Key Points
CSF reversibly attenuates the efficacy of antifolate drugs, including methotrexate, against leukemia cells.
Reduced proliferation and integrated stress response activation in leukemia cells in CSF may contribute to this resistance.
Visual Abstract
Treatment and prophylaxis of the central nervous system (CNS) is a standard component of acute lymphoblastic leukemia (ALL) therapy. However, CNS-directed therapies are a significant cause of morbidity, and CNS relapse remains a cause of treatment failure. CNS-directed ALL therapies must target leukemia cells within cerebrospinal fluid (CSF), a fluid that is compositionally distinct from plasma and has been shown to affect leukemia biology. Herein, we demonstrate that human CSF attenuates the potency and efficacy of antifolate drugs including methotrexate, the primary CNS-directed chemotherapeutic for >6 decades. Importantly, this effect of CSF on leukemia methotrexate sensitivity was reversible. Additional mechanistic studies support that diminished proliferation and activation of the integrated stress response in leukemia cells in the CSF may contribute to this resistance. Our findings suggest potential strategies to enhance methotrexate efficacy in CNS-directed ALL therapy and highlight the need to critically reassess even established standards of care.
Introduction
Acute lymphoblastic leukemia (ALL) involvement of the leptomeninges is common at diagnosis and relapse.1-3 As a result, central nervous system (CNS)–directed therapy and prophylaxis have been a critical component of ALL treatment since, starting in the late 1950s, antifolates such as methotrexate were administered directly into the cerebrospinal fluid (CSF) by intrathecal (IT) injection.4 The blood-brain barrier and neurotoxicity risks have hindered the development of many CNS-directed therapy alternatives to methotrexate.5,6 Although cranial radiation bypasses the blood-brain barrier and is an effective therapy for CNS leukemia, its use is accompanied by significant acute and long-term toxicities.7 Consequently, methotrexate has remained the standard of care for >6 decades despite failing to prevent CNS relapse in some patients and risks for methotrexate-related neurotoxicity.1,2
Developing more efficacious and less toxic CNS-directed leukemia therapies requires defining how the leptomeningeal microenvironment influences leukemia biology. Studies using patient-derived xenograft models of ALL or limited patient samples have identified significant transcriptional, proteomic, and metabolic differences between leukemia cells isolated from the leptomeninges/CSF and the bone marrow.8-14 In addition, targeting these pathways enriched in leukemia cells within the CNS has shown promise in preclinical models, emphasizing the need for tailored CNS-directed ALL therapies.8,9,11-13
The distinctive effects the leptomeningeal niche on leukemia cells may be attributed, at least in part, to the unique composition of the CSF that bathes the leptomeninges.15 Notably, CSF has significantly lower levels of protein, lipids, and glucose than plasma. This unique composition potentially influences leukemia cell behavior, as evidenced by prior studies demonstrating decreased proliferation and increased cell death of leukemia cells in the CSF.16
Herein, we postulated that the unique composition of CSF may also modulate leukemia drug sensitivity patterns. To investigate this hypothesis, we conducted leukemia drug testing in human CSF and unexpectedly discovered that CSF attenuates the efficacy of methotrexate, the long-standing foundation of CNS leukemia treatment and prophylaxis.
Methods
Cell culture
Leukemia cell lines were from the American Type Culture Collection or the Leibniz Institute DSMZ-German Collection of Microorganisms and Cell Cultures, and were cultured in RPMI 1640 media supplemented with fetal bovine serum (FBS; Seradigm) 10% and penicillin-streptomycin. Dialyzed FBS was from R&D Systems. The A549 lung carcinoma cell line was provided by Martin Felices (University of Minnesota). Human CSF was obtained from 3 different sources: (1) pooled CSF from donors (Medix Biochemica USA), (2) patients with extraventricular drains at the University of Minnesota Masonic Children’s Hospital (intuitional review board approval #00001210), and (3) pediatric patients with leukemia in remission undergoing routine lumbar punctures during the maintenance phase of therapy (intuitional review board approval #1512M80866). All pediatric patients with leukemia had received systemic and IT methotrexate as part of their therapy, with the most recent IT dose administered ∼3 months before CSF collection. CSF was filtered and centrifuged to remove any cellular components or debris and then used undiluted or stored at −80°C.
Drugs and reagents
Methotrexate, pralatrexate, trimetrexate, raltitrexed, cytarabine, clofarabine, doxorubicin, L-asparaginase, brefeldin A, palbociclib, tunicamycin, and 5-methyltetrahydrofolic acid were purchased from MedChemExpress. Talotrexin and piritrexim were purchased from TargetMol. Fluorescein-methotrexate was purchased from Fisher Scientific.
Proliferation, apoptosis, protein synthesis, and cell cycle analyses
To assess proliferation/viability, leukemia cells were cultured in 96-well plates and then viability assessed with the CellTiter-Glo Luminescent cell viability assay (PromegaI) and a Tecan Infinite M200 Pro plate reader. All experiments were performed with at least 3 wells per condition. To assess apoptosis and cell death, leukemia cells were stained with annexin-V antibody (eBioscience), a fixable viability dye (ThermoFisher Scientific), and analyzed by flow cytometry using a BD Accuri C6, Canto, or Symphony instrument. Protein synthesis was assessed using the Click-iT Plus OPP Alexa Fluor 488 protein synthesis assay kit (ThermoFisher Scientific) according to the manufacturer’s instructions. Cell cycle and proliferation were assessed using the Click-iT Plus EdU flow cytometry kit (ThermoFisher Scientific) according to the manufacturer’s instructions.
Fluoroscein-conjugated methotrexate
Leukemia cells were cultured in regular media with fluoroscein-conjugated methotrexate 2.5 μM for 2 hours. The leukemia cells were then washed 3 times with phosphate-buffered saline and resuspended in either CSF or regular media. The leukemia cells were then treated with regular, unlabeled methotrexate 500 nM or dimethyl sulfoxide (DMSO) and leukemia cell fluorescence assessed by flow cytometry at 0, 30, 45, 60, 90, and 120 minutes.
Protein isolation and immunoblotting
Leukemia cell lysates for immunoblotting were prepared by incubating cells in radioimmunoprecipitation assay buffer (ThermoFisher Scientific) with protease and phosphatase inhibitors (Halt protease and phosphatase inhibitor cocktail, ThermoFisher) for 20 minutes. Supernatants were collected after centrifuging (17 000g at 4°C) for 15 minutes. Protein concentrations were determined using the Rapid Gold bicinchoninic acid (BCA) protein assay kit (Pierce). Sodium dodecyl sulfate–polyacrylamide gel electrophoresis was used to separate proteins, which were then transferred to polyvinylidene difluoride membranes (Millipore). Antibodies to the following proteins were used in the immunoblots: phosphorylated eukaryotic translation initiation factor 2 α (phospho-eIF2α [Ser51]; Cell Signaling, catalog no. 3597, 1:1000), eIF2α (Cell Signaling, catalog no. 9722, 1:1000), dihydrofolate reductase (DHFR; Cell Signaling, catalog no. 43497, 1:1000), thymidylate synthase (TYMS; Cell Signaling, catalog no. 5449, 1:1000), AICAR transformylase (ATIC; Sigma-Aldrich, HPA021012, 1:1000), and β-actin (Cell Signaling, catalog no. 4970, 1:1000), goat anti-mouse immunoglobulin G Alexa Fluor Plus 680 (Invitrogen), and goat anti-rabbit Alexa Fluor Plus 800 (Invitrogen). Blot imaging was performed using a LI-COR Imaging instrument.
