Key Points
Targeting cyclin-dependent kinase 9 with enitociclib induces apoptotic cell death in MM and demonstrates synergy in drug combinations.
Enitociclib may be an effective treatment for MM as a single agent or in combination therapies in future clinical studies.
Visual Abstract
Multiple myeloma (MM) is a cancer of plasma cells that remains incurable despite advances in treatment options. In this study, a library of 216 clinically feasible small-molecule inhibitors was screened to identify agents that selectively inhibit MM cell proliferation. Enitociclib, a cyclin-dependent kinase 9–specific small-molecule inhibitor, was found to be highly effective in decreasing cell viability and inducing apoptosis in 4 MM cell lines. Enitociclib inhibited the phosphorylation of the carboxy-terminal domain (CTD) of RNA polymerase II at Ser2/Ser5 and repressed the protein expression of oncogenes c-Myc, myeloid cell leukemia-1 (Mcl-1), and proliferating cell nuclear antigen (PCNA) in MM cells. Additionally, enitociclib demonstrated synergistic effects with several anti-MM agents, including bortezomib, lenalidomide, pomalidomide, and venetoclax. These results suggest that enitociclib may represent a promising therapeutic option for the treatment of MM, either as a single agent or in combination with other anti-MM agents.
Introduction
Multiple myeloma (MM) is a blood cancer characterized by the uncontrolled growth of clonal plasma cells.1 This leads to clinical symptoms such as hypercalcemia, renal failure, anemia, and bone lesions. The disease follows a multistep process, starting with premalignant monoclonal gammopathy of undetermined significance, progressing through smoldering MM, and then reaching active MM. The progression of MM is associated with the accumulation of genomic alterations and immune dysfunction, which increases the risk of infectious complications and promotes tumor progression. Anticancer efficacy has greatly improved in recent years, with 5-year overall survival now exceeding 50%.2 Despite significant progress in MM treatment, active disease remains incurable. Aberrant expression of cyclin-dependent kinases (CDKs) plays a role in MM by causing loss of control over cell proliferation and enhancing survival. CDK9, a serine/threonine kinase and a subunit of the positive transcription elongation factor (P-TEFb) that phosphorylates the carboxy-terminal domain (CTD) of RNA polymerase II (Pol II; Rpb1), is a major transcriptional regulator of various oncogenes relevant in the pathogenesis of MM, including MYC, MCL1, and PCNA which encode c-Myc, myeloid cell leukemia-1 (Mcl-1), and proliferating cell nuclear anitgen (PCNA), respectively.3,4 Understanding the role of CDKs in MM could potentially lead to more effective treatments for this disease.
The small-molecule enitociclib (VIP152) is a CDK9 inhibitor currently undergoing early phase clinical trials for various hematologic malignancies, such as diffuse large B-cell lymphoma.5,6 We hypothesized that CDK9 inhibition by enitociclib would represent a rational pharmacologic approach to target the transcription of critical oncogenic effector proteins in MM. In this study, we describe that enitociclib induces cytotoxicity in vitro and responses in vivo against aggressive MM cells. The responses were due to the downregulation of RNA Pol II–mediated transcription of MM-related oncogenes, including MYC, MCL1, and PCNA, followed by rapid drug-induced apoptosis. The potential clinical benefits of enitociclib warrant further investigation in the context of treating patients with MM.
Methods
Cell culture
MM cell lines were obtained from the German Collection of Microorganisms and Cell Cultures (DSMZ) or the American Type Culture Collection (ATCC). NCI-H929 (DSMZ catalog no. ACC-163; accession: CVCL_1600), MM1.S (ATCC catalog no. CRL-2974; accession: CVCL_8792), OPM-2 (DSMZ catalog no. ACC-50; accession: CVCL_1625), and U266B1 (ATCC catalog no. TIB-196; accession: CVCL_0566) were cultured in RPMI-1640 (Gibco) supplemented with 10% fetal bovine serum (FBS; Gibco) and 1% penicillin/streptomycin/L-glutamine (Gibco) at 37°C and 5% CO2. JJN-3 myeloma cells (DSMZ catalog no. ACC-541; accession: CVCL_2078) were cultured in 50% Dulbecco's Modified Eagle Medium (DMEM; Biochrom)/50% Iscove's Modified Dulbecco's Medium (IMDM; Gibco), supplemented with 10% FBS (Gibco).
