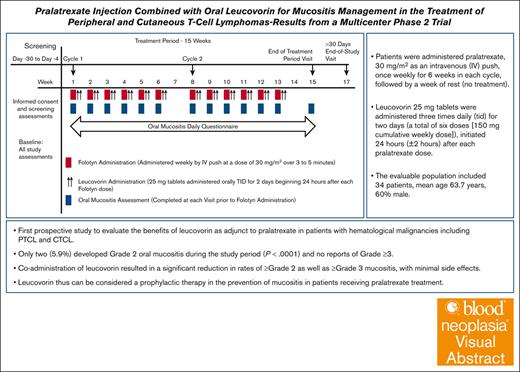
Key Points
Coadministration of leucovorin resulted in significant reduction in rates of grade 2 and grade 3 mucositis, with minimal side effects.
Leucovorin thus can be considered a prophylactic therapy in the prevention of mucositis in patients receiving pralatrexate treatment.
Visual Abstract
Pralatrexate (Folotyn) is an antifolate indicated for the treatment of relapsed/refractory peripheral T-cell lymphoma (PTCL), and although durable clinical benefit has been demonstrated, oral and gastrointestinal mucositis and/or skin reactions are frequent toxicity complications associated with pralatrexate treatment. Leucovorin (d,l-folinic acid) administration has been used as a standard rescue for patients receiving high-dose methotrexate therapy and has recently been studied in patients with PTCL and cutaneous T-cell lymphoma receiving pralatrexate. We describe results from a multicenter, phase 2, single-arm, open-label trial, conducted with the primary objective of evaluating the effect of leucovorin in preventing or reducing the incidence of grade 2 or higher oral mucositis associated with pralatrexate treatment in cycle 1. Patients were administered pralatrexate, 30 mg/m2 as an IV push, once weekly for 6 weeks in each cycle, followed by a week of rest (no treatment). Leucovorin 25 mg tablets were administered 3 times daily for 2 days (a total of 6 doses [150 mg cumulative weekly dose]), initiated 24 hours (±2 hours) after each pralatrexate dose. The evaluable population included 34 patients, with a mean age of 63.7 years and 60% males, of whom 2 (5.9%) developed grade 2 oral mucositis during the study period (P < .0001) and there were no reports of grade 3 or higher oral mucositis. Dose modifications, including omissions, delays, or reductions, due to oral mucositis were limited to 1 patient. Coadministration of leucovorin resulted in a significant reduction in mucositis and can be considered a prophylactic therapy in patients receiving pralatrexate treatment. This trial was registered at www.clinicaltrials.gov as #NCT02106650.
Introduction
T-cell lymphomas are a broad collection of aggressive or indolent heterogeneous lymphoid malignancies and comprise ∼12% of all non-Hodgkin lymphoma diagnoses.1,2 T-cell lymphomas can be further categorized into peripheral (PTCL) and cutaneous T-cell lymphomas (CTCL) and distinguished by their diagnostic, phenotypic, molecular, and prognostic characteristics. PTCL is rare (∼5% of all non-Hodgkin lymphoma diagnoses) and generally aggressive, developing from mature post-thymic T cells or natural killer cells. The most common type of CTCL is mycosis fungoides, which is a malignancy of mature T cells that tends to appear on the skin, due to the expression of the cutaneous lymphocyte antigen.3 Achieving durable clinical responses in PTCL or advanced-stage CTCL is a challenge for clinicians, and prognoses remain poor for those with advanced disease.
Pralatrexate (Folotyn) is an antifolate indicated for the treatment of relapsed/refractory PTCL.4 The PROPEL study was a phase 2 trial of pralatrexate monotherapy in relapsed or refractory PTCL that demonstrated durable clinical benefit (objective response rate of 29%).5 Within the PROPEL cohort, patients tolerated the target dose of 30 mg/m2 weekly for the duration of treatment (6 weeks of a 7-week cycle), however, 68% had 1 or more dose omissions due to adverse events (AEs). Despite vitamin supplementation (vitamin B12 1 mg intramuscularly every 8-10 weeks and daily oral folic acid 1.0-1.25 mg) to attempt to ameliorate mucositis in the trial, 71% of patients experienced some grade of mucositis (18% grade 3/4% grade 4), which contributed to dose reduction in 23% of patients. In addition to mucositis, skin reactions were also noted with pralatrexate treatment in 41% of patients.
