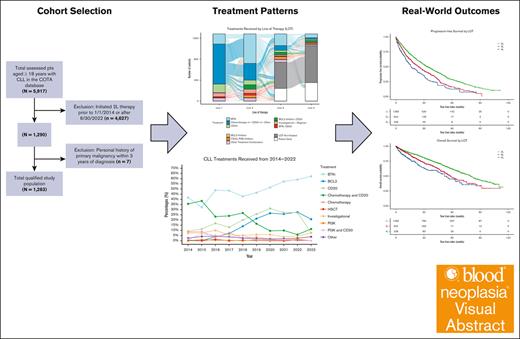
Key Points
Targeted agents have revolutionized CLL treatment, shown by decreased use of chemoimmunotherapy and increased use of targeted inhibitors from 2014 to 2023.
Median times to events were generally shorter for each subsequent LOT; unmet need persists among patients with CLL receiving ≥2L LOT.
Visual Abstract
The development of targeted agents for chronic lymphocytic leukemia (CLL) has transformed the treatment paradigm for patients with CLL. Because of this evolving treatment landscape, contemporaneous evidence was needed related to US treatment patterns and outcomes among patients treated in the real-world. Using COTA’s electronic health records–based database, we examined characteristics, treatment patterns, and outcomes of patients receiving ≥2 lines of therapy (LOTs). A total of 1283 adult patients with CLL were identified who initiated second LOT (2L) between 1 January 2014 and 30 June 2022. Of those patients, 542 (42.2%) later received third-line (3L) therapy, of whom 228 (42.1%) went on to receive fourth-line (4L) therapy. Overall, >18% of patients died after 2L initiation and before 3L initiation, and more than a quarter died before 4L initiation. Most patients were White (77.7%), male (60.6%), aged ≥65 years (68.8%), and treated in a community practice setting (87.8%). From 2014 to 2023, the use of chemoimmunotherapy in any ≥2L LOT decreased, whereas use of Bruton tyrosine kinase inhibitor and B-cell lymphoma 2 inhibitor therapy increased. Across endpoints, median times to event(s) were generally shorter with each subsequent LOT received, both in the overall population and among patients receiving a given therapy in different LOTs. With a median follow-up time from 2L initiation of 38.0 months, median real-world time to next treatment, progression-free survival, and overall survival was 31.9, 33.8, and 80.1 months, respectively. Despite great advancements in CLL treatments since 2014, unmet need persists for patients receiving late LOT.
Introduction
Despite the development of highly active targeted therapies over the past decade, chronic lymphocytic leukemia (CLL) remains generally incurable.1 Historically, chemoimmunotherapy (CIT) was the main therapy used for CLL treatment, but beginning in 2014 with the approval of Bruton tyrosine kinase inhibitors (BTKis) and phosphoinositide-3-kinase inhibitors (PI3Kis) and, later, a B-cell lymphoma 2 inhibitor (BCL2i), CLL has experienced a fast-evolving treatment landscape. Some patients have more aggressive disease and are at higher risk of disease progression after first-line (1L) treatment, especially those with CLL with known adverse molecular characteristics, such as deletion and/or mutation of TP53 or unmutated immunoglobulin heavy-chain variable region (IGHV) gene.2
The development of the targeted agents, such as ibrutinib, idelalisib, venetoclax, duvelisib, acalabrutinib, zanubrutinib, and pirtobrutinib (first approved by the US Food and Drug Administration in 2014, 2014, 2016, 2018, 2019, 2023, and 2023, respectively), has transformed the treatment paradigm and improved health outcomes for patients with CLL/small lymphocytic lymphoma (SLL) both in the 1L setting and for previously treated patients. Despite improvements in treatment outcomes for patients with relapsed/refractory (R/R) CLL/SLL with the development of targeted agents in recent years, those with R/R disease, especially those with high-risk molecular characteristics, continue to have worse outcomes across each subsequent line of therapy (LOT).3
Because of the rapidly evolving treatment landscape in CLL, there is a need for real-world (rw) evidence related to treatment patterns and outcomes among patients who received second or later LOT (≥2L) for CLL. The main objective of this study was to examine the characteristics, treatment patterns, and outcomes of a large, contemporaneous cohort of rw patients with CLL/SLL receiving ≥2L by LOT in the United States. We sought to understand the evolution of treatment patterns over this dynamic time period in the history of CLL management.