Lentiviral production and transduction of leukemia cells
Lentiviral vectors and particles (>108 transduction units per mL) expressing human DHFR (containing L22F and F31S mutations) and TYMS (containing T51S and G52S mutations) under control of the spleen focus-forming virus (SFFV) promoter were purchased from VectorBuilder (VectorBuilder Inc). The vectors also expressed either enhanced green fluorescent protein (DHFR) or mCherry (TYMS) fluorescent proteins and puromycin to facilitate cell selection. Vectors and mutations were verified by sequencing. Leukemia cells adhered to retronectin-coated tissue culture plates were transduced with virus particles (a multiplicity of infection of 10) for 48 hours. Transduced cells were then selected with puromycin and sorted by flow cytometry (BD fluorescence-activated cell sorter Aria II) for green fluorescent protein or mCherry expression.
Mass spectrometry
Methotrexate polyglutamate species were quantitated by the University of Minnesota Center for Metabolomics and Proteomics using liquid chromatography–tandem mass spectrometry (LC-MS/MS) based on a previously described protocol.17 Methotrexate polyglutamate standards (MTXPG2-7) and deuterated MTXPG5 were obtained from Toronto Research Chemicals and dissolved in methanol. Jurkat cells (5 × 105 cells per mL) were cultured with methotrexate 215 nM in CSF or regular media. After 24 hours, cells were washed twice with phosphate-buffered saline, and viable cells (5 × 106 cells per sample) pelleted and stored at −80°C. Cell pellets were lysed and a deuterated MTXPG5-d3 internal standard added at a final concentration of 0.1 ng/mL. Proteins were then precipitated with ice-cold acetonitrile and methanol solution (90:10) and centrifugation. The supernatant was evaporated to dryness. Samples were reconstituted in mobile phase A, vortexed for 1 minute, placed in an ultrasonic bath for 10 minutes, centrifuged for 15 minutes at 14 000g, and supernatant transferred to an autosampler vial. Samples were then analyzed on a QTrap 6500+ LC-MS/MS system (Sciex). Chromatography was performed with a Waters Acquity BEH C18 Column (300Å, 1.7 μm, 1 mm × 50 mm) at 55°C. The mobile phase consisted of (A) 10 mM ammonium acetate, pH 10; and (B) methanol. A flow rate of 10 μL/min was maintained and the analytes were eluted with the following program: 0 to 1 minutes, isocratic hold 5% B; 1 to 8 minutes, linear gradient 5% to 60% B; 8 to 8.5 minutes, isocratic hold 60% B; 8.5 to 9 minutes, linear gradient 60% to 95% B; 9 to 9.5 minutes, isocratic hold 95% B; and 9.5 to 10 minutes, 95% to 5% B. The mass spectrometer used an Optiflow Turbo V electron spray ionization ion source operating in positive ionization mode. The gas temperature was maintained at 350°C and the ionization spray voltage was 4.5 kV. Multiple reaction monitoring mode was used, and peaks integrated using Skyline. Quantification was based on the ratio of area counts of analyte to internal standard.
Statistical analysis
Results are the mean ± standard deviation. All experiments were performed at least twice, and most often at least 3 times, with representative data of 1 experiment presented. Student t test or analysis of variance were used for statistical comparisons. P values <.05 were considered statistically significant. All graphing, curve fitting (nonlinear regression, sigmoidal, 4-parameter logistic, X is log[concentration]), and statistical significance testing were performed using GraphPad Prism 10.1.1 software (GraphPad Software, La Jolla, CA).
Results
CSF attenuates the efficacy of antifolates
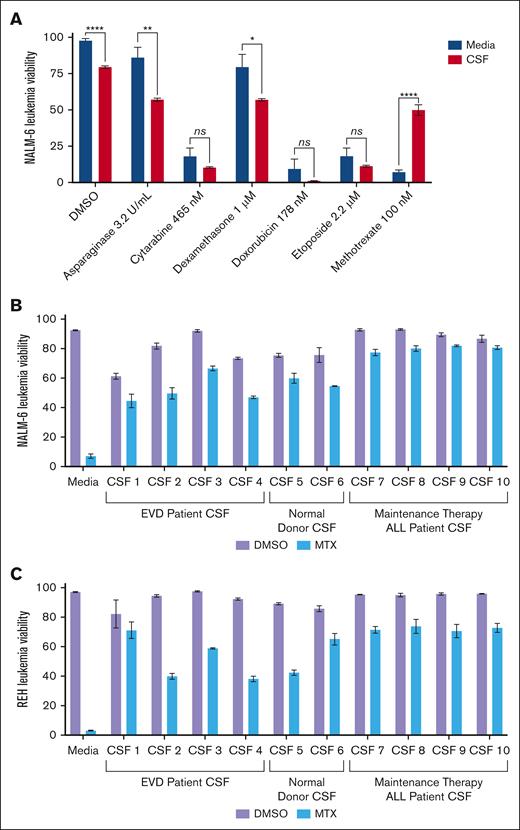
We screened the efficacy of multiple chemotherapeutics used in ALL therapy in both regular tissue culture media (RPMI 1640 with 10% fetal calf serum) and human CSF with the NALM-6 (B-cell ALL) cell line. Leukemia cell viability was assessed after 48 hours of treatment with DMSO control, asparaginase, cytarabine, dexamethasone, doxorubicin, etoposide, or methotrexate (Figure 1A). Drug concentrations of ∼75% inhibitory concentration (IC75) were used except for asparaginase and dexamethasone because of the more limited potency of these drugs. Similar to prior results, NALM-6 cells showed decreased viability in CSF relative to regular media in the absence of any drug (DMSO control).16 Strikingly, NALM-6 cells showed significant resistance to methotrexate in CSF relative to regular media, whereas for the other chemotherapeutics tested NALM-6 sensitivity was either unchanged or modestly increased in CSF. To ensure that the methotrexate resistance was not a unique feature of the CSF sample or leukemia cell line, we tested methotrexate efficacy with REH (B-cell ALL) and NALM-6 cells in 10 different CSF samples obtained from patients with extraventricular CSF drains, patients with ALL in remission undergoing routine lumbar punctures during maintenance therapy, and pooled CSF from normal donors (Figure 1B-C). All CSF samples significantly attenuated the efficacy of methotrexate, although to varying degrees, compared with tissue culture media for both NALM-6 and REH leukemia cells.