Cytotoxicity assays
Cells were seeded in 96-well plates (Greiner Bio-One) at 5 × 103 cells per well in 100 μL of RPMI-1640 media (Gibco) supplemented with 10% FBS. The cells were treated with either enitociclib or dimethyl sulfoxide diluted in media, and 100 μL was added to each well (1:2 final dilution). All treatments were run in triplicate at final concentrations ranging from 12.5 to 200 nM. After 96 hours in culture, cell viability was evaluated using the alamarBlue Cell Viability (Alamar Blue) reagent (Thermo Fisher Scientific) as per the manufacturer’s instructions. Half-maximal inhibitory concentrations (IC50) were determined by nonlinear regression using GraphPad Prism v9.1.0 analysis software (GraphPad Software). Matrix dosing of agents in combinations was used to assess drug synergy using the SynergyFinder v3.0 online web application tool (https://synergyfinder.fimm.fi/).7 Synergy scores were determined by the zero-interaction potency (ZIP) method.
Small-molecule inhibitor screening
Cells were seeded in 96-well plates (Greiner Bio-One) at 5 × 103 cells per well and treated with a library containing 216 small-molecule compounds or corresponding dimethyl sulfoxide treatment at a final concentration of 1 μM. After 96 hours, cell viability was measured using Alamar Blue reagent.
Western blotting
MM cells were lysed in radioimmunoprecipitation assay (RIPA) buffer supplemented with 0.1% protease/phosphatase inhibitors. Protein concentration was quantified using the DC Protein Assay (Bio-Rad) as per the manufacturer’s instructions. Appropriate volumes of samples containing 30 μg of protein were mixed with loading buffer (0.125 M Tris-HCl [6.8 pH], 20% glycerol, 10% 2-mercaptoethanol, 4% sodium dodecyl sulfate (SDS), and 0.005% bromophenol blue). Samples were then resolved by denaturing polyacrylamide gel electrophoresis (PAGE; SDS-PAGE) using various percentages of acrylamide (7.5%-12%), depending on the protein of interest. SDS-PAGE was performed in running buffer (25 mM Tris, 192 mM glycine, and 0.1% SDS), followed by transfer to nitrocellulose membranes in 1X Trans-Blot Turbo transfer buffer (Bio-Rad) at 25 V for 10 minutes at room temperature using the Trans-Blot Turbo transfer system (Bio-Rad). The membranes were blocked in 5% (weight-to-volume ratio) skim milk or bovine serum albumin serum in Tris-buffered saline with 0.1% (volume to volume ratio) Tween-20 (TBS-T; 50-mM Tris-HCl [pH 7.5], 150-mM NaCl, and 0.1% [volume-to-volume ratio] Tween-20) at room temperature for 1 hour. The membranes were then incubated overnight at 4°C with the following primary antibodies diluted in TBS-T with 5% (weight-to-volume ratio) skim milk or bovine serum albumin: anti–caspase-3 (1:1000, 9662S), anti–poly-(adenosine diphosphate-ribose) polymerase (PARP; 1:1000; 9542S), anti-Bim (1:1000; 2933S), anti–c-Myc (1:1000; 18583S), anti-Mcl-1 (1:1000; 94296S), anti-PCNA (1:1000; 13110S), anti–phospho-Rpb1 CTD (Ser2/Ser5; 1:1000; 13546S), anti-Rpb1 CTD (1:1000, 2629S), and anti–β-actin (1:1000; 4967S; all from Cell Signaling Technology). After, the membranes were washed 3 times with TBS-T, incubated with anti-rabbit secondary antibody (1:10 000; 7074S; Cell Signaling Technology), washed 3 times with TBS-T, incubated with Clarity Western ECL substrates (Bio-Rad), and developed using chemiluminescence on a ChemiDoc MP imaging system (Bio-Rad).
Gene expression quantification
Total RNA was extracted from homogenized frozen tumor with RNeasy Plus Mini kit (QIAGEN) from 2 animals at 1, 3, 7, and 24 hours after in vivo dosing and from 3 predose animals. The quality and concentration of the RNA samples was tested by ultraviolet absorption at 260/280 nm in Nano Drop system and electrophoresis on denaturized 1% agarose gel to determine the concentration. BCL2, CCND1, MYC, MCL1, HEXIM1, and PCNA messenger RNA were quantified using TaqMan probes (Thermo Fisher Scientific) as per the manufacturer’s instructions. The real-time polymerase chain reaction results are expressed as the mean from 3 independent experiments for vehicle-treated animals and 2 independent experiments for drug-treated animals. Readouts were normalized to L32 transcript primers.
