Key Points
Ruxolitinib resulted in complete response, which is the disappearance of disease activity, in 22.2% (2/9) of patients with sCAEBV.
Ruxolitinib is a potent treatment drug that may improve HSCT outcomes by suppressing disease activity in sCAEBV.
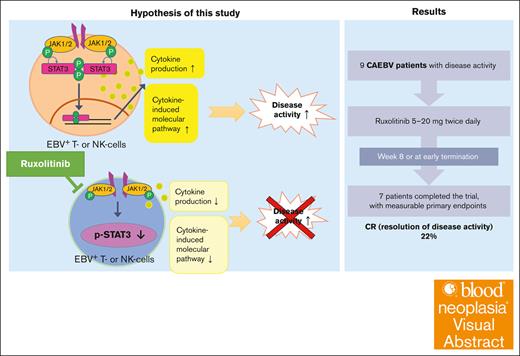
Visual Abstract
Systemic chronic active Epstein-Barr virus disease (sCAEBV) is a rare intractable EBV-positive T-cell or natural killer (NK)-cell lymphoid neoplasm with systemic inflammation. The only curative treatment is allogeneic hematopoietic stem cell transplantation (allo-HSCT). Disease activity defined by multiple inflammatory symptoms is associated with poor survival. In sCAEBV, signal transducer and activator of transcription 3 (STAT3) is constitutively activated in EBV-infected T and NK cells and promotes their activation and survival. Ruxolitinib, a Janus kinase 1/2 inhibitor, suppresses the STAT3 activation in vitro, suggesting its clinical potential. We conducted a phase 2, multicenter, open-label study to investigate the effects of ruxolitinib on disease activity of sCAEBV. Complete response (CR) and partial response were defined as complete and partial resolution of disease activity, respectively. Nine patients received ruxolitinib, and 7 patients completed the study. The primary end point, CR rate (%) 56 days after the administration or at early termination as defined in the protocol, was 22.2% (2/9). No patient showed hematopoietic toxicity nor disease progression. Notably, 71.4% (5/7) of patients who completed the study were treated at our outpatient clinic. One patient developed a severe adverse event of oral bleeding due to lesion shrinkage during the treatment, and ruxolitinib was discontinued. EBV-DNA levels in whole blood did not change significantly whether the treatment was effective or not. After ruxolitinib treatment, 7 patients received allo-HSCT, and 5 of them achieved CR with undetectable EBV-DNA levels in whole blood. Ruxolitinib is a potent treatment drug that may improve allo-HSCT outcomes by suppressing disease activity of sCAEBV. The trial was registered at University hospital Medical Information Network (UMIN), numbered UMIN000035121.
Introduction
Systemic chronic active Epstein-Barr virus disease (sCAEBV) is a rare and fatal disorder accompanying persistent and/or recurrent inflammation and clonal proliferation of EBV-infected T or natural killer (NK) cells (EBV-T/NK cells).1 The World Health Organization (WHO) has classified sCAEBV as a T-cell and NK-cell neoplasm.2 Increased levels of proinflammatory cytokines (ie, interferon gamma [IFN-γ], tumor necrosis factor α [TNF-α], and interleukin-6 [IL-6]) cause systemic inflammatory symptoms (ie, recurrent or sustained fever and liver dysfunction)3 and ultimately develop hemophagocytic lymphohistiocytosis in patients with sCAEBV.4 Furthermore, clonal evolution of EBV-T/NK cells can lead to chemotherapy-resistant EBV-positive T-cell or NK-cell lymphoma.1,5 Although sCAEBV was initially known as a children’s disease, a Japanese study in 2020 revealed that over half of the cases were of adults.6
Optimal medical treatment has not been established for sCAEBV. In immunochemotherapy, quantification levels of EBV-DNA in whole blood (WB), indicative of the quantity of infected cells, do not reach undetectable levels.6 The 3-year overall survival rate of patients treated only with chemotherapy is 0%.6 Allogeneic hematopoietic stem cell transplantation (allo-HSCT) has a 3-year overall survival rate of 72.5%.7 However, the presence of disease activity, which we defined as the manifestation of inflammatory symptoms such as fever, liver dysfunction, progressive skin lesions, vasculitis, and uveitis, at the time of allo-HSCT is significantly associated with poor outcomes.7,8 Hence, an optimal chemotherapy regimen needs to be established to control the disease activity of sCAEBV.
