. Jerkeman received research support from Roche, AbbVie, AstraZeneca, and Bristol Myers Squibb; and honoraria from Kite/Gilead, Roche, AbbVie, Janssen, AstraZeneca, and Bristol Myers Squibb. K.G. received research support from Janssen as funding paid to the institution; and was a consultant for Otsuka Pharma and GlaxoSmithKline. The remaining authors declare no competing financial interests.
Correspondence: Kirsten Grønbæk, Department of Hematology, Rigshospitalet, Ole Maaløes Vej 5, Building 2, 3rd Floor, 2200 Copenhagen, Denmark; email: kirsten.groenbaek@regionh.dk.
References
Author notes
T.C.E.-G. and K.G. contributed equally as joint last authors.
Data are not available because we are not allowed to share individual-level data according to General Data Protection Regulation and the Danish Data Protection Act.
The full-text version of this article contains a data supplement.