Effects of CSF on ALL drug sensitivity. (A) NALM-6 cells (B-cell ALL) were treated with asparaginase, cytarabine, dexamethasone, doxorubicin, etoposide, or methotrexate in either CSF or regular tissue culture media. Leukemia cell viability was assessed after 48 hours of drug treatment using fixable viability dye staining and flow cytometry. Error bars represent the mean ± standard deviation (SD) of 3 technical replicates. ∗P < .05; ∗∗P < .01; ∗∗∗∗P < .0001 by t test. (B-C) NALM-6 (B) and REH (C) cells were treated with methotrexate 220 nM and 180 nM, respectively, or DMSO in regular media or CSF obtained from patients with extraventricular CSF drains (CSF 1-4), normal donors (CSF 5-6), or patients with ALL undergoing routine lumbar punctures during maintenance therapy (CSF 7-10). Leukemia cell viability was assessed after 48 hours of drug treatment using annexin-V and viability dye staining and flow cytometry. Error bars represent the mean ± SD of 3 technical replicates. When comparing methotrexate toxicity in media vs each CSF sample, P < .0001 by analysis of variance with post hoc Dunnett multiple comparisons test. EVD, extraventricular drain; ns, not significant.
Effects of CSF on ALL drug sensitivity. (A) NALM-6 cells (B-cell ALL) were treated with asparaginase, cytarabine, dexamethasone, doxorubicin, etoposide, or methotrexate in either CSF or regular tissue culture media. Leukemia cell viability was assessed after 48 hours of drug treatment using fixable viability dye staining and flow cytometry. Error bars represent the mean ± standard deviation (SD) of 3 technical replicates. ∗P < .05; ∗∗P < .01; ∗∗∗∗P < .0001 by t test. (B-C) NALM-6 (B) and REH (C) cells were treated with methotrexate 220 nM and 180 nM, respectively, or DMSO in regular media or CSF obtained from patients with extraventricular CSF drains (CSF 1-4), normal donors (CSF 5-6), or patients with ALL undergoing routine lumbar punctures during maintenance therapy (CSF 7-10). Leukemia cell viability was assessed after 48 hours of drug treatment using annexin-V and viability dye staining and flow cytometry. Error bars represent the mean ± SD of 3 technical replicates. When comparing methotrexate toxicity in media vs each CSF sample, P < .0001 by analysis of variance with post hoc Dunnett multiple comparisons test. EVD, extraventricular drain; ns, not significant.
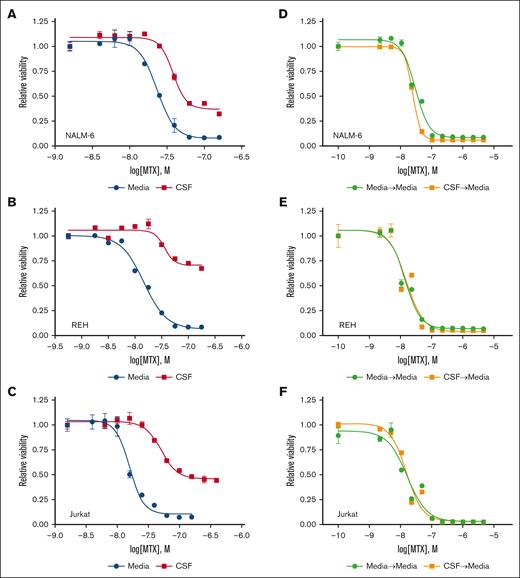
We then generated methotrexate dose-response curves for REH, NALM-6, and Jurkat (T-cell ALL) cells in CSF and regular media to better characterize the effect of CSF on methotrexate sensitivity (Figure 2A-C; Supplemental Table 1). For all leukemia cell lines, the methotrexate dose-response curve, and corresponding IC50, was shifted to higher methotrexate concentrations in CSF relative to media. This shift in IC50 is indicative of decreased methotrexate potency in CSF. CSF also significantly attenuated the toxicity of methotrexate at maximal drug doses relative to media, indicative of reduced overall efficacy in CSF. Together, these data suggest that CSF modulates both the potency and efficacy of methotrexate against leukemia cells. Notably this effect of CSF on methotrexate sensitivity was reversible because leukemia cells cultured in CSF for 48 hours but then returned to tissue culture media regained their sensitivity to methotrexate (Figure 2D-F).
CSF attenuates leukemia cell sensitivity to methotrexate. (A-C) Methotrexate dose-response curves for NALM-6 (A), REH (B), and Jurkat (C) leukemia cells in either regular media or CSF. Leukemia cell viability was assessed after 48 hours of drug treatment using the CellTiter-Glo luminescent cell viability assay, which quantitates adenosine triphosphate as an indicator of viable and metabolically active cells. Error bars represent the mean ± SD of 3 technical replicates. LogIC50 and bottom values with confidence intervals were calculated from the dose-response curves and are shown in the supplemental Table. (D-F) Methotrexate dose-response curves for NALM-6 (D), REH (E), and Jurkat (F) leukemia cells cultured in regular media after 48 hours of preculture in either regular media or CSF. Leukemia cell viability was assessed after 48 hours of drug treatment using the CellTiter-Glo luminescent cell viability assay. Error bars represent the mean ± SD of 3 technical replicates.
CSF attenuates leukemia cell sensitivity to methotrexate. (A-C) Methotrexate dose-response curves for NALM-6 (A), REH (B), and Jurkat (C) leukemia cells in either regular media or CSF. Leukemia cell viability was assessed after 48 hours of drug treatment using the CellTiter-Glo luminescent cell viability assay, which quantitates adenosine triphosphate as an indicator of viable and metabolically active cells. Error bars represent the mean ± SD of 3 technical replicates. LogIC50 and bottom values with confidence intervals were calculated from the dose-response curves and are shown in the supplemental Table. (D-F) Methotrexate dose-response curves for NALM-6 (D), REH (E), and Jurkat (F) leukemia cells cultured in regular media after 48 hours of preculture in either regular media or CSF. Leukemia cell viability was assessed after 48 hours of drug treatment using the CellTiter-Glo luminescent cell viability assay. Error bars represent the mean ± SD of 3 technical replicates.
To assess whether this effect of CSF on methotrexate efficacy was generalizable to other antifolates, we next generated dose-response curves for the antifolates pralatrexate and raltitrexed in regular media and CSF. As with methotrexate, CSF attenuated the potency and efficacy of both drugs against leukemia cell lines, although the extent of the effect did differ between drugs and cell lines (supplemental Figure 1; supplemental Table). For example, although the efficacy of pralatrexate significantly diminished in CSF for all 3 ALL cell lines tested, the IC50 for pralatrexate in CSF only increased in 1 cell line. Both the potency and maximal efficacy of raltitrexed was attenuated in CSF for all 3 cell lines.