Signal transducer and activator of transcription 3 (STAT3) can regulate inflammation by mediating intracellular signaling downstream of cytokine receptors.9 We had reported previously that STAT3 is constitutively activated in neoplastic EBV-T/NK cells from patients with sCAEBV,10 that Janus kinase 1/2 (JAK1/2/) inhibitors block STAT3 activation, and that ruxolitinib (an oral JAK1/2 inhibitor) suppresses IFN-γ, TNF-α, and IL-6 messenger RNA (mRNA) expression in EBV-T/NK cells.10 Thus, we hypothesized that ruxolitinib may suppress EBV-positive neoplastic cell proliferation, proinflammatory cytokine production, and associated signaling.
In this investigator-initiated phase 2 study, we examined the effectiveness of ruxolitinib on disease activity and its safety profile in patients with sCAEBV.
Methods
Study design
This open-label, phase 2, prospective, multicenter trial was designed to assess the efficacy and the safety of ruxolitinib in patients with sCAEBV and was conducted in accordance with the Japanese Ministerial Ordinance on Japanese good clinical practice for drugs (Ministry of Health, Labour, and Welfare) and the principles of the Declaration of Helsinki. The protocol was approved by the Pharmaceuticals and Medical Devices Agency in 2018 (University hospital Medical Information Network (UMIN) trial number: UMIN000035121) and is available with the full-text version of this article. The study was conducted at St Marianna University Hospital, Tokyo Medical and Dental University Hospital, Kyushu University Hospital; and Osaka Women’s and Children’s Hospital, with approvals from the institutional review boards of each hospital. All participants provided written informed consent before enrollment. We purchased ruxolitinib from Novartis Pharma K.K.
Diagnostic criteria of sCAEBV
All enrolled patients were diagnosed based on the following published criteria: elevated EBV-DNA in WB (>102.5 copies per μg DNA), EBV-infected T or NK cells in affected tissues or the peripheral blood (PB), systemic inflammatory symptoms (ie, fever, lymphadenopathy, liver dysfunction, progressive skin lesions, vasculitis, or uveitis) persisting for >3 months, and the exclusion of other diagnosed diseases (ie, congenital and acquired immune deficiencies, other iatrogenic immune deficiencies, autoimmune diseases, lymphoma, or leukemia).4,6 The criteria meet the WHO definition of sCAEBV.11 Patients meeting all of the above criteria were diagnosed with sCAEBV.
Detection of EBV-infected cells in diagnosing patients with sCAEBV
Infected cells were isolated and detected as described previously.10 In brief, PB mononuclear cells of patients were isolated by density-gradient centrifugation using Separate-L (Muto Pure Chemical Co, Ltd, Tokyo, Japan) and sorted into CD19+, CD4+, CD8+, and CD56+ fractions using antibody-conjugated magnetic beads (Miltenyi Biotec, Bergisch Gladbach, Germany). The EBV-DNA level in each fraction was measured by real-time polymerase chain reaction (RT-PCR) using a TaqMan assay (Applied Biosystems, Foster City, CA).12 The fraction with the highest titer was considered as to contain infected cells.
Eligibility and exclusion criteria
The eligibility criteria were as follows: sCAEBV diagnosis (described above); exhibiting any 1 of the following 2 symptoms indicative of disease activity, fever (≥37.5°C) and/or liver dysfunction (alanine aminotransferase [ALT] ≥2.0× the upper normal limit); adequate bone marrow reserve (absolute neutrophil count of >500/μL, platelet count of >50 × 103/μL without the assistance of growth factors); age of ≥13 years; and comprehension of, and willingness to, sign the informed consent form. The definitions of fever and liver dysfunction are described in detail in supplemental Data, 1.6.
The age cutoff was set because ruxolitinib pharmacokinetics (PK) in 13-year-olds are identical to those in adults.13 Sex was not a criterion of eligibility.
Only the patients who fulfilled the inclusion criteria were eligible for the enrollment. Patients who met any of the exclusion criteria provided in supplemental Data were not enrolled.
Detection of the clonality
The clonal proliferation of EBV-infected cells was detected by Southern blotting for EBV-terminal repeat.14
Treatment
The initiation dose was 5 to 20 mg twice daily (supplemental Table 1), based on WB platelet counts. Doses were adjusted according to platelet and neutrophil counts (supplemental Tables 2-4; see supplemental Data). When a potent CYP3A4 inhibitor was administered concomitantly, ruxolitinib was administered orally once daily every 24 hours. Patients were administered ruxolitinib in hospital for at least 2 weeks.
The standard treatment lasted for 8 weeks. When a complete response (CR; described below) was observed and allo-HSCT was scheduled, ruxolitinib administration was terminated early, even if these events occurred within 8 weeks after treatment initiation. The criteria to withdraw and to restart ruxolitinib are shown in supplemental Data. The administration period could be extended up to 16 weeks if significant efficacy was observed after 8 weeks and allo-HSCT was scheduled.