Mucositis contributes to significant morbidity and severely affects patients’ quality of life, and no standard preventative medical management strategy exists, with the exception of early discontinuation or dosage remodulation of active treatment. Overall, it has been estimated that ∼15% of patients modify their active treatment due to antineoplastic therapy–induced oral mucositis, thus affecting therapeutic responses and survival outcomes.6
Leucovorin (d,l-folinic acid) administration has been used as a standard rescue for patients receiving high-dose methotrexate therapy and has recently been studied in patients with PTCL and CTCL receiving pralatrexate. Haddad et al7 conducted an observational study of 17 patients with PTCL with grade 2+ mucositis who were given 25 mg leucovorin every 6 hours for the first 5 days of a week before pralatrexate treatment. They found that no patients discontinued pralatrexate treatment due to mucositis and there was no decline in clinical response. Koch et al8 presented the findings of prophylactic leucovorin administration in 3 patients with CTCL who were given 50 mg leucovorin IV 24 hours after pralatrexate treatment. They reported a good clinical response and no occurrence of dose-limiting mucositis. More recently, a retrospective review of 34 patients (7 PTCL, 27 CTCL) treated by Foss et al9 compared patients who had previously received either (1) pralatrexate only or (2) pralatrexate plus oral leucovorin (50 mg every 6 hours × 4 doses starting 24 hours after pralatrexate). In the pralatrexate only group, 46% experienced mucositis, whereas in the group receiving leucovorin only 10% (n = 1) had mucositis, and no reduction in response rates was observed for the pralatrexate + leucovorin group compared with pralatrexate alone.
Although previous studies have demonstrated the therapeutic benefits of pralatrexate, prospective studies to evaluate the addition of leucovorin to pralatrexate treatment in patients with PTCL or CTCL in preventing or reducing the severity of mucositis are limited.
Herein we describe the results of a phase 2, multicenter trial investigating the effect of leucovorin in preventing or reducing grade 2 or higher oral mucositis for patients with PTCL and CTCL receiving treatment with pralatrexate.
Methods
Study design and oversight
This multicenter, phase 2, single-arm, open-label trial was conducted with the primary objective to evaluate the effect of leucovorin in preventing or reducing the incidence of grade 2 or higher oral mucositis associated with pralatrexate treatment in cycle 1.
The study was reviewed and approved by the institutional review board at each of the 4 participating sites. The study conduct complied with the Declaration of Helsinki and followed the International Conference on Harmonization Guidelines for Good Clinical Practice. All participating patients provided a written informed consent and understood that study participation was voluntary.
Selection of patients
Patients were eligible to be included in this study if they were aged 18 years or older and had been diagnosed as having hematologic malignancies including PTCL or CTCL and were eligible for treatment with a pralatrexate dose of 30 mg/m2. Patients were required to have adequate hematologic, hepatic, and renal function (absolute neutrophil count ≥103/μL; platelet count ≥100 x109/L; total bilirubin ≤1.5 mg/dL; aspartate aminotransferase/serum glutamic-oxaloacetic transaminase, alanine aminotransferase/serum glutamic-pyruvic transaminase, and gamma-glutamyltransferase ≤2.5 × upper limit of normal [aspartate aminotransferase/alanine aminotransferase/gamma-glutamyltransferase ≤5 × upper limit of normal if documented hepatic involvement with lymphoma]; and creatinine ≤2.0 mg/dL or calculated creatinine clearance ≥50 mL/min). Patients also had to be at least 30 days from their most recent cytotoxic therapy and have an Eastern Cooperative Oncology Group performance status ≤2. Patients were excluded if they had congestive heart failure, uncontrolled hypertension, uncontrolled HIV, central nervous system metastases, uncontrolled infection, or underlying medical condition that would prevent protocol treatment, major surgery, or therapy with any investigational therapies within 14 days of study treatment; had exposure to pralatrexate within 6 months before study enrollment; or were pregnant or breastfeeding.