Mechanisms of methotrexate resistance
Next, we sought to investigate the mechanism by which CSF affects methotrexate resistance in ALL. Multiple mechanisms of methotrexate resistance in both ALL and other cancers are well described and include impaired import, dysregulated expression of methotrexate target proteins, diminished intracellular retention secondary to alterations in polyglutamylation and increased efflux, folate analog rescue, and changes in cell cycle and proliferation.18,19
Changes in leukemia cell proliferation
Leukemia cells proliferate slowly in CSF ex vivo (supplemental Figure 2) and in vivo when assessed in a small number of patients with ALL after the administration of tritiated thymidine directly into the CSF via cerebroventricular injection.8,16,20,21 This reduced proliferation may render leukemia cells less susceptible to antimetabolites, such as methotrexate, which inhibit the synthesis of purines, thymidylate, and amino acids such as methionine required for actively dividing and proliferating cells.22,23 To address this possibility, we first tested the efficacy of cytarabine and clofarabine in CSF. Cytarabine and clofarabine are pyrimidine and purine antimetabolites, respectively, used in ALL therapy. Unlike methotrexate, leukemia cells were equally sensitive to both cytarabine and clofarabine in CSF and regular media (supplemental Figure 3; supplemental Table). These data suggest that this effect of CSF on methotrexate does not generally extend to all drugs targeting actively dividing cells.
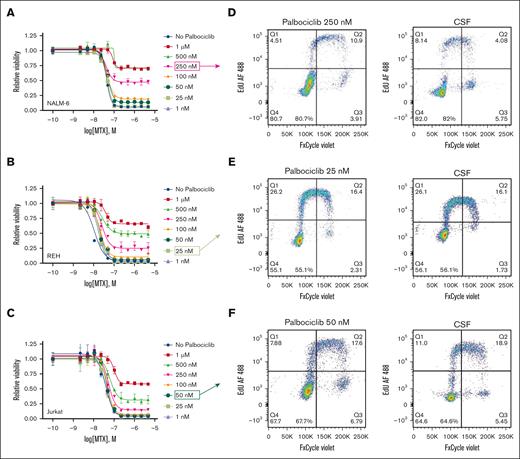
We then used the cyclin-dependent kinase 4/6 inhibitor palbociclib to trigger a G1 cell cycle arrest in ALL cells. Palbociclib as a single agent did not cause leukemic cell death but decreased proliferation (supplemental Figure 4). Palbociclib, at higher doses, also significantly decreased methotrexate potency and efficacy in a dose-dependent manner that was similar in extent to that of CSF (Figure 3A-C). However, the effect of CSF on methotrexate resistance was more pronounced than the palbociclib dose that exerted roughly comparable effects on cell cycle (Figures 2 and 3D-F). This was particularly seen for the REH and Jurkat cell lines in which palbociclib doses that caused cell cycle effects similar to CSF had only a minimal effect on methotrexate efficacy. Together these data suggest that effects of CSF on ALL proliferation may influence methotrexate sensitivity, but this is unlikely to be the sole explanation.
Palbociclib decreases leukemia cell proliferation and attenuates leukemia cell sensitivity to methotrexate. (A-C) Methotrexate dose-response curves for NALM-6 (A), REH (B), and Jurkat (C) leukemia cells in the absence or presence of palbociclib at varying concentrations. Leukemia cell viability was assessed after 48 hours of drug treatment using the CellTiter-Glo luminescent cell viability assay. Error bars represent the mean ± SD of 3 technical replicates. (D-F) Cell cycle analysis of NALM-6 (D), REH (E), and Jurkat (F) leukemia cells cultured in either CSF or regular media with a palbociclib concentration that caused a level of G0/G1 arrest similar to CSF. Leukemia cells were treated with EdU (5-ethynyl 2’-deoxyuridine) for 30 minutes before fixation, permeabilization, staining, and analysis by flow cytometry. Representative flow cytometry histograms for both CSF and the comparable palbociclib dose are shown. AF, Alexa Fluor.
Palbociclib decreases leukemia cell proliferation and attenuates leukemia cell sensitivity to methotrexate. (A-C) Methotrexate dose-response curves for NALM-6 (A), REH (B), and Jurkat (C) leukemia cells in the absence or presence of palbociclib at varying concentrations. Leukemia cell viability was assessed after 48 hours of drug treatment using the CellTiter-Glo luminescent cell viability assay. Error bars represent the mean ± SD of 3 technical replicates. (D-F) Cell cycle analysis of NALM-6 (D), REH (E), and Jurkat (F) leukemia cells cultured in either CSF or regular media with a palbociclib concentration that caused a level of G0/G1 arrest similar to CSF. Leukemia cells were treated with EdU (5-ethynyl 2’-deoxyuridine) for 30 minutes before fixation, permeabilization, staining, and analysis by flow cytometry. Representative flow cytometry histograms for both CSF and the comparable palbociclib dose are shown. AF, Alexa Fluor.
Alterations in methotrexate uptake, retention, or efflux
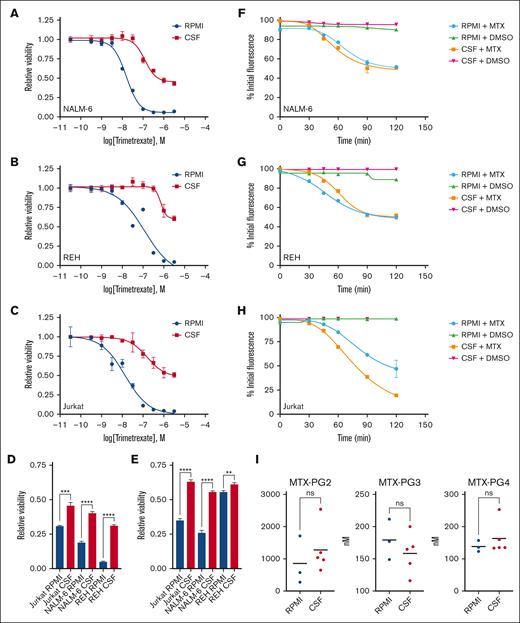
Methotrexate, a close structural analog of folate, enters cells primarily via the reduced folate carrier (RFC/SLC19A1).24 After cell entry, methotrexate is reversibly polyglutamylated to enhance its binding to target enzymes and prevent cellular efflux by various ATP-binding cassette (ABC) transporters. We used several complementary approaches to assess the potential role of methotrexate influx/efflux in CSF-mediated methotrexate resistance. First, trimetrexate is an antifolate that does not require the reduced folate carrier for uptake or polyglutamylation for cellular retention.19 Accordingly, we generated dose-response curves for trimetrexate in regular media and CSF. As shown in Figure 4A-C and supplemental Table, CSF diminished trimetrexate potency and efficacy against leukemia cells. Further supporting this result, leukemia cells also showed resistance to 2 additional nonpolyglutamatable antifolates (talotrexin and piritrexim19) in CSF relative to media (Figure 4D-E). Second, we used fluorescein-conjugated methotrexate to further assess methotrexate uptake and retention.25 Leukemia cells were loaded with fluorescein-conjugated methotrexate in regular media, washed, transferred to either regular media or CSF, and unlabeled methotrexate or DMSO added. Leukemia cell fluorescence was then measured at multiple time points by flow cytometry. If the unlabeled methotrexate is taken up by the cells, it competes with the fluorescein-conjugated methotrexate for cellular retention and ALL cell fluorescence decreases with time. As shown in Figure 4F-H, DMSO had no effect on leukemia cell fluorescence, but leukemia cell fluorescence diminished at a similar rate in both media and CSF in the presence of unlabeled methotrexate. These data are consistent with effective methotrexate uptake by ALL cells in both media and CSF. Finally, we used LC-MS/MS to measure methotrexate polyglutamate species in leukemia cells treated with methotrexate for 24 hours, and before the onset of significant cell death, in either regular media or CSF. Because of the large volume of CSF required for this experiment, we tested only a single leukemia cell line (Jurkat). We were able to measure methotrexate polyglutamate species 2 to 4 and observed no significant differences in levels between Jurkat cells from media or CSF (Figure 4I). Together these data do not support that significant changes in methotrexate uptake, retention, or efflux are occurring in leukemia cells in CSF.