The rules on concomitant use of drugs is also provided in detail in supplemental Data.
Definitions of disease-activity symptoms
Disease activity was determined by evaluating 5 symptoms: fever, liver dysfunction, progressive skin lesions, vasculitis, and uveitis.
Fever
Fever was defined as an axillary temperature of ≥37.5°C for ≥2 days, without any other known cause within 5 days before enrollment.
Liver dysfunction
Liver dysfunction was defined by the plasma ALT level exceeding double the maximum value of reference ranges of each testing institution at 2 time points. Any measurement interval between the 2 points could be used, but the latest plasma ALT level (≤7 days before enrollment) was required to be used.
Progressive skin lesions
Progressive skin lesions were defined as follows:
Skin lesions exacerbated in parallel with 2 symptoms of disease activity (fever and/or liver dysfunction) and were clearly not symptoms of other diseases.
Skin lesions histopathologically associated with sCAEBV and diagnosed as nonlymphomatous.
Vasculitis
Vasculitis was defined histopathologically based on vasculitis exacerbation in parallel with 2 symptoms of disease activity (fever and/or liver dysfunction) and was evaluated further based on organ damage and peripheral ischemic lesions.
Uveitis
Uveitis was defined as the characteristic finding of detectable EBV DNA in the eyes.
Disease evaluation and end points
Outcomes were evaluated based on the improvements of disease activity6 including fever, liver dysfunction, uveitis, progressive skin lesions, and vasculitis. The treatment effects were evaluated based on the conditions of CR, partial response (PR), progressive disease (PD), and stable disease (SD). CR was defined as the disappearance of all symptoms of disease activity. The disappearance of symptoms is explained in supplemental Data, 1.6. PR was defined as the disappearance of at least 1 symptom of disease activity after treatment. Symptoms not observed during the preobservation period were evaluated as “disappearance” if reappearances were not observed. PD was defined as the appearance of a new disease activity or the presence of lymphoma. SD was judged when a patient did not show any change in disease activity of lymphoma development after treatment nor the pathogenesis of lymphoma. Further details are presented in supplemental Table 5. Virological CR (vCR) was defined as a CR with a WB EBV-DNA of <102.5 copies per μg DNA. Relapse was defined as a change from CR to active disease.
The primary efficacy end point was the CR rate at 8 weeks or early termination. The secondary end points were CR rate at 4 weeks, an overall response (OR) rate at 4 weeks, and an OR rate at 8 weeks or early termination. OR was defined as improved disease activity, which is equivalent to the sum of CR and PR.
We assessed safety based on the frequency of adverse events in reference to common terminology criteria for adverse events, version 4.0, which was the latest version at the time when we finalized our protocol.
Exploratory assessments
PK data, EBV-DNA in the WB, EBV mRNA expression, and the concentration of inflammatory cytokines were examined as exploratory assessments. EBV-DNA quantitative levels in WB were measured by RT-PCR.12 During the administration of ruxolitinib, we could not measure the levels of EBV mRNA of patients I-1, II-1, and II-2 at Tokyo Medical and Dental University because of the coronavirus disease pandemic in 2019. EBV-DNA levels (copies per mL) were measured at an alternative laboratory (SRL, Inc). The expression levels of EBV genes (latent membrane protein 1 [LMP1], BamHI Z fragment leftward open reading frame 1 [BZLF1], and envelope glycoprotein [gp] 350 and 220) were measured by RT-PCR.15 The levels of inflammatory cytokines IFN-γ, TNF-α, and IL-6 were measured by commercial Quantikine enzyme-linked immunosorbent assay kits (R&D Systems, Minneapolis, MN).
Statistical analysis
Because sCAEBV is a rare disease, analyzing a large number of cases was not possible. We initially planned to enroll 5 to 10 patients in the full analysis set (FAS), based on prior distributions and a Bayesian design.16 The definition of FAS is shown in supplemental Data. We assumed a mean no-effect response rate of 5% and a β-distribution with shape parameters of 10 and 190, after considering that the spontaneous disappearance of the disease activity was 0%.6 We also assumed a mean ruxolitinib response rate of 30% and used a β-distribution of 0.6 to 1.4. Under these settings, we required that 2 responders achieved a posterior probability of >95% and that the ruxolitinib response rate was ≥5% for 5 to 10 patients. This design controlled the type-1 error rate at a target level of <0.10. We estimated the response rate and 95% confidence interval (CI) using the Clopper-Pearson method. Details are provided in “Statistical analysis” in supplemental Data. All statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC).