Study treatment
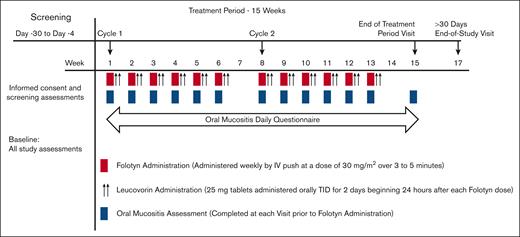
The duration of the primary study period was ∼13 weeks, which included a screening period of up to 30 days, 1 7-week treatment cycle (6 weeks of treatment, 1 week of no treatment), and an end-of-study visit, ∼14 days after the last dose of pralatrexate. At the investigator’s discretion, treatment could continue for up to 5 cycles (Figure 1).
Patients were administered pralatrexate, 30 mg/m2 as an IV push, once weekly for 6 weeks in each cycle, followed by a week of rest (no treatment). This dose was selected based on the dose used in the PROPEL study.5 Leucovorin 25 mg tablets were administered 3 times daily for 2 days (a total of 6 doses [150 mg cumulative weekly dose]), initiated 24 hours (±2 hours) after each pralatrexate dose. All patients were recommended to initiate vitamin supplementation, per currently approved label guidance, with folic acid (1.0 mg by mouth daily) initiated at least 10 days before pralatrexate administration and vitamin B12 (1 mg intramuscularly) administered within 10 weeks before initiation of pralatrexate, which was allowed to be administered during screening.
Folotyn (pralatrexate injection) was provided by Acrotech Biopharma Inc, and leucovorin tablets were obtained by investigators from commercial sources.
Pralatrexate dose modifications and delays were permitted, based on mucositis grade on the day of treatment or defined hematologic or other treatment-related toxicities.
Study assessments
Oral mucositis was assessed at baseline (day 1 of cycle 1) and before each subsequent dose of pralatrexate by the investigator and graded according to National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03 and World Health Organization criteria.10,11 In addition, the assessment of oral mucositis was documented daily by the patient, using an Oral Mucositis Daily Questionnaire (OMDQ) worksheet. The OMDQ was provided to the patient on day 1 of cycle 1 and they were instructed by the investigator how and when to complete the questionnaire each day. The OMDQ was reviewed by the investigator at each subsequent visit and was collected at the end-of-study visit.
Safety was assessed by AE reporting and clinical laboratory evaluations. Treatment emergent AEs (TEAEs) were defined as new AEs occurring from the time of the first dose of pralatrexate to 14 days (±3 days) after the last dose or worsening of AEs from before the first dose of pralatrexate.
Statistical analysis
The primary objective of the study was to evaluate the number and proportion of patients experiencing onset of pralatrexate-related grade 2 or higher oral mucositis during cycle 1. This study planned to enroll 37 patients that would provide 89% statistical power to detect a statistically significant reduction for the grade 2 or higher oral mucositis, assuming 25% in the current study and historic rate of 51%. The power was calculated based on the exact binomial test at a 5% nominal significance level.
The study included 2 analysis populations, the safety analysis population who received at least 1 dose of pralatrexate and the evaluable analysis population who consisted of all patients who received at least 1 dose of pralatrexate, 1 dose of leucovorin, and at least 1 postbaseline assessment of oral mucositis. Missing data were not imputed.
Appropriate descriptive statistics were performed. In addition, a generalized linear model with Poisson distribution was used by considering the number of pralatrexate doses as a dependent variable and occurrence of mucositis, leucovorin dose, and duration of treatment as independent variables. Statistical analyses were performed using SAS version 9.4 (SAS Institute).