CSF does not influence methotrexate uptake and retention by leukemia cells. (A-C) Trimetrexate dose-response curves for NALM-6 (A), REH (B), and Jurkat (C) leukemia cells treated in either regular media or CSF. Leukemia cell viability was assessed after 48 hours of drug treatment using the CellTiter-Glo luminescent cell viability assay. Error bars represent the mean ± SD of 3 technical replicates. (D-E) Leukemia cells were treated with talotrexin 1 μm (D) or piritrexim 1 μM (E) in either regular tissue culture media or CSF. Leukemia cell viability was assessed after 48 hours of drug treatment using the CellTiter-Glo luminescent cell viability assay. Error bars represent the mean ± SD of 3 technical replicates. ∗∗P < .01; ∗∗∗P < .001; and ∗∗∗∗P < .0001 by t test. (F-H) Fluorescent methotrexate retention in leukemia cells. NALM-6 (F), REH (G), and Jurkat (H) cells loaded with fluorescein-conjugated methotrexate were treated with unlabeled methotrexate 500 nM or DMSO in regular media or CSF. Leukemia cell fluorescence was then measured by flow cytometry at 0, 30, 45, 60, 90, and 120 minutes. Error bars represent the mean ± SD of 3 technical replicates. (I) Methotrexate polyglutamate species 2-4 were measured using LC-MS/MS in Jurkat leukemia cells after treatment with methotrexate 215 nM for 24 hours in either regular media or CSF. ns, not significant by Student t test.
CSF does not influence methotrexate uptake and retention by leukemia cells. (A-C) Trimetrexate dose-response curves for NALM-6 (A), REH (B), and Jurkat (C) leukemia cells treated in either regular media or CSF. Leukemia cell viability was assessed after 48 hours of drug treatment using the CellTiter-Glo luminescent cell viability assay. Error bars represent the mean ± SD of 3 technical replicates. (D-E) Leukemia cells were treated with talotrexin 1 μm (D) or piritrexim 1 μM (E) in either regular tissue culture media or CSF. Leukemia cell viability was assessed after 48 hours of drug treatment using the CellTiter-Glo luminescent cell viability assay. Error bars represent the mean ± SD of 3 technical replicates. ∗∗P < .01; ∗∗∗P < .001; and ∗∗∗∗P < .0001 by t test. (F-H) Fluorescent methotrexate retention in leukemia cells. NALM-6 (F), REH (G), and Jurkat (H) cells loaded with fluorescein-conjugated methotrexate were treated with unlabeled methotrexate 500 nM or DMSO in regular media or CSF. Leukemia cell fluorescence was then measured by flow cytometry at 0, 30, 45, 60, 90, and 120 minutes. Error bars represent the mean ± SD of 3 technical replicates. (I) Methotrexate polyglutamate species 2-4 were measured using LC-MS/MS in Jurkat leukemia cells after treatment with methotrexate 215 nM for 24 hours in either regular media or CSF. ns, not significant by Student t test.
Altered expression of methotrexate target proteins
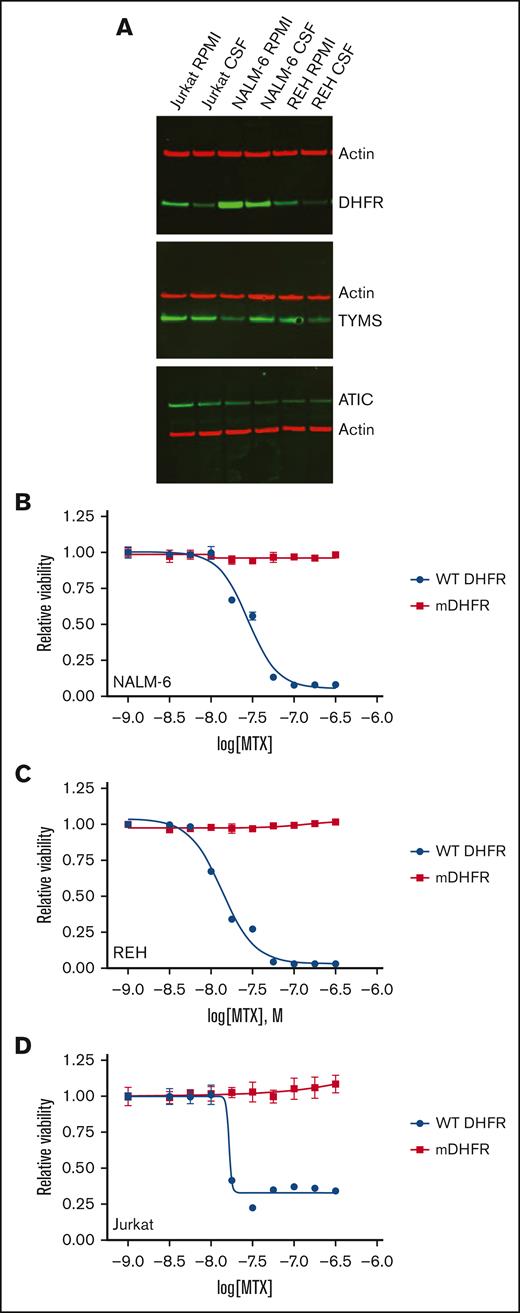
Methotrexate primarily inhibits DHFR, which converts DHF to tetrahydrofolate (THF). THF, and derivatives including the primary biologically active form 5-methyltetrahydrofolic acid (5-MTHF), serve as cofactors for many 1-carbon metabolic reactions.24 In addition to DHFR, methotrexate also directly inhibits TYMS and ATIC, which are involved in the de novo synthesis of thymidylate and purines, respectively. Overexpression of DHFR or other methotrexate target proteins can enable methotrexate resistance. To test this possibility, we compared expression of DHFR, ATIC, and TYMS proteins in media and CSF (Figure 5A). None of these proteins showed increased expression in CSF relative to media, with the exception of TYMS for the NALM-6 cell line. In fact, expression of DHFR, the primary methotrexate target, decreased in leukemia cells cultured in CSF. Although an increase rather than decrease in DHFR has been typically shown to enable methotrexate resistance,19 we assessed whether this decrease in DHFR protein expression could affect methotrexate sensitivity using short hairpin RNAs to attenuate DHFR levels in NALM-6, Jurkat, and REH leukemia cell lines. However, significantly decreasing DHFR levels in leukemia cells caused only a small increase in methotrexate potency and had no effect on overall efficacy, suggesting the moderate decrease in DHFR levels observed in CSF were not significantly contributing to methotrexate resistance (supplemental Figure 5).