Results
Patients
We enrolled 5 male and 4 female patients (aged 13-64 years) between January 2019 and August 2021 (Table 1). The types of EBV-infected cells were CD4+ (n = 4), CD8+ (n = 1), and CD56+ (n = 4) cells. Three patients had received chemotherapy before enrollment. As for disease activity symptoms, 6 patients had liver dysfunction, 5 had fever, and 1 had progressive skin lesions when the treatment was initiated. Figure 1 shows the clinical course of each patient. The initial ruxolitinib dose was 5 to 20 mg twice daily (Figure 1). At 4 weeks after ruxolitinib initiation, patient II-4 showed SD and patient III-6 showed PR; thus, we increased their doses from 15 to 20 and 20 to 25 mg twice daily, respectively. Ruxolitinib was administered twice daily to all patients. We administered ruxolitinib to 3 patients, I-1, II-1, and II-4, beyond week 8. We terminated ruxolitinib on day 13 for patient III-4 because of a severe adverse event (SAE) described later. Ruxolitinib administration to patient II-5 was terminated at week 4, on day 29, despite a PR, based on the attending physician’s decision to treat the patient with allo-HSCT. Seven patients completed the trial with measurable primary end points. Among them, 2 patients (II-2 and II-3) terminated treatments early at week 5, on day 35, and at week 1.5, on day 12, respectively, because they showed CRs and had allo-HSCT scheduled. We evaluated the efficacy of ruxolitinib on day 56 in the remaining 5 patients.
Characteristics of the enrolled patients with sCAEBV disease
| Patient number . | Sex . | Age (y) . | EBV-infected cells . | Ruxolitinib initiation . | Treatment before ruxolitinib . | HSCT . | |||
|---|---|---|---|---|---|---|---|---|---|
| Clonality of the infected cells . | Disease activity symptom(s) . | Other clinical findings . | EBV-DNA (copies per 103/μL DNA) . | ||||||
| I-1 | M | 13 | CD56 | Monoclonal | Liver dysfunction | Coronary aneurysm | 710∗ | (−) | (+) |
| II-1 | M | 46 | CD56 | Monoclonal | Liver dysfunction | ND | 2 400∗ | (−) | (−) |
| II-2 | F | 40 | CD56 | Monoclonal | Liver dysfunction | ND | 1 200∗ | CHOP × 2, Cytarabine 150 mg/d ×7 d | (+) |
| II-3 | F | 24 | CD8 | Monoclonal | Fever | ND | 0.89 | mPSL pulse, VP16 + PSL, DeVIC, SMILE | (+) |
| II-4 | F | 64 | CD56 | Monoclonal | Liver dysfunction | ND | 51 | (−) | (+) |
| II-5 | M | 63 | CD4 | Monoclonal | Fever, liver dysfunction, progressive skin lesions | ND | 8.4 | (−) | (+) |
| III-1 | M | 33 | CD4 | Monoclonal | Fever | ND | 0.43 | (−) | (+) |
| III-4 | M | 49 | CD4 | Monoclonal | Fever, liver dysfunction | ND | 2 | (−) | (−) |
| III-6 | F | 21 | CD4 | Monoclonal | Fever | Cerebral aneurysm | 37 | CHOP ×3 | (+) |
| Patient number . | Sex . | Age (y) . | EBV-infected cells . | Ruxolitinib initiation . | Treatment before ruxolitinib . | HSCT . | |||
|---|---|---|---|---|---|---|---|---|---|
| Clonality of the infected cells . | Disease activity symptom(s) . | Other clinical findings . | EBV-DNA (copies per 103/μL DNA) . | ||||||
| I-1 | M | 13 | CD56 | Monoclonal | Liver dysfunction | Coronary aneurysm | 710∗ | (−) | (+) |
| II-1 | M | 46 | CD56 | Monoclonal | Liver dysfunction | ND | 2 400∗ | (−) | (−) |
| II-2 | F | 40 | CD56 | Monoclonal | Liver dysfunction | ND | 1 200∗ | CHOP × 2, Cytarabine 150 mg/d ×7 d | (+) |
| II-3 | F | 24 | CD8 | Monoclonal | Fever | ND | 0.89 | mPSL pulse, VP16 + PSL, DeVIC, SMILE | (+) |
| II-4 | F | 64 | CD56 | Monoclonal | Liver dysfunction | ND | 51 | (−) | (+) |
| II-5 | M | 63 | CD4 | Monoclonal | Fever, liver dysfunction, progressive skin lesions | ND | 8.4 | (−) | (+) |
| III-1 | M | 33 | CD4 | Monoclonal | Fever | ND | 0.43 | (−) | (+) |
| III-4 | M | 49 | CD4 | Monoclonal | Fever, liver dysfunction | ND | 2 | (−) | (−) |
| III-6 | F | 21 | CD4 | Monoclonal | Fever | Cerebral aneurysm | 37 | CHOP ×3 | (+) |
HSCT, Hematopoietic stem cell transplantation; CHOP, cyclophosphamide; doxorubicin; vincristine; prednisolone; DeVIC, dexamethasone; etoposide; ifosfamide; carboplatin; F, female; M, male; ND, not detected; PSL, prednisolone; SMILE, dexamethasone; etoposide; ifosfamide; methotrexate; L-asparaginase.