Animal studies
All animal studies were conducted at Bayer Pharma AG in accordance with German animal welfare law and with approval from local authorities. For JJN-3 xenografts, 5.0 × 106 cells in 0.1-mL 50% Matrigel suspension were injected subcutaneously into severe combined immunodeficiency disease (SCID)/Beige mice (Taconic Biosciences) and treated with 10 mL/kg 80% PEG400 control or 15 mg/kg enitociclib IV once per week. For OPM-2 xenografts, 5.0 × 106 cells in 0.1-mL medium suspension were injected subcutaneously into non-obese diabetic (NOD)/SCID mice (Taconic Biosciences) and treated with 5 mL/kg PEG400-ethanol-water (60/10/30) vehicle control or 15 mg/kg enitociclib once per week by IV injection. For NCI-H929 xenografts, 3- to 4-mm tumor fragments obtained from serially passaged xenografts were implanted subcutaneously in the flank of non-obese diabetic (NOD)/SCID mice (Harlan Laboratories) and treated with 5 mL/kg PEG400-ethanol-water (30/10/60) vehicle control or 15 mg/kg enitociclib once per week by IV injection. For drug combinations, mice bearing OPM-2 xenografts were treated with 50 mg/kg lenalidomide (vehicle, 10 mL/kg 1% cMC/0.5% Tween in water) daily by oral gavage, 0.8 mg/kg bortezomib (vehicle, 10 mL/kg saline) twice per week by intraperitoneal injection, or lenalidomide/bortezomib in combination with 15 mg/kg enitociclib as described above. Animals were monitored daily, and 2-dimensional tumor measurements were determined twice weekly using calipers.
Statistical analysis
The Student t test or analysis of variance statistical analyses were performed using GraphPad Prism v9.1.0 software. P values <.05 were deemed statistically significant.
Results
Enitociclib inhibits MM cell proliferation and induces apoptosis in vitro
We examined the activity of agents from a library comprising 216 individual drugs, including clinically feasible small-molecule inhibitors, against various components of cancer growth and survival pathways. In this experiment, OPM-2 cells were treated with 1 μM of each therapeutic agent, followed by cell viability measurement under each experimental condition by Alamar Blue assay after 96 hours in culture (supplemental Table 1). OPM-2 cells show high sensitivity to CDK inhibitors (Figure 1A), with CDK9-specific inhibition from enitociclib identified as a candidate agent due to its inhibition of oncogenes expressed in MM (Figure 1B).
Enitociclib decreases cell viability in MM cells. (A) Cell viability of small-molecule inhibitors from the pharmaceutical pipeline library treated at 1 μM for 96 hours in OPM-2 cells. A breakdown of molecular targets with a mean cell viability inhibition of >50% are shown. (B) Cell viability values of various CDK inhibitors from the library treated at 1 μM for 96 hours in OPM-2 cells are shown. (C) Western blotting of MM cell lines MM1.S, NCI-H929, OPM-2, and U266B1. Total cell lysates were prepared and analyzed by immunoblotting to detect the level of CDK9. β-actin was used as a loading control. Molecular masses are indicated in kilodaltons (kDa). (D) Dose response curves of MM cell lines treated with increasing concentrations (12.5-200 nM) of enitociclib for 96 hours. Cell viability was measured by Alamar Blue assay. Percent cell viability was normalized to corresponding treatment with dimethyl sulfoxide (DMSO; vehicle control). Mean percentages of cell viability were calculated from 3 technical replicates and standard deviations are shown. BET, Bromodomain and extraterminal; EZH1/2, Enhancer of zeste homolog 1/2; FLT3, Fms-like tyrosine kinase 3; HDAC, Histone deacetylase; HSP, Heat shock protein; IGF-1R, Insulin-like growth factor 1 receptor; KDM5A, Lysine (K)-specific demethylase 5A; mTOR, Mammalian target of rapamycin; NAE, NEDD8-activating enzyme; WNK, With no lysine (K); XPO1, Exportin-1.