Results
Patient characteristics
From April 2015 to June 2019, 45 patients were screened at the 4 participating institutions. Patient disposition is shown in Figure 2. Thirty-six patients were enrolled; 1 patient withdrew before dosing and 35 initiated study treatment and were included in analysis. Reasons for screen failure included patients not meeting the criteria for pathology requirements (n = 1), not meeting the criteria for laboratory requirements (n = 5), patient’s decision to withdraw (n = 1), and development of an AE before cycle 1, day 1 (n = 2). Twenty-four patients completed cycle 1 of pralatrexate treatment, and 11 patients discontinued before completing cycle 1. The most common reason for discontinuation was progression of disease (n = 8), followed by investigator decision (n = 2) and patient death (n = 1).
The mean age of patients included was 63.7 years and 60% were male. Most (60%) had an Eastern Cooperative Oncology Group performance status of 0 (fully active). Patient characteristics are presented in Table 1.
Summary of patient baseline characteristics of safety population
| Parameter/statistic, n (%) . | Total (N = 35) . |
|---|---|
| Age, n (y) | |
| Mean (SD) | 63.7 (13.88) |
| Min, max | 31, 85 |
| Sex, n (%) | |
| Male | 21 (60) |
| Female | 14 (40) |
| Race, n (%) | |
| White or Caucasian | 30 (85.7) |
| Black or African American | 2 (5.7) |
| Native Hawaiian or other Pacific Islander | 1 (2.9) |
| Asian | 2 (5.7) |
| Ethnicity, n (%) | |
| Hispanic or Latino | 4 (11.4) |
| Not Hispanic or Latino | 31 (88.6) |
| Diagnosis, n (%) | |
| All nodal T-cell lymphomas (PTCL, AITL, ALCL, cALCL/LyP, ENKTL, T-ALL) | 20 (57.1) |
| CTCL | 9 (25.7) |
| B-cell lymphoma | 3 (8.6) |
| Missing | 3 (8.6) |
| ECOG performance status, n (%) | |
| 0, fully active | 21 (60) |
| 1, restricted in physically strenuous activity but ambulatory | 9 (25.7) |
| 2, ambulatory and capable of all self-care but unable to perform work | 5 (14.3) |
| Parameter/statistic, n (%) . | Total (N = 35) . |
|---|---|
| Age, n (y) | |
| Mean (SD) | 63.7 (13.88) |
| Min, max | 31, 85 |
| Sex, n (%) | |
| Male | 21 (60) |
| Female | 14 (40) |
| Race, n (%) | |
| White or Caucasian | 30 (85.7) |
| Black or African American | 2 (5.7) |
| Native Hawaiian or other Pacific Islander | 1 (2.9) |
| Asian | 2 (5.7) |
| Ethnicity, n (%) | |
| Hispanic or Latino | 4 (11.4) |
| Not Hispanic or Latino | 31 (88.6) |
| Diagnosis, n (%) | |
| All nodal T-cell lymphomas (PTCL, AITL, ALCL, cALCL/LyP, ENKTL, T-ALL) | 20 (57.1) |
| CTCL | 9 (25.7) |
| B-cell lymphoma | 3 (8.6) |
| Missing | 3 (8.6) |
| ECOG performance status, n (%) | |
| 0, fully active | 21 (60) |
| 1, restricted in physically strenuous activity but ambulatory | 9 (25.7) |
| 2, ambulatory and capable of all self-care but unable to perform work | 5 (14.3) |
AITL, angioimmunoblastic T-cell lymphoma; ALCL, anaplastic large cell lymphoma; cALCL, cutaneous anaplastic large cell lymphoma; ECOG, Eastern Cooperative Oncology Group; ENKTL, Extranodal natural killer/T-cell lymphoma; LyP, lymphomatoid papulosis; Max, maximum; Min, minimum; SD, standard deviation; T-ALL, T-cell acute lymphoblastic leukemia.
Drug exposure
A summary of pralatrexate and leucovorin exposure including the cumulative dose and relative dose intensity of pralatrexate are presented in Table 2.