EBV-DNA quantitative levels were measured at an alternative laboratory, SRL, Inc. The units were copies per mL.
Responses and clinical course during the trial period. Swimmer plots showing the clinical course of each patient during the trial. CR: disappearance of all of symptoms of disease activity, including fever, liver dysfunction, uveitis, progressive skin lesions, and vasculitis; PR: disappearance of at least 1 of the symptoms listed above; SD: no disease progression; PD: conversion to active disease, exacerbation of active disease, or development of overt T-cell or NK-cell lymphoma; vCR: a CR with a WB EBV-DNA of <102.5 copies per μg DNA.
Responses and clinical course during the trial period. Swimmer plots showing the clinical course of each patient during the trial. CR: disappearance of all of symptoms of disease activity, including fever, liver dysfunction, uveitis, progressive skin lesions, and vasculitis; PR: disappearance of at least 1 of the symptoms listed above; SD: no disease progression; PD: conversion to active disease, exacerbation of active disease, or development of overt T-cell or NK-cell lymphoma; vCR: a CR with a WB EBV-DNA of <102.5 copies per μg DNA.
Evaluation of the disease
Responses
Figure 1 shows the response of each patient based on our definition described in the “Methods.” The response rates of the FAS are shown in Figure 2. The CR rate at week 8 or at early termination (the primary end point) was 2 of 9 patients (22.2%; 95% CI, 2.8-60.0; the middle bar of Figure 2). The CR rate at week 4 (the secondary end point) was 1 of 9 patients (11.1%; 95% CI, 0.3-48.3: the left bar of Figure 2). Regarding the other secondary end points, the OR rate at week 4 was 3 of 9 (33.3%; 95% CI, 7.5-70.1), and the OR rate at week 8 or at early termination was 3 of 9 (33.3%; 95% CI, 7.5-70.1). No patients achieved a vCR with ruxolitinib. Three patients were administered ruxolitinib for >8 weeks; we observed SD for patient II-1 at week 10, SD for patient II-4 at week 11, and CR for patient I-1 at week 16. Responses at the end of ruxolitinib administration are shown in the right bar of Figure 2: 3 patients achieved CR at weeks 1.5, 5, and 16, respectively; 2 achieved PR at week 4 and 8, respectively; and 3 achieved SD at weeks 10, 11, and 8, respectively. No patients showed PD during ruxolitinib administration. The transition of disease activity during the treatment by patient is shown in supplemental Table 7. Of 6 cases with elevated ALT, 4, including 2 cases that normalized, showed a decrease in ALT levels during the treatment. The skin lesions of patient II-5 are shown in supplemental Figure 1. The lesions resolved after treatment.
Response rates of FAS in this study. Response rates of all 9 patients of FAS at each treatment point. The CR rate at week 8 or at the time of early termination (the primary end point) was 2 of 9 (22.2%; 95% CI, 2.8-60.0; the middle bar. ∗NE owing to short-term administration (<4 weeks); ∗∗NE owing to ruxolitinib discontinuation before evaluation. NE, not evaluable.
Response rates of FAS in this study. Response rates of all 9 patients of FAS at each treatment point. The CR rate at week 8 or at the time of early termination (the primary end point) was 2 of 9 (22.2%; 95% CI, 2.8-60.0; the middle bar. ∗NE owing to short-term administration (<4 weeks); ∗∗NE owing to ruxolitinib discontinuation before evaluation. NE, not evaluable.