Enitociclib decreases cell viability in MM cells. (A) Cell viability of small-molecule inhibitors from the pharmaceutical pipeline library treated at 1 μM for 96 hours in OPM-2 cells. A breakdown of molecular targets with a mean cell viability inhibition of >50% are shown. (B) Cell viability values of various CDK inhibitors from the library treated at 1 μM for 96 hours in OPM-2 cells are shown. (C) Western blotting of MM cell lines MM1.S, NCI-H929, OPM-2, and U266B1. Total cell lysates were prepared and analyzed by immunoblotting to detect the level of CDK9. β-actin was used as a loading control. Molecular masses are indicated in kilodaltons (kDa). (D) Dose response curves of MM cell lines treated with increasing concentrations (12.5-200 nM) of enitociclib for 96 hours. Cell viability was measured by Alamar Blue assay. Percent cell viability was normalized to corresponding treatment with dimethyl sulfoxide (DMSO; vehicle control). Mean percentages of cell viability were calculated from 3 technical replicates and standard deviations are shown. BET, Bromodomain and extraterminal; EZH1/2, Enhancer of zeste homolog 1/2; FLT3, Fms-like tyrosine kinase 3; HDAC, Histone deacetylase; HSP, Heat shock protein; IGF-1R, Insulin-like growth factor 1 receptor; KDM5A, Lysine (K)-specific demethylase 5A; mTOR, Mammalian target of rapamycin; NAE, NEDD8-activating enzyme; WNK, With no lysine (K); XPO1, Exportin-1.
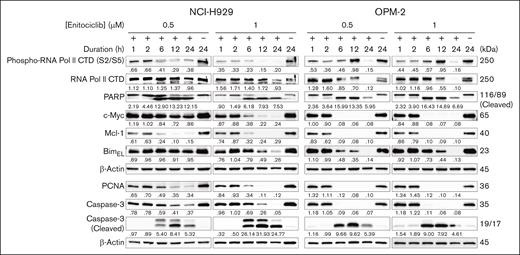
To further investigate the antimyeloma activity of enitociclib, we tested various concentrations in 4 MM cell lines that express CDK9 (Figure 1C) and different molecular features (supplemental Table 2) with a range of enitociclib concentrations from 12.5 to 200 nM for 96 hours, and cell viability was measured using the Alamar Blue assay. Enitociclib decreased cell viability in a concentration-dependent manner in all cell lines tested, with IC50 values ranging from 36 to 78 nM (Figure 1D). Notably, enitociclib exhibited similar activity in cells with MCL1 gene amplification and cells without chromosome 1q gains. We also found that enitociclib potently induced mechanism-based apoptosis in NCI-H929 and OPM-2 cells, which have distinct molecular alterations. Western blot analysis showed that cleavage of apoptosis markers, caspase-3 and PARP proteins, occurred in a dose- and time-dependent manner, with significant activity observed as early as 6 hours after treatment at the tested concentrations (Figure 2). Similarly, persistent repression of the antiapoptosis marker Mcl-1 was observed from 6 hours onward after treatment at both dose levels (Figure 2).
Enitociclib induces apoptosis by inhibition of RNA Pol II phosphorylation and oncogene expression in MM cells. Western blotting of NCI-H929 and OPM-2 MM cells treated with either DMSO (vehicle control; “–”) or 0.5 to 1 μM of enitociclib for up to 24 hours. Total cell lysates were prepared and analyzed by immunoblotting to detect levels of markers associated with apoptosis (total and cleaved PARP and caspase-3, Mcl-1, and BimEL), total and phosphorylated RNA Pol II CTD (S2/S5), and short half-life oncogene proteins c-Myc and PCNA. β-actin was used as a loading control. Molecular masses are indicated in kDa.
Enitociclib induces apoptosis by inhibition of RNA Pol II phosphorylation and oncogene expression in MM cells. Western blotting of NCI-H929 and OPM-2 MM cells treated with either DMSO (vehicle control; “–”) or 0.5 to 1 μM of enitociclib for up to 24 hours. Total cell lysates were prepared and analyzed by immunoblotting to detect levels of markers associated with apoptosis (total and cleaved PARP and caspase-3, Mcl-1, and BimEL), total and phosphorylated RNA Pol II CTD (S2/S5), and short half-life oncogene proteins c-Myc and PCNA. β-actin was used as a loading control. Molecular masses are indicated in kDa.