Pralatrexate and leucovorin exposure
| . | Pralatrexate (n = 35) . | Leucovorin (n = 34) . |
|---|---|---|
| No. of cycles started | ||
| n | 35 | 34 |
| Mean (SD) | 1.89 (1.568) | 1.91 (1.583) |
| Median | 1.00 | 1.00 |
| Min, max | 1.0, 6.0 | 1.0, 6.0 |
| Cumulative dose received (mg/m2) | ||
| n | 35 | 34 |
| Mean (SD) | 304.05 (285.064) | 1499.26 (1327.727) |
| Median | 180.00 | 900.00 |
| Min, max | 29.4, 1046.9 | 300.0, 5100.0 |
| Relative dose intensity (%) | ||
| n | 35 | 34 |
| Mean (SD) | 86.56 (22.004) | 95.76 (5.727) |
| Median | 98.68 | 100.00 |
| Min, max | 52.2, 130.0 | 85.0, 100.0 |
| Dose reduced (%) | ||
| n | 1 | 0 |
| AE | 1 (2.86) | - |
| Investigator decision | 1 (2.86) | - |
| Dose delayed (%) | ||
| n | 1 | 0 |
| Oral mucositis | 1 (2.86) | - |
| . | Pralatrexate (n = 35) . | Leucovorin (n = 34) . |
|---|---|---|
| No. of cycles started | ||
| n | 35 | 34 |
| Mean (SD) | 1.89 (1.568) | 1.91 (1.583) |
| Median | 1.00 | 1.00 |
| Min, max | 1.0, 6.0 | 1.0, 6.0 |
| Cumulative dose received (mg/m2) | ||
| n | 35 | 34 |
| Mean (SD) | 304.05 (285.064) | 1499.26 (1327.727) |
| Median | 180.00 | 900.00 |
| Min, max | 29.4, 1046.9 | 300.0, 5100.0 |
| Relative dose intensity (%) | ||
| n | 35 | 34 |
| Mean (SD) | 86.56 (22.004) | 95.76 (5.727) |
| Median | 98.68 | 100.00 |
| Min, max | 52.2, 130.0 | 85.0, 100.0 |
| Dose reduced (%) | ||
| n | 1 | 0 |
| AE | 1 (2.86) | - |
| Investigator decision | 1 (2.86) | - |
| Dose delayed (%) | ||
| n | 1 | 0 |
| Oral mucositis | 1 (2.86) | - |
Primary end point: reduction in mucositis
Of the 34 patients included in the evaluable analysis population, 2 (5.9%) developed grade 2 oral mucositis during the study period (P < .0001). The time to occurrence ranged from 1.00 to 9.29 weeks. There were no reports of grade 3 or higher oral mucositis. Dose modifications, including omissions, delays, or reductions, due to oral mucositis were limited to 1 patient who had their dose delayed on weeks 3 and 4.
An analysis was performed using the Poisson distribution to assess the relationship between the number of pralatrexate doses and the leucovorin doses received and occurrence of oral mucositis. The number of pralatrexate doses was considered as a dependent variable, whereas occurrence of mucositis, leucovorin dose, and duration of treatment as independent variables. At the end of cycle 1, there was no statistically significant correlation between the number of pralatrexate doses received and occurrence of mucositis.
Tumor response was assessed by each investigator using International Harmonization Project/Implementation Working Group criteria. Although the study was not powered to formally analyze the efficacy of pralatrexate in PTCL or CTCL, 1 patient (2.9%) showed a complete response, whereas 6 (17.6%) showed an overall response, and 12 (35.3%) were assessed to have progressive disease, based on data available at the end of the study period.
Safety
AEs that occurred from the first dose of pralatrexate until at least 30 days after the last dose of either pralatrexate or leucovorin were defined as TEAEs. Within the protocol, during the optional treatment period (after cycle 1), only serious AEs (SAEs) were to be recorded. During the study period, 31 patients (88.6%) reported at least 1 TEAE. Of these 24 (68.6%) had pralatrexate-related events and 9 (25.7%) were related to leucovorin, with the most common being dizziness and pyrexia. Two patients (5.7%) had grade 4 TEAEs, 12 (34.2%) reported grade 3 TEAEs, and 28 (90.3%) reported at least 1 grade 1 or 2 TEAE. Ten patients (28.6%) reported SAEs, 2 of these leading to drug discontinuation. No SAEs were related to pralatrexate or leucovorin.