Safety
Three adverse events associated with ruxolitinib occurred in 2 of 9 patients (Table 2). There were no cases of ruxolitinib being discontinued or dose-reduced because of thrombocytopenia or neutropenia. Patient III-4 also had a grade 4 hematemesis, which we considered as a SAE. He experienced bleeding from the swollen nasopharynx mucosa at the onset of sCAEBV. As shown in supplemental Figure 2A-D, infiltration of lymphocytes without morphological atypia was observed by a histopathological examination. We detected no CD56+ lymphocytes but only a few EBV-positive cells and thereby ruled out extranodal NK/T-cell lymphoma. Bleeding resolved spontaneously at the time of trial enrollment. However, on day 13 of ruxolitinib administration, the same lesion started bleeding again, which caused respiratory distress. The platelet count at the time of the event was 102 000 × 103/μL. Fibrinogen level, prothrombin time, and activated partial-thromboplastin time were within normal ranges. The nasopharyngeal swelling decreased during treatment (supplemental Figure 2E-F). Ruxolitinib administration was discontinued, and the embolization of the hyoid artery achieved hemostasis. Our data and safety monitoring committee determined that the bleeding was caused by the contraction of the mucosal swelling that led to vessel exposure. The committee permitted the clinical trial to continue, but the principal investigator decided to discontinue the administration of the investigational drug to this patient.
Adverse events observed in the study
| Patient number . | Event . | Grade . | Associated with ruxolitinib . | Dosage change . | Outcome . |
|---|---|---|---|---|---|
| I-1 | Constipation | 2 | No | None | Continued |
| Cerebrospinal fluid rhinorrhea | 1 | No | None | Recovered | |
| II-2 | Neutropenia | 2 | Yes | None | Recovered |
| III-1 | Oral mucositis | 2 | No | None | Recovered |
| Shoulder impingement syndrome | 1 | No | None | Recovered | |
| Folliculitis | 1 | No | None | Recovered | |
| III-4 | Night steroid delirium | 1 | No | None | Remission |
| Hematemesis | 4 | Yes | Discontinuation (day 13) | Recovered | |
| Lymphocytopenia | 3 | Yes | None | Recovered | |
| III-6 | Upper respiratory inflammation | 2 | No | None | Recovered |
| Fever caused by odontectomy | 1 | No | None | Recovered |
| Patient number . | Event . | Grade . | Associated with ruxolitinib . | Dosage change . | Outcome . |
|---|---|---|---|---|---|
| I-1 | Constipation | 2 | No | None | Continued |
| Cerebrospinal fluid rhinorrhea | 1 | No | None | Recovered | |
| II-2 | Neutropenia | 2 | Yes | None | Recovered |
| III-1 | Oral mucositis | 2 | No | None | Recovered |
| Shoulder impingement syndrome | 1 | No | None | Recovered | |
| Folliculitis | 1 | No | None | Recovered | |
| III-4 | Night steroid delirium | 1 | No | None | Remission |
| Hematemesis | 4 | Yes | Discontinuation (day 13) | Recovered | |
| Lymphocytopenia | 3 | Yes | None | Recovered | |
| III-6 | Upper respiratory inflammation | 2 | No | None | Recovered |
| Fever caused by odontectomy | 1 | No | None | Recovered |
All patients started ruxolitinib administration while hospitalized. After day 15, administration was allowed during outpatient visits if patients could reserve environment equivalent to hospitalization at home. Of 7 patients, 5 received ruxolitinib as outpatients.
Exploratory analysis
PK analysis
We performed ruxolitinib PK analysis on all 9 patients (see supplemental Data). The maximum concentration (Cmax) of ruxolitinib in the plasma reached ∼0.5 hour after administration, and the half-life (t1/2) was 2.7 ± 1.0 hour (supplemental Figure 3; supplemental Table 8). The findings were similar to those of a previous Japanese single-dose and repeated-administration study of ruxolitinib in healthy donors.17
Biomarkers, ferritin, SIL-2R, and EBV-DNA in the WB
The patients’ values of these factors at administration initiation are shown in supplemental Table 9. The ferritin levels of the 2 patients in the high-ferritin group decreased after ruxolitinib treatment (supplemental Figure 4A). However, we observed no correlation between the ferritin levels and treatment effects in the low-ferritin group (supplemental Figure 4B). Although soluble IL-2 receptor (SIL-2R) is a prognostic marker for allo-HSCT in sCAEBV,6 no significant correlation between SIL-2R levels and the effects of ruxolitinib was found (supplemental Figure 4C). C-reactive protein was also not associated with the efficacy (supplemental Figure 4D).