Enitociclib treatment leads to inhibition of CTD phosphorylation and short half-life transcript proteins
To confirm that the antiproliferative effects of enitociclib were due to on-target inhibition of CDK9, we next examined the effects of CDK9-mediated transcriptional regulation in NCI-H929 and OPM-2 cells. The selectivity of enitociclib to inhibit the phosphorylation of the CTD of RNA Pol II at Ser2 (RNA Pol II S2) has been shown previously,6,8 and the results showed that enitociclib could potently block the CTD phosphorylation of RNA Pol II S2/S5 in a dose- and time-dependent manner (Figure 2). We next investigated the impact of enitociclib treatment on the expression of oncogenes that are known to drive the progression of MM. Regarding the MM cell lines used in this study, MYC is highly expressed in NCI-H929 and OPM-2 cells.3 We found that treatment with enitociclib repressed c-Myc protein levels in NCI-H929 and OPM-2 cells (Figure 2). Enitociclib also repressed the protein expression of PCNA in these cells, which is a known disease-related factor in MM that is associated with cellular proliferation and cancer progression.9 Together, these findings suggest that enitociclib exerts its antiproliferative effects in MM cells by inhibiting CDK9-mediated transcriptional regulation of oncogenes.
CDK9-selective inhibition by enitociclib synergizes with several anti-MM agents in vitro
Provided the ability of enitociclib to disrupt higher-order transcriptional machinery through selective CDK9 inhibition,8 we hypothesized that the downregulation of molecular factors related to MM-related and antiapoptotic-driven drug resistance may enable effective combinatory activity with other chemotherapeutic agents. Thus, we evaluated the potential of enitociclib to synergize with current conventional and experimental agents used in the treatment of MM. A panel of MM cell lines were treated with increasing concentrations of enitociclib and 1 of 4 different agents, including bortezomib, lenalidomide, pomalidomide, and venetoclax, for 96 hours in culture before cell viability measurement using the Alamar Blue assay (supplemental Figure 1).
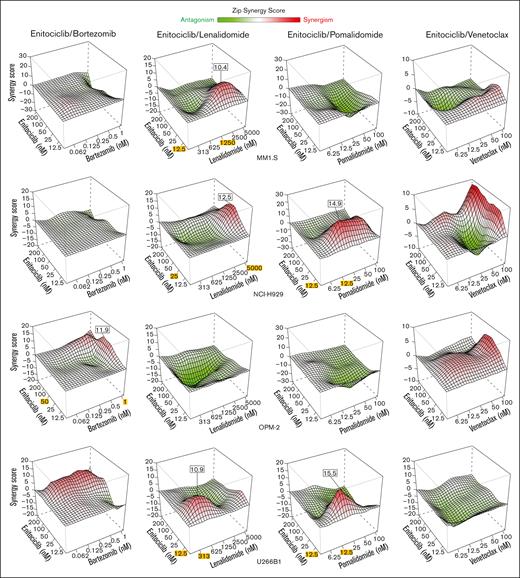
The proteasome inhibitor bortezomib is approved by the US Food and Drug Administration (FDA) for first-line therapy in MM.10 Despite high initial response rates, bortezomib eventually loses its efficacy. Therefore, we next determined whether enitociclib could enhance the activity of bortezomib in MM cells. In OPM-2 cells, synergistic effects (>10 ZIP synergy score) were observed with 50 nM of enitociclib in combination with bortezomib treated at 1 nM (Figure 3). Lenalidomide is another FDA-approved first-line therapy in newly diagnosed MM, which acts as both an immunomodulatory and anticancer drug by altering proinflammatory cytokine production and exerting antiproliferative and proapoptotic effects on MM cells through transcriptional control of MYC and other oncogenes.11,12 When used in combination with enitociclib, synergistic effects were identified in 3 of 4 cell lines tested (MM1.S, NCI-H929, and U266B1) at enitociclib concentrations between 12.5 and 25 nM and lenalidomide concentrations between 313 nM and 5 μM (Figure 3). Pomalidomide is an FDA-approved immunomodulatory drug for patients with refractory MM, who have been treated with 2 prior therapies including lenalidomide and bortezomib. The drug acts by inhibiting cell proliferation and promoting apoptosis in MM cells, in addition to antiangiogenic properties.13 NCI-H929 and U266B1 cells showed synergistic effects of enitociclib with pomalidomide at 12.5 nM and at 12.5 to 50 nM for each agent, respectively (Figure 3). Lastly, the selective B-cell lymphoma 2 (Bcl-2) inhibitor venetoclax is an experimental drug for MM that has demonstrated antiproliferative activity in patients with relapse/refractory MM positive for t(11;14) translocation.14 In general, enitociclib with venetoclax showed modest additive effects (ZIP synergy scores <10) across all 4 cell lines (Figure 3).