The most common TEAEs were reported as general disorders and administration site conditions (n = 15, 42.9%), of which fatigue was the most common (n = 7, 20%). Mucosal inflammation was also reported by 4 patients (11.4%). Gastrointestinal disorders were reported for 14 patients (40.0%), with diarrhea (n = 5, 14.3%), nausea (n = 4, 11.4%), and stomatitis (n = 4, 11.4%), being among the most common.
Ten patients (28.6%) had reported events categorized as respiratory, thoracic, and mediastinal disorders, with hypoxia (n = 3, 8.6%) and epistaxis (n = 2, 5.7%) being reported more than once. Nine patients (25.7%) reported events categorized as infections and infestations, whereas 7 patients (20.0%) reported metabolism and nutrition disorders, with decreased appetite (n = 3, 8.6%) being the most common. Six patient (17.1%) reported nervous system disorders, which included 3 patients (8.6%) each who reported dizziness and headaches. Six patients also reported skin and subcutaneous tissue disorders, with pruritis and rash being the most common (n = 2 each, 5.7%).
Discussion
Pralatrexate is an antifolate, specifically designed to be efficiently internalized by the reduced folate carrier, which is an oncofetal protein expressed on both embryonic and malignant tissues that regulates the internalization of natural folates required for purine and pyrimidine biosynthesis. Pralatrexate is also a superior substrate for folylpolyglutamyl synthetase, making pralatrexate more effectively polyglutamylated and retained, minimizing extrusion via natural efflux pumps. This high affinity of pralatrexate for the reduced folate carrier is hypothesized to lead to selective tumor cell accumulation.12 Early experience with pralatrexate in patients with lymphoma suggested the risk of mucositis was greatest in those patients with markedly elevated levels of homocysteine and methylmalonic acid, leading to a requirement for folic acid and vitamin B12 pretreatment.4 Although the 111 patients with relapsed/refractory PTCL in the pivotal PROPEL trial received folic acid and vitamin B12 supplementation before treatment with pralatrexate (30 mg/m2 per week for 6 weeks in 7-week cycles), 70% reported either stomatitis or mucosal inflammation of the gastrointestinal or genitourinary tracts (grade 1-2, 50%; grade 3-4, 21%), which generally occurred early in the first cycle of treatment.5 Mucositis was also the most common reason given for dose omission, reduction, and treatment withdrawal in PROPEL.
Leucovorin is a well-recognized rescue therapy for high-dose methotrexate therapy, mitigating gastrointestinal lining and bone marrow cell toxicity, and is typically initiated 24 to 36 hours after methotrexate. In in vitro preclinical studies, the effects of leucovorin on the antitumor activity of pralatrexate were compared with methotrexate in HeLa cells. It was observed that exposure to leucovorin diminished the activity of methotrexate, but did not affect pralatrexate accumulation intracellular or antitumor activity as long as a sufficient interval between exposure to leucovorin and the next dose of antifolate was allowed. The authors conjectured that because of the high therapeutic index of pralatrexate relative to methotrexate, modest doses of leucovorin administered over short intervals may abrogate the pralatrexate-associated mucositis without diminishing its therapeutic efficacy.13 In addition, a mouse model of mesothelioma xenografts examined the use of leucovorin, administered 24 hours after pralatrexate. No decrease in tumor regression was seen, and less toxicity was observed, assessed in part by less reduction in body weight (a surrogate end point for mucositis in mice) vs that observed in controls. The authors suggested that the incorporation of leucovorin rescue with current pralatrexate dosing regimens may prove an effective method of preventing mucositis as a dose-limiting toxicity.14
When patients in this study received 25 mg leucovorin thrice daily for 2 days, initiated 24 hours after each pralatrexate dose, there were 12 instances of mucositis, all of which were mild and did not require any change in pralatrexate dose. One patient who experienced moderate mucositis (grade 2) and dosing was interrupted. Our findings are similar to the recently published FOL-CHOP study, in which patients with PTCL were instructed to take leucovorin tablets (25 mg) thrice daily for 2 days beginning 24 hours after each pralatrexate treatment as mucositis prophylaxis, with the subsequent dose of pralatrexate at least 72 hours after the last dose of leucovorin. The FOL-CHOP study reported an overall incidence of any mucositis of 51.9%, 7.7% grade 3, and no grade 4.15