Impact of CSF on the expression of methotrexate target proteins in leukemia cells. (A) Immunoblots showing the effects of CSF on methotrexate target proteins. Leukemia cell lines were cultured in regular media or CSF for 48 hours. Protein lysates were then collected for immunoblotting with DHFR, TYMS, ATIC, or β-actin antibodies. (B-D) Dose-response curves for wild-type (WT) DHFR or mutant DHFR (mDHFR; L22F, F31S) NALM-6 (B), REH (C), and Jurkat (D) leukemia cells treated with different concentrations of the methotrexate. Leukemia cell viability was assessed after 48 hours of drug treatment using the CellTiter-Glo luminescent cell viability assay. Error bars represent the mean ± SD of 3 technical replicates. LogIC50 and bottom values with confidence intervals were calculated from the dose-response curves and are shown in the supplemental Table.
Impact of CSF on the expression of methotrexate target proteins in leukemia cells. (A) Immunoblots showing the effects of CSF on methotrexate target proteins. Leukemia cell lines were cultured in regular media or CSF for 48 hours. Protein lysates were then collected for immunoblotting with DHFR, TYMS, ATIC, or β-actin antibodies. (B-D) Dose-response curves for wild-type (WT) DHFR or mutant DHFR (mDHFR; L22F, F31S) NALM-6 (B), REH (C), and Jurkat (D) leukemia cells treated with different concentrations of the methotrexate. Leukemia cell viability was assessed after 48 hours of drug treatment using the CellTiter-Glo luminescent cell viability assay. Error bars represent the mean ± SD of 3 technical replicates. LogIC50 and bottom values with confidence intervals were calculated from the dose-response curves and are shown in the supplemental Table.
Finally, to better understand the relative contribution of DHFR and TYMS inhibition to the ability of methotrexate to target leukemia cells, we overexpressed methotrexate-resistant mutants of DHFR (L22F and F31S mutations) and TYMS (T51S and G52S mutations) in leukemia cells. Leukemia cells expressing the methotrexate-resistant DHFR mutant were completely resistant to methotrexate (Figure 5B-D). In contrast, cells expressing the methotrexate-resistant TYMS mutant retained methotrexate sensitivity (supplemental Figure 6). These data suggest that DHFR is the most critical target in leukemia cells during short term (48 hour) exposure to methotrexate and, combined with the DHFR protein expression data, argue against changes in DHFR expression contributing to CSF-mediated methotrexate resistance.
Methotrexate rescue by metabolically active folate species
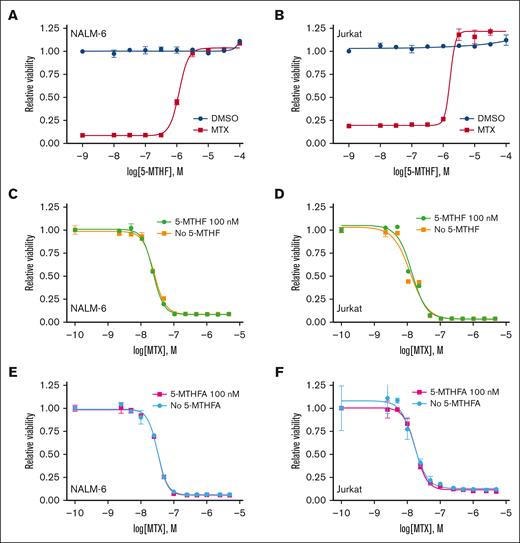
Inhibition of DHFR prevents the generation of metabolically active forms of folate. As a result, exogenous activated folate species can rescue the effects of methotrexate. 5-MTHF is the primary metabolically active form of folate in plasma and CSF.26 5-MTHF dose-response curves were generated in the presence of high-dose methotrexate (∼IC80-90) or DMSO control (Figure 6A-B). 5-MTHF had no effect on baseline leukemia cell proliferation but was able to completely rescue methotrexate toxicity in a dose-dependent fashion. However, the 5-MTHF concentration required for rescue was significantly higher than found in CSF (∼100 nM).27,28 In agreement, physiologically relevant 5-MTHF 100 nM was insufficient to rescue methotrexate toxicity (Figure 6C-D). Similar results were observed in folate-free RPMI 1640 media and dialyzed FBS, suggesting that folic acid present in regular RPMI 1640 (∼ 2.3 μM) and unmodified FBS were not confounding the previous results (Figure 6E-F).
High concentrations of a metabolically active folate derivative rescue methotrexate toxicity. (A-B) Dose-response curves for NALM-6 (A) and Jurkat (B) leukemia cells treated with different concentrations of the folate derivative 5-MTHF in either the absence or presence of methotrexate. Leukemia cell viability was assessed after 48 hours of drug treatment using the CellTiter-Glo luminescent cell viability assay. Error bars represent the mean ± SD of 3 technical replicates. (C-F) Methotrexate dose-response curves for NALM-6 (C,E) and Jurkat (D,F) leukemia cells in either the absence or presence of 5-MTHF 100 nM in either regular media (C-D; RPMI 1640 and 10% FBS) or folate-free media (E-F; folate-free RPMI 1640 and dialyzed 10 % FBS). Leukemia cell viability was assessed after 48 hours of drug treatment using the CellTiter-Glo luminescent cell viability assay. Error bars represent the mean ± SD of 3 technical replicates.
High concentrations of a metabolically active folate derivative rescue methotrexate toxicity. (A-B) Dose-response curves for NALM-6 (A) and Jurkat (B) leukemia cells treated with different concentrations of the folate derivative 5-MTHF in either the absence or presence of methotrexate. Leukemia cell viability was assessed after 48 hours of drug treatment using the CellTiter-Glo luminescent cell viability assay. Error bars represent the mean ± SD of 3 technical replicates. (C-F) Methotrexate dose-response curves for NALM-6 (C,E) and Jurkat (D,F) leukemia cells in either the absence or presence of 5-MTHF 100 nM in either regular media (C-D; RPMI 1640 and 10% FBS) or folate-free media (E-F; folate-free RPMI 1640 and dialyzed 10 % FBS). Leukemia cell viability was assessed after 48 hours of drug treatment using the CellTiter-Glo luminescent cell viability assay. Error bars represent the mean ± SD of 3 technical replicates.