Enitociclib is synergistic with anti-MM chemotherapies. Three-dimensional (3D) response surface plots for combinatory activity of enitociclib with bortezomib, lenalidomide, pomalidomide, or venetoclax treated in MM cell lines for 96 hours. Cell viability was measured by Alamar Blue assay. Percent cell viability was normalized to corresponding treatment with DMSO (vehicle control). Synergy score is calculated by SynergyFinder based on the ZIP interaction model.7 Red indicates synergism, and green indicates antagonism of the respective drug combinations. Maximal synergistic effects (MSEs) are indicated when the synergy score is >10, and the drug concentrations at which the MSE occurs are highlighted in yellow.
Enitociclib is synergistic with anti-MM chemotherapies. Three-dimensional (3D) response surface plots for combinatory activity of enitociclib with bortezomib, lenalidomide, pomalidomide, or venetoclax treated in MM cell lines for 96 hours. Cell viability was measured by Alamar Blue assay. Percent cell viability was normalized to corresponding treatment with DMSO (vehicle control). Synergy score is calculated by SynergyFinder based on the ZIP interaction model.7 Red indicates synergism, and green indicates antagonism of the respective drug combinations. Maximal synergistic effects (MSEs) are indicated when the synergy score is >10, and the drug concentrations at which the MSE occurs are highlighted in yellow.
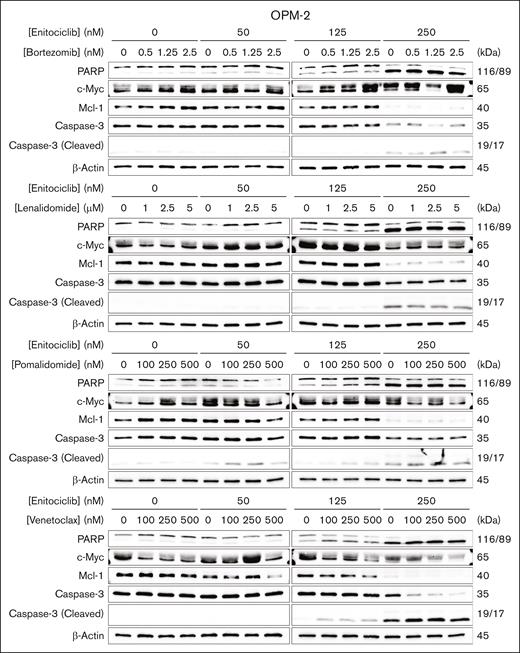
We then sought to examine the changes in protein expression levels of MM-related oncogenes and apoptosis markers in response to the 2-drug combinations in a representative MM cell. Interestingly, we observed a dose-dependent repression of c-Myc protein in OPM-2 cells treated in combination with enitociclib and pomalidomide or venetoclax (Figure 4). The most robust inhibition of Mcl-1 protein was observed for the venetoclax combination in a dose-dependant manner in OPM-2 cells, which has MCL1 gene amplification (Figure 4). Increased cleavage of PARP and caspase-3 were also seen in these combinations in a dose-dependent manner (Figure 4). Overall, these data suggest clinical potential for enitociclib to be used in combination with conventional anti-MM agents.
Enitociclib enhances apoptosis and oncogene repression. Western blotting of OPM-2 cells treated with enitociclib in combination with bortezomib, lenalidomide, pomalidomide, or venetoclax for 6 hours. Total cell lysates were prepared and analyzed by immunoblotting to detect the levels of markers associated with apoptosis (total and cleaved PARP and caspase-3 and Mcl-1) and short half-life oncogene proteins (c-Myc and PCNA). β-actin was used as a loading control. Molecular masses are indicated in kDa.
Enitociclib enhances apoptosis and oncogene repression. Western blotting of OPM-2 cells treated with enitociclib in combination with bortezomib, lenalidomide, pomalidomide, or venetoclax for 6 hours. Total cell lysates were prepared and analyzed by immunoblotting to detect the levels of markers associated with apoptosis (total and cleaved PARP and caspase-3 and Mcl-1) and short half-life oncogene proteins (c-Myc and PCNA). β-actin was used as a loading control. Molecular masses are indicated in kDa.