Methods
Study design
We conducted a retrospective, observational cohort study using the COTA rw database, a Health Insurance Portability and Accountability Act (HIPAA)–compliant database comprising longitudinal data pertaining to the diagnosis, clinical management, and outcomes of patients with cancer. Data are abstracted from the electronic health records of partnered healthcare provider sites in the United States, leveraging both human abstraction and technologic methods to transform structured and unstructured data into a standardized data model. The COTA provider network represents diverse treatment settings including both community and academic practices, with a primary geographic concentration in the Northeast and Southern regions of the United States. COTA’s deidentified rw database is approved by Western Institutional Review Board (WIRB)-Copernicus Group (WCG) for use in research.
Patients were identified in COTA’s rw database and were eligible for the study if they met the following criteria: aged ≥18 years at diagnosis with confirmed CLL/SLL and who initiated 2L therapy between 1 January 2014 and 30 June 2022. Patients were ineligible if they had a documented diagnosis of an additional concurrent primary malignancy at the time of CLL/SLL diagnosis or Richter transformation any time before 2L initiation or a history of other primary malignancies, excluding localized basal and squamous cell skin cancers, within 3 years before CLL/SLL diagnosis. Characteristics, treatment patterns, and rw outcomes were examined overall, by LOT, by most common treatment received by LOT, and by 2L initiation year (prepandemic vs pandemic [2014-2019 vs 2020-2022]).
The LOT algorithm used in this study is disease specific and incorporates internal and external clinical guidance to programmatically assign LOTs to abstracted data. LOT is assigned and advanced based on various parameters. LOT begins with the first regimen received for the cancer diagnosis, and all regimens received within a specified timeframe of the initial regimen are combined into a single LOT, in the absence of any documented progression. LOT will continue until a regimen is discontinued because of progression or inadequate response, a new drug or regimen is added outside of the prespecified window, or a gap in treatment occurs, all of which will end the current LOT and initiate a new LOT. Conditioning regimens, maintenance regimens, stem cell transplants within 1 year of preceding therapy, and the de-escalation or dropping of drugs from a regimen will not advance the LOT.
The index date for the study was the date of initiation of the relevant LOT, and the observation period was defined as the time from index date to the date of patient death or last visit date (if date of death was not available) before 25 August 2023 (data-lock date). The window for assessment of baseline characteristics was from 30 days before the date of diagnosis to the date of initiation of 2L therapy.
Statistical analysis
Patient and clinical characteristics and treatment patterns were summarized as appropriate using means, medians, and/or patient counts and percentages. The 10 most common treatment regimen(s) by LOT were identified. Characteristics, reasons for treatment discontinuation, and RW outcomes were examined by most common treatment regimens.
Time-to-event (TTE) endpoints, including rw time to next treatment (rwTTNT; defined as the time from index date to initiation of the next LOT or death), rw time to treatment discontinuation (rwTTD; defined as the time from index date to treatment discontinuation for any reason), rw duration of response (rwDOR; defined as the time from the first clinician-assessed complete response or partial response to the first assessment of progressive disease or the initiation of subsequent treatment, if no progression documented), rw progression-free survival (rwPFS; defined as the time from index date to progression event, Richter transformation, or death), and rw overall survival (rwOS; defined as the time from index date to date of death), were assessed using the Kaplan-Meier (KM) method. Patients without an event were censored at the date of last visit for all endpoints except rwOS for which patients were censored at the last clinically relevant date (ie, date of laboratory analysis).
The association of treatment with all time-to-event endpoints was evaluated using univariate and multivariable models. The multivariable Cox proportional-hazards regression model adjusted for the following variables chosen a priori: age (continuous), sex, race, practice type, Charlson Comorbidity Index (CCI) score, Eastern Cooperative Oncology Group (ECOG) performance status, and cytogenetic risk (defined as high risk [at least 1 of: del(17p) and/or TP53 mutation and/or IGHV unmutated], low risk [defined as none of the aforementioned mutations], or unknown [defined as at least 1 unknown test result and none of the aforementioned mutations]).