ISR activation by CSF
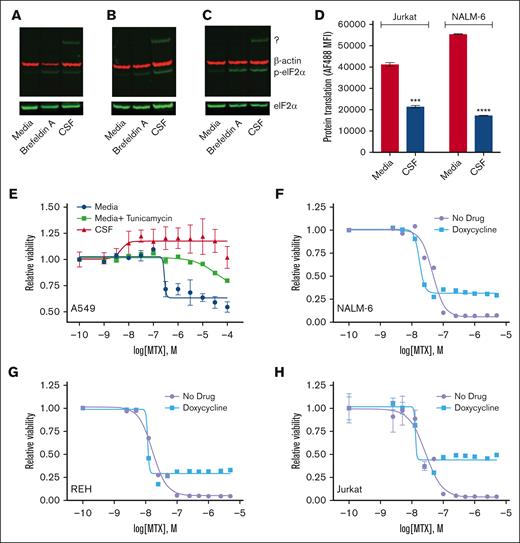
We also considered other less commonly described mechanisms of methotrexate resistance, including activation of the integrated stress response (ISR).29 Activation of the ISR occurs in response to diverse extrinsic and intrinsic stressors.30,31 The hallmark event in this pathway is the phosphorylation of eIF2α, which inhibits global protein synthesis while allowing the translation of selected genes that restore homeostasis and promote cellular recovery. It seemed plausible that nutrient-poor CSF could cause cellular stress and trigger ISR pathway activation in leukemia cells. Supporting this hypothesis, leukemia cells in CSF had increased phospho-eIF2α levels and attenuated global protein translation (Figure 7A-D). We then performed gene expression profiling on REH and NALM-6 leukemia cells in either regular media or CSF. Importantly, multiple genes involved in the ISR were among the genes that were differentially regulated in CSF relative to media (supplemental Figures 7 and 8). Together these data support that CSF can trigger the activation of the ISR in leukemia cells.
CSF activates the ISR. (A-C) Immunoblots showing the effects of CSF on eIF2α levels. NALM-6 (A), REH (B), and Jurkat (C) leukemia cell lines were cultured in regular media or CSF for 24 hours. Cells treated with brefeldin A 2.5 μg/mL in regular media served as a positive control. Protein lysates were then collected for immunoblotting with phospho-eIF2α (p-eIF2α), total eIF2α, or β-actin antibodies. Representative western blots are shown. The p-eIF2α antibody reproducibly detected a higher molecular weight protein of unclear etiology in leukemia cells in CSF, which is denoted by “?” (D) Protein synthesis in leukemia cells was assessed using O-propargyl-puromycin after 48 hours of culture in either regular media or CSF. Error bars represent the mean ± SD of 3 technical replicates. ∗∗∗P < .001 and ∗∗∗∗P < .0001 by t test. (E) Methotrexate dose-response curves for A549 lung carcinoma cells in regular media (Dulbecco modified Eagle medium + 10% FBS), regular media plus tunicamycin 2.5 μg/mL, or CSF. A549 cell viability was assessed after 48 hours of drug treatment using the CellTiter-Glo luminescent cell viability assay. Error bars represent the mean ± SD of 3 technical replicates. (F-H) Methotrexate dose-response curves for NALM-6 (F), REH (G), and Jurkat (H) leukemia cells in the absence or presence of doxycycline 7.5 μM. Leukemia cell viability was assessed after 48 hours of drug treatment using the CellTiter-Glo luminescent cell viability assay. Error bars represent the mean ± SD of 3 technical replicates. LogIC50 and bottom values with confidence intervals were calculated from the dose-response curves and are shown in the supplemental Table.
CSF activates the ISR. (A-C) Immunoblots showing the effects of CSF on eIF2α levels. NALM-6 (A), REH (B), and Jurkat (C) leukemia cell lines were cultured in regular media or CSF for 24 hours. Cells treated with brefeldin A 2.5 μg/mL in regular media served as a positive control. Protein lysates were then collected for immunoblotting with phospho-eIF2α (p-eIF2α), total eIF2α, or β-actin antibodies. Representative western blots are shown. The p-eIF2α antibody reproducibly detected a higher molecular weight protein of unclear etiology in leukemia cells in CSF, which is denoted by “?” (D) Protein synthesis in leukemia cells was assessed using O-propargyl-puromycin after 48 hours of culture in either regular media or CSF. Error bars represent the mean ± SD of 3 technical replicates. ∗∗∗P < .001 and ∗∗∗∗P < .0001 by t test. (E) Methotrexate dose-response curves for A549 lung carcinoma cells in regular media (Dulbecco modified Eagle medium + 10% FBS), regular media plus tunicamycin 2.5 μg/mL, or CSF. A549 cell viability was assessed after 48 hours of drug treatment using the CellTiter-Glo luminescent cell viability assay. Error bars represent the mean ± SD of 3 technical replicates. (F-H) Methotrexate dose-response curves for NALM-6 (F), REH (G), and Jurkat (H) leukemia cells in the absence or presence of doxycycline 7.5 μM. Leukemia cell viability was assessed after 48 hours of drug treatment using the CellTiter-Glo luminescent cell viability assay. Error bars represent the mean ± SD of 3 technical replicates. LogIC50 and bottom values with confidence intervals were calculated from the dose-response curves and are shown in the supplemental Table.
It was previously shown that the A549 lung carcinoma cell line became methotrexate resistant upon induction of the ISR with the drug tunicamycin, which induces endoplasmic reticulum stress.29 We confirmed this result and, at the same time, showed that CSF also caused significant methotrexate resistance in A549 cells (Figure 7E). We then tested whether activation of the ISR in leukemia cells by other mechanisms also attenuated methotrexate sensitivity. Unlike the original report, we were unable to use tunicamycin to initiate the ISR because of significant toxicity to leukemia cells (data not shown).29 Rather we used doxycycline, because tetracycline antibiotics have been shown to activate the ISR by inhibiting mitochondrial ribosome translation and inducing mitochondrial stress.32-34 High-dose doxycycline monotherapy did not cause significant leukemia cell death and had only a very modest (NALM-6), or insignificant (REH and Jurkat), impact on leukemia cell line proliferation (supplemental Figure 9). Similar to CSF, doxycycline enhanced the resistance of leukemia cells to methotrexate, although the effect was primarily on methotrexate efficacy rather than potency (Figure 7F-H). Although this result further supports a potential role for ISR activation in methotrexate resistance in leukemia cells, it is also possible that a downstream effect of mitochondrial dysfunction besides, or in addition to, ISR activation is also contributing.