Enitociclib shows mechanism-based efficacy in vivo as a single agent and in combination against murine models of MM
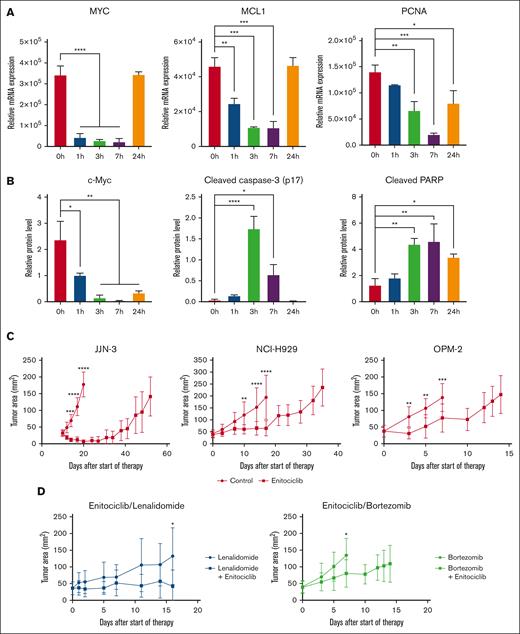
To determine the mechanism of action of enitociclib in vivo, a single dose of 15 mg/kg enitociclib was administered IV in mice bearing JJN-3 cells, and tumors were extracted at several time points up to 24 hours. Enitociclib was able to transiently inhibit the transcription of MYC, MCL1, and PCNA (Figure 5A) and promote apoptosis by the induction of caspase-3 and PARP cleavage (Figure 5B), with the onset of drug-induced effects observed as early as 1 hour after treatment. Protein levels of c-Myc were also abated for up to 24 hours (Figure 5B). Interestingly, BCL2 levels remained consistent after treatment (supplemental Figure 2). These results suggest that enitociclib maintains its mechanistic efficacy in MM when administered IV.
Enitociclib is effective against MM in vivo. (A-B) In vivo mechanism of action of enitociclib in mice bearing JJN-3 MM xenografts upon a single dose of 15 mg/kg enitociclib administered IV compared with 80% PEG400 vehicle. Messenger RNA transcript (A) and protein levels (B) were normalized to housekeeping genes in L32 and β-actin, respectively. (C) In vivo antitumor activity of enitociclib administered as a single agent. For JJN-3, NCI-H929, and OPM-2 MM xenografts, enitociclib was dosed 15 mg/kg IV once weekly compared with vehicle control. (D) To study combinations, enitociclib was dosed 15 mg/kg IV once weekly in combination with 50 mg/kg lenalidomide orally daily or 0.8 mg/kg bortezomib intraperitoneally twice weekly in OPM-2 MM xenografts. ∗P < .05.
Enitociclib is effective against MM in vivo. (A-B) In vivo mechanism of action of enitociclib in mice bearing JJN-3 MM xenografts upon a single dose of 15 mg/kg enitociclib administered IV compared with 80% PEG400 vehicle. Messenger RNA transcript (A) and protein levels (B) were normalized to housekeeping genes in L32 and β-actin, respectively. (C) In vivo antitumor activity of enitociclib administered as a single agent. For JJN-3, NCI-H929, and OPM-2 MM xenografts, enitociclib was dosed 15 mg/kg IV once weekly compared with vehicle control. (D) To study combinations, enitociclib was dosed 15 mg/kg IV once weekly in combination with 50 mg/kg lenalidomide orally daily or 0.8 mg/kg bortezomib intraperitoneally twice weekly in OPM-2 MM xenografts. ∗P < .05.
Next, we sought to assess the efficacy of enitociclib to reduce tumor burden in vivo to better evaluate its potential clinical utility in patients with MM. Murine models of MM were generated using JJN-3, NCI-H929, and OPM-2 cells that readily established xenograft tumors. Mice were randomized to vehicle vs enitociclib at the time disease was evident and treated until end point. Enitociclib was dosed 15 mg/kg administered IV once per week. As a single agent, enitociclib-treated mice had reduced tumor volumes and prolonged survival time compared with vehicle-treated mice (Figure 5C). To examine the therapeutic benefit of combining enitociclib with conventional anti-MM agents, mice bearing OPM-2 cells were randomly administered with lenalidomide (50 mg/kg taken orally daily), bortezomib (0.8 mg/kg intraperitoneally twice weekly), or the respective combination with enitociclib. Increased efficacy of enitociclib in combination with lenalidomide or bortezomib was observed (Figure 5D). No significant changes in weight were observed with treatment (supplemental Figure 3). These data provide evidence that the anti-MM activity of enitociclib is maintained in vivo and provides effective therapeutic relief by delaying tumor cell growth.