The infection of EBV-T/NK cells in sCAEBV is a latent infection without virus particle production,15 and EBV-DNA quantitative levels in the WB reflect the number of infected cells.7 EBV-DNA levels in the WB at ruxolitinib initiation are shown in Table 1. As shown in Figure 3, EBV-DNA quantitative levels did not change during ruxolitinib administration. These findings indicate that ruxolitinib did not decrease the number of EBV-T/NK cells. The mRNA expression of viral genes encoding BZLF1 and gp350/220 (necessary for virus production) in the WB was not induced by ruxolitinib (supplemental Figure 5). These findings indicate that ruxolitinib did not induce the production of EBV.
Changes in EBV-DNA quantitative levels in the WB of enrolled patients. During the administration of ruxolitinib, we could not measure the levels of EBV mRNA of patients I-1, II-1, and II-2 in our laboratory at Tokyo Medical and Dental University because of the coronavirus disease pandemic in 2019. EBV-DNA quantitative levels (copies per mL) were measured at an alternative laboratory (SRL, Inc). The responses to ruxolitinib at each examination point are represented as follows: CR; PR; PD; SD; and NE, not evaluable.
Changes in EBV-DNA quantitative levels in the WB of enrolled patients. During the administration of ruxolitinib, we could not measure the levels of EBV mRNA of patients I-1, II-1, and II-2 in our laboratory at Tokyo Medical and Dental University because of the coronavirus disease pandemic in 2019. EBV-DNA quantitative levels (copies per mL) were measured at an alternative laboratory (SRL, Inc). The responses to ruxolitinib at each examination point are represented as follows: CR; PR; PD; SD; and NE, not evaluable.
Cytokines
Disease activity and the plasma levels of inflammatory cytokines (IFN-γ, TNF-α, and IL-6) were not correlated (supplemental Figure 6). The number of patients who achieved CR remained high, and the correlation with the therapeutic effect was unclear.
Outcomes
Of 9 patients in the FAS, 7 underwent allo-HSCT, and 5 patients, including the 3 who achieved CR with ruxolitinib, achieved post-HSCT vCR (Figure 1). Four patients survived and maintained vCR until the end of the trial period (March 2022). Three patients died after the allo-HSCT. Supplemental Figure 7 shows the survival curves at the end of the trial period.
Discussion
Our study is, to our knowledge, the first multicenter prospective clinical trial conducted on the basis of the WHO definition of sCAEBV and that used a consistent protocol for analysis. We assessed the efficacy of ruxolitinib against sCAEBV by monitoring inflammatory symptoms. The primary end point (CR rate after 8 weeks of ruxolitinib treatment or at early termination) was 22.2%, suggesting the validity of ruxolitinib based on Bayesian analysis.16 In 2021, Song et al18 conducted a single-center retrospective analysis of 9 cases of sCAEBV treated with ruxolitinib and reported improved fever and liver dysfunction in 7 cases. These results suggest that ruxolitinib is a potent therapeutic agent against the disease activity of sCAEBV. We retrospectively analyzed immunochemotherapies’ effects on disease activity of sCAEBV using the same criteria of this study.6 The CR rate of a conventional immunochemotherapy with prednisolone, cyclosporine, and etoposide was 17%. However, the analysis of this retrospective study was based on a questionnaire survey, and there was no unified protocol of immunochemotherapies. Furthermore, although the conventional immunochemotherapy resulted in grade ≥4 hematological toxicity in 23% of the cases, ruxolitinib did not show any hematological toxicity. As a result, all 5 patients who completed the 8-week administration of ruxolitinib in our trial were treated as outpatients. Compared with the conventional chemotherapies, which require prolonged hospitalization,19 ruxolitinib can improve the quality of life of patients.
In this study, 7 patients underwent allo-HSCT after ruxolitinib treatment. Among the 7 patients, 5, including the 3 who achieved CR with ruxolitinib, achieved vCR after allo-HSCT. In our previous retrospective analysis of the Japanese registry data, we calculated that 62.2% of the patients achieved vCR after allo-HSCT. 7 These findings suggest that ruxolitinib can reduce the disease activity of sCAEBV and enable favorable prognosis after allo-HSCT.
The results of our PK analysis were similar to those of previous studies.17,20 The Cmax values of ruxolitinib ranged from 344 to 1730 nM. In vitro analysis has shown that 250 nM ruxolitinib can suppress the survival of PB mononuclear cells from patients with sCAEBV, including EBV-T/NK cells.10 Furthermore, 500 nM ruxolitinib can suppress IFN-γ and TNF-α production in vitro.10 The Cmax values of all patients (except for 1) were higher than these in vitro concentrations. Patient II-2 (whose Cmax value was <500 nM) achieved CR; thus, we assumed that Cmax values and efficacy were not correlated. The in vitro effects of ruxolitinib on EBV-T/NK cells are dose-dependent.10 As no dose-limiting toxicity was observed in any of the enrolled patients, the ruxolitinib dose could potentially be increased in the cases of insufficient efficacy.