Discussion
The ability of CSF to attenuate methotrexate efficacy was an unexpected finding given methotrexate’s long-standing role in CNS-directed ALL therapy. Importantly, this resistance occurred despite CSF making leukemia cells generally more prone to apoptosis16 and is unique among antimetabolites, with cytarabine and clofarabine retaining their efficacy in CSF. The rapid onset and reversibility of methotrexate resistance in CSF support it is not due to genetic or epigenetic changes. In addition, our data suggest that alterations in methotrexate influx/efflux, DHFR expression, and rescue by metabolically active folate species are less likely to be driving methotrexate resistance in CSF.
Rather, our data support further investigations into how cell cycle alterations and ISR activation in leukemia cells within CSF may contribute to methotrexate resistance. Notably, reducing leukemia proliferation with palbociclib to levels comparable with those seen with CSF had only a moderate impact on methotrexate efficacy and, in some cell lines, the effect was minimal. This suggests that the decreased proliferation of leukemia cells in CSF may play a more minor role in methotrexate resistance compared with ISR activation or other as-yet-unknown mechanisms of resistance.
Although cancer cell proliferation affects the efficacy of many cytotoxic drugs, it was only recently shown that ISR activation enables methotrexate resistance by diverting metabolites from glycolysis to mitochondrial 1-carbon metabolism.29 Defining which unique characteristics of CSF, such as its low glucose, protein, and lipid levels and its pro-oxidant nature, trigger ISR activation is an important next step. Similarly, future experiments will need to use genetic and pharmacologic approaches to more rigorously define ISR’s role in methotrexate resistance and determine whether targeting the ISR can sensitize leukemia cells to methotrexate in CSF.
Although the mechanism of CSF-mediated methotrexate resistance is not fully understood, our results suggest potential ways to enhance methotrexate’s efficacy against leukemia cells in CSF. First, CSF methotrexate levels drop quickly after lumbar IT administration, staying above the minimal cytotoxic level (∼0.5-1 μM) for only ∼24 hours.35,36 Prolonged methotrexate exposure, via high-dose systemic infusions or slow-release formulations, could better target slowly proliferating leukemia cells in the CSF.37,38 In rats, intracisternal injection of liposomal methotrexate extended its CSF half-life by 18-fold without neurotoxicity, maintaining effective concentrations for 7 to 14 days vs ∼1 day for unencapsulated methotrexate.38 Analogously, liposomal cytarabine, which also provides prolonged exposure, has been used intrathecally in patients with CNS leukemia.39
Second, targeting the ISR could enhance methotrexate efficacy in CSF. Although the ISR is a highly complex and incompletely understood pathway, pharmacologic approaches for modulating this pathway are emerging. This includes targeting the kinases that initiate the ISR through phosphorylation of eIF2α, the eIF2α complex itself, or pathways activated downstream of eIF2α.31,40,41
Third, the efficacy of asparaginase, cytarabine, and dexamethasone were not significantly affected by CSF. This result highlights the potential importance of the continued incorporation and optimization of these other agents in CNS-directed leukemia therapy, especially as cranial radiation is less frequently used. Regarding cytarabine, although many ALL treatment protocols and clinical trials use IT methotrexate monotherapy, others combine cytarabine with methotrexate and hydrocortisone (triple IT therapy) or have used liposomal cytarabine monotherapy.39,42,43 Direct comparisons of the efficacies of these different IT therapies in not just preventing CNS relapse but also bone marrow relapse, overall survival, and risk for both acute and late toxicities, are limited.39,42,44,45 As a result, if, when, and how to incorporate IT cytarabine into CNS-directed therapy remains a complex clinical question but warrants continued consideration.
This work is not without limitations. First, although all CSF samples significantly reduced the efficacy of methotrexate, the extent of this effect varied (Figure 1B-C). However, because of limited sample volumes and the absence of detailed patient or donor information, we cannot speculate on the potential causes of this variability. Second, as described above, additional work is necessary to better define the role and contribution of ISR activation in CSF-mediated methotrexate resistance. Third, other less-common mechanisms of methotrexate resistance, such as oxidative stress pathways, were not explored in this work.46,47 Accordingly, further unbiased gene and protein expression and metabolic studies on leukemia cells in CSF in the presence and absence of methotrexate are planned and may guide future mechanistic studies. Finally, these experiments were all performed ex vivo, albeit in human CSF. Experiments testing for relative methotrexate resistance in different microenvironments in vivo are challenging but once the resistance pathways are better defined at the molecular level ex vivo it may be possible to compare these pathways in leukemia cells from paired CSF and blood samples, or xenografts, derived from patients with leukemia. In addition to these limitations, we also recognize that methotrexate is clearly an efficacious and critical component of CNS leukemia treatment and prophylaxis.
In conclusion, our work demonstrates that leukemia cell sensitivity to methotrexate is rapidly and reversibly affected by CSF and the microenvironment. This may have clinical implications given the important role methotrexate plays in leukemia therapy and that leukemia is a systemic disease and occupies many diverse microenvironments in vivo. Moreover, we anticipate that our work underway in defining the mechanisms driving this resistance may identify novel approaches for maximizing methotrexate efficacy and more completely eradicating leukemia cells in CSF and the CNS. Increasing methotrexate efficacy in the CNS also may enable dose reductions that would potentially decrease methotrexate-related neurotoxicity risks. Finally, this work highlights the importance of critically evaluating even long-established standards of care.
Acknowledgments
The authors thank the University of Minnesota Center for Metabolomics and Proteomics for quantitating methotrexate polyglutamate species using liquid chromatography–tandem mass spectrometry. The authors thank the University of Minnesota Genomics Center for performing RNA-sequencing services, including bioinformatic analyses (Juan E. Abrahante Lloréns).
This study was supported by National Institutes of Health (NIH) R37CA240846-01A1 (P.M.G.), a Hyundai Hope on Wheels Scholar grant (P.M.G.), and the Timothy O’Connell Foundation (P.M.G.). This study used the University of Minnesota Masonic Cancer Center shared flow cytometry core, which is supported, in part, by the National Institutes of Health National Cancer Institute (P30 CA77598).
Authorship
Contribution: J.K., J.O., and X.W. designed and performed the experiments, analyzed data, and prepared the figures; P.M.G. designed the study, oversaw the laboratory investigations, prepared figures, and wrote the manuscript; and all authors have reviewed, edited, and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Peter M. Gordon, Division of Pediatric Hematology/Oncology, University of Minnesota, 420 Delaware St SE, MMC 366, Minneapolis, MN, 55455; email: gord0047@umn.edu.
References
Author notes
J.K. and J.O. contributed equally to this study.
RNA sequencing data were deposited in the Gene Expression Omnibus repository (accession number GSE274857).
The data sets generated and analyzed during this study are available upon reasonable request from the corresponding author, Peter M. Gordon (gord0047@umn.edu).
The full-text version of this article contains a data supplement.