Discussion
We took a candidate approach to determining the anti-MM mechanism of action of the CDK9-specific small-molecule inhibitor enitociclib. Our small-molecule library screening approach revealed that MM cells were remarkably sensitive to single-agent CDK inhibitors, particularly CDK9 inhibition, providing the rationale for this study to characterize and investigate the molecular mechanism of activity of enitociclib in MM. Enitociclib activity in MM cells was similar to the IC50 values reported in lymphoma and leukemia cells.6,8 We found that enitociclib potently and directly inhibited CDK9-mediated phosphorylation of RNA polymerase II S2/S5 and repressed the expression of oncogenes that are crucial for MM cell survival and proliferation. Combinatory approaches can reduce the amount of drug required to produce an anticancer effect, therefore lessening the potential of on- and off-target drug toxicities. We explored a panel of conventional anti-MM drugs in combination with enitociclib and found that varying additive or synergistic interactions were observed in each combination at clinically relevant concentrations. However, antagonistic effects at certain concentrations were also noted in some cell lines, highlighting the importance of optimizing the dose range for each combination. Notably, enitociclib reduced the expression of MCL1, which has been shown to confer sensitivity to venetoclax.15,16 Accordingly, CDK9 inhibition has also demonstrated synergy with venetoclax in leukemia models.17 Lastly, enitociclib showed significant in vivo efficacy as a single agent and in combination with lenalidomide or bortezomib across multiple murine MM xenograft models, and its mechanism of action was recapitulated in vivo.
MM cells are highly sensitive to enitociclib. The high sensitivity may be due to the instability of oncoproteins expressed in MM cells, which require perpetual replenishment through de novo synthesis.18,19 As a result, their expression and activity may be significantly affected by an agent that disrupts transcription. Enitociclib inhibits CDK9 function, leading to a disruption in gene transcription.20 This disruption results in a quick degradation of MM oncoproteins because of their instability and short protein half-lives. Because MM cells are highly dependent on these oncogenic signals, their loss of expression leads to rapid apoptosis. Therefore, enitociclib shows promise as a potential treatment option for MM due to its ability to disrupt transcription and rapidly degrade MM oncoproteins, resulting in the induction of apoptosis.
The results of our studies are, to our knowledge, the first to demonstrate that enitociclib has anti-MM activity. It also targets specific oncogenic pathways related to oncoproteins such as c-Myc, Mcl-1, and PCNA, resulting in the suppression of growth and the rapid induction of apoptosis. We also identify potential effective combinations for use with enitociclib in MM. These results demonstrate that enitociclib is effective in cell lines with unfavorable prognostic factors, such as p53 deletion and t(4; 14), and not restricted to the chromosome 1q gain subset where one of the genes at the 1q21 locus is MCL1.21-24 These findings provide a solid basis and biological rationale for conducting further optimization studies of CDK9 inhibitors, which could have important clinical implications for improving outcomes in patients with MM.
Acknowledgments
Visual abstract was created with BioRender.com.
This study was supported by funding from the Kids Cancer Care foundation (project #10024168) and in part by a research grant from Vincerx Pharma (project #10036310) to the study laboratory that was administered by the University of Calgary.
Authorship
Contribution: All authors contributed to the conceptualization and design of the study; A.N., P.N., and N.J.B. provided supervision; S.T., P.S., R.M., B.S.-L., and J.B. performed experiments and analyzed data; S.T., M.M.F., B.S.-L., and A.J.J. wrote and edited the manuscript; P.S. revised the final version; and all authors reviewed and approved the final manuscript.
Conflict-of-interest disclosure: M.M.F., B.S.-L., A.J.J., J.B., R.I., and A.H. report employment with Vincerx Pharma. The remaining authors declare no competing financial interests.
Correspondence: Aru Narendran, University of Calgary, 3330 Hospital Dr NW, HMRB 326, Calgary, AB, Canada, T2N 4N1; email: a.narendran@ucalgary.ca.
References
Author notes
Data may be available on request from the corresponding author, Aru Narendran (a.narendran@ucalgary.ca).
The full-text version of this article contains a data supplement.