Within the current study, 11 patients discontinued study treatment before the completion of cycle 1, which we acknowledge limits the final study population for analyses. Within the previous PROPEL study, 14 participants went off treatment in cycle 1% and 58% received fewer than 8 courses of treatment over the study period.5
Administration of leucovorin at 24 hours after pralatrexate and at least 72 hours before the next dose is done so to mitigate the toxicity of pralatrexate, without affecting efficacy. An investigation of the clinical pharmacology of leucovorin by Koch et al8 determined that based on ∼6-hour terminal half-life of leucovorin (both IV and oral), a minimum of 2 days is required for the elimination of the biologically active isomer from the plasma, minimizing the potential of decreasing efficacy of the subsequent antifolate dose.
Published case series have also examined mucositis rates and intensity in association with leucovorin administration at different doses and routes of administration. A comparative retrospective review of 34 patients with PTCL or CTCL by Foss et al16 demonstrated that of patients who received pralatrexate plus 50 mg oral leucovorin every 6 hours for 4 doses starting 24 hours after pralatrexate, only 1 of 10 experienced mucositis compared with 11 of 24 patients who received pralatrexate only. The authors concluded that addition of leucovorin significantly reduced the incidence of mucositis, with no reduction in response rates. A retrospective series by Haddad et al7 described short oral leucovorin rescue in 17 patients with PTCL with recurrent grade 2 or higher mucositis after pralatrexate treatment. This group had already experienced pralatrexate dose reductions or omissions in an attempt to ameliorate the mucositis. Patients received 25 mg oral leucovorin every 6 hours for 5 consecutive days, stopping at least 48 hours before their next pralatrexate dose. All experienced resolution of symptoms by day 7 regardless of mucositis grade with subjective improvement at day 4. Koch et al8 described 3 cases in which pre-emptive 50 mg leucovorin was administered, by IV infusion, 24 hours after 50 mg pralatrexate administration.
Good clinical responses were observed without the occurrence of mucositis or significant hematologic toxicities. Although this study was not designed to compare response rates of pralatrexate with and without leucovorin or evaluate long-term efficacy, we acknowledge that future studies and real-world data would be helpful to clarify this very important question. An additional limitation of the current study is that final analyses did not separately categorize participants by PTCL or CTCL diagnoses, and therefore, any distinction between results for the 2 groups cannot be made.
This is the first prospective study, to our knowledge, to evaluate the benefits of leucovorin as adjunct to pralatrexate in patients with hematologic malignancies including PTCL and CTCL. Coadministration of leucovorin resulted in a significant reduction in rates of grade or higher as well as grade 3 or higher mucositis, with minimal side effects. Thus, leucovorin can be considered a prophylactic therapy in the prevention of mucositis in patients receiving pralatrexate treatment.
Acknowledgments
The authors thank April Ingram of Avlis International for her valuable editorial and writing contributions.
This work was supported by Acrotech Biopharma Inc.
Authorship
Contribution: S.P.I., S.D., M.M.S., J.Z., and F.F. were investigators in the study, contributed data, reviewed data, and reviewed and edited the submitted manuscript; and M.A. designed the study, analyzed the data, and reviewed and edited the submitted manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Swaminathan P. Iyer, Department of Lymphoma & Myeloma, The University of Texas MD Anderson Cancer Center, 1400 Holcombe Blvd, Unit 429, Houston, TX 77030; email: spiyer@mdanderson.org.
References
Author notes
All data generated or analyzed during this study are included in this published article.