In this study, the quantitative levels of EBV-DNA were maintained at the same level either with or without improvement in disease activity. In other words, disease activity did not correlate with EBV-DNA levels, the number of infected cells. We can postulate that uninfected immune cells orchestrate the inflammation associated with sCAEBV. In addition, BZLF1 mRNA was absent in most cases suggesting that, at least, short-term ruxolitinib administration did not induce the production of EBV. However, patient III-6 showed BZLF1 and gp350/220 mRNA at the termination of ruxolitinib treatment (supplemental Figure 3). The increase in EBV-DNA quantitative levels during ruxolitinib administration in the PB of a myelofibrosis patient was monitored.21 Lytic infection may have caused the transformation of EBV-positive cells.22 We need to further investigate the association of ruxolitinib and the type of infection; latent vs lytic, especially in long-term ruxolitinib administration.
One patient had an SAE (grade 4 hematemesis). Bleeding occurred in the same lesion identified at the time of sCAEBV diagnosis. Ruxolitinib administration could have reduced the mucosal swelling rapidly therefore exposed the blood vessels and promoted bleeding. Approximately 9% of patients with sCAEBV have aneurysms and 7% have vasculitis.6 Angiopathy could have caused bleeding. The possibility of bleeding must be taken into consideration when administering ruxolitinib to patients with sCAEBV.
We were unable to identify any significant markers that reflect the treatment efficacy of sCAEBV. Potential biomarkers of our interest include chemokine (C-X-C motif), ligand 9 (CXCL9), and interferon-inducible protein 10 (IP-10). CXCL9, which is inducible by IFN-γ, has demonstrated to outperform IFN-γ in assessing disease activity of poststeroid treatment in adult Still disease, a disease characterized primarily by inflammatory symptoms.23 Moreover, clinical trials utilizing emapalumab, an anti–IFN-γ antibody, for hemophagocytic lymphohistiocytosis, have identified a correlation between the serum levels of CXCL9 and therapeutic effects.24 Consequently, CXCL9 is anticipated to be valuable to sCAEBV, which predominantly manifests inflammatory symptoms. IP-10 has been recognized also for its biomarker potential in lymphoma-associated hemophagocytic syndrome. This potential suggests its utility in gauging the severity of sCAEBV that can precipitate hemophagocytic lymphohistiocytosis.25 At the time of the present study, the potential values of CXCL9 and IP-10 were not known. Further research involving a broader spectrum of samples is essential to substantiate the utility of these biomarkers particularly in more severe cases.
Further investigation of other cytokines is needed to identify markers that reflect disease activity.
One of the limitations of this study was the small number of patients. Another is a possible bias because of the study being a single-arm, open-label trial. Because sCAEBV is rare, we need to expand our study to a broader geographical area to enroll more patients. Some patients were not eligible because of their thrombocyte counts. Because we did not observe ruxolitinib-dependent thrombocytopenia, we should consider including these patients in future studies.
In conclusion, ruxolitinib is a potent treatment drug that may improve HSCT outcomes by suppressing disease activity in sCAEBV.
Acknowledgments
The authors thank Ayako Komoto, an assistant supported by funding from the Japan Agency for Medical Research and Development (AMED), for her excellent editorial support during manuscript preparation.
This study was supported by grants from the Practical Research Project for Rare/Intractable Diseases (18ek0109334h0001, 19ek0109334h0002, 20ek0109334h0003, 21ek0109334h0004, 22ek0109612h0001, 23ek0109612h0002, and 24ek0109612h0003) of AMED.
Authorship
Contribution: Y.U. treated the patients, analyzed the data, and wrote the manuscript; A.A. organized the project, designed the research, analyzed the data, and wrote the manuscript; M.Y. and M.I. treated the patients and analyzed the data; H.K., A.S., K.I.-I., M.Y., M.N., and N.S. analyzed the data; K.Y. performed the pathological diagnosis; A.H. and R.K. designed the research and analyzed the data; and all authors have contributed to the final version of the manuscript and approved its submission for publication.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ayako Arai, Department of Hematology and Oncology, St. Marianna University School of Medicine, 2-16-1 Sugao, Miyamae-Ku, Kawasaki 216-8511, Japan; email: ara.hema@marianna-u.ac.jp.
References
Author notes
The data that support the findings of this study are available, upon reasonable request, from the corresponding author Ayako Arai (ara.hema@marianna-u.ac.jp).
The full-text version of this article contains a data supplement.