Results
Population characteristics
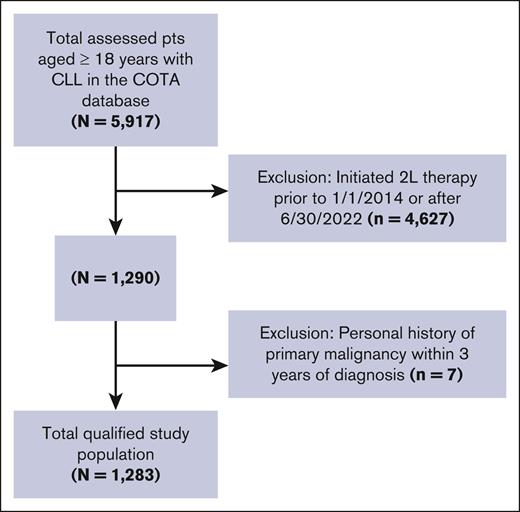
From the COTA database, 1283 patients were identified who met the study criteria (Figure 1). Of the 2L study population, 542 patients (42.2%) went on to receive 3L therapy, of whom 228 (42.1%) went on to receive 4L therapy. Ninety-five patients (41.7% of 4L) received 5L therapy, of which 38 (40%) received 6L. More than 18% of patients (n = 242) died after the initiation of 2L and before initiation of 3L therapy, and more than a quarter died before initiation of 4L. Forty-one patients (3.2% of the study population) experienced Richter transformation during the study period after 2L initiation, and 30 patients (73.2% of those with Richter transformation) died. The majority of the study population was White (77.7%), male (60.6%), aged ≥65 years (68.8%), and treated in a community practice setting (87.8%; Table 1). Patients were most commonly diagnosed in 2014 or earlier (71.4%), and median ages at diagnosis and 1L initiation were 64 (interquartile range [IQR]: 56-72) and 67 years (IQR: 59-75), respectively. Median time from initial diagnosis to 2L initiation was 64.0 months (IQR: 34.7-102.9), and median follow-up time after 2L initiation was 38.0 months (IQR: 17.0-61.4).
Attrition diagram. Population attrition based on inclusion/exclusion criteria.
Characteristics by LOT
| Characteristics . | 2L . | 3L . | 4L . |
|---|---|---|---|
| N = 1283 . | N = 542 . | N = 228 . | |
| Age (y) at initiation of LOT, n (%) | |||
| <65 | 400 (31.2) | 145 (26.8) | 62 (27.2) |
| ≥65 | 883 (68.8) | 397 (73.2) | 166 (72.8) |
| Age (y) at initiation of LOT, median (IQR) | 70.0 (63.0-78.0) | 71.0 (64.0-79.0) | 72.0 (64.0-78.3) |
| Sex, n (%) | |||
| Female | 506 (39.4) | 230 (42.4) | 92 (40.4) |
| Race, n (%) | |||
| Black or African American | 99 (7.7) | 44 (8.1) | 21 (9.2) |
| Asian | 8 (0.6) | 6 (1.1) | 1 (0.4) |
| White | 997 (77.7) | 406 (74.9) | 173 (75.9) |
| Other race | 154 (12.0) | 69 (12.7) | 30 (13.2) |
| Unknown | 25 (1.9) | 17 (3.1) | 3 (1.3) |
| Ethnicity, n (%) | |||
| Hispanic | 107 (8.3) | 45 (8.3) | 24 (10.5) |
| Non-Hispanic | 1,134 (88.4) | 479 (88.4) | 200 (87.7) |
| Unknown | 42 (3.3) | 18 (3.3) | 4 (1.8) |
| Practice type, n (%) | |||
| Academic | 156 (12.2) | 76 (14.0) | 39 (17.1) |
| Community | 1,127 (87.8) | 466 (86.0) | 189 (82.9) |
| Year of initial diagnosis, n (%) | |||
| ≤2014 | 916 (71.4) | 413 (76.2) | 188 (82.5) |
| 2015-2019 | 349 (27.2) | 126 (23.2) | 39 (17.1) |
| ≥2020 | 18 (1.4) | 3 (0.6) | 1 (0.4) |
| Follow-up time from initial diagnosis (mo), median (IQR) | 106.0 (71.9-150.1) | 120.0 (85.3-160.4) | 128.2 (94.3-164.9) |
| Time from initial diagnosis to 2L initiation (mo), median (IQR) | 64.0 (34.7-102.9) | 61.0 (33.1-100.4) | 57.5 (32.6-102.2) |
| Follow-up time from 2L initiation (mo), median (IQR) | 38.0 (17.0-61.4) | 53.7 (34.6-73.1) | 61.8 (44.2-82.1) |
| ECOG performance status, n (%) | |||
| 0-1 | 833 (64.9) | 323 (59.6) | 127 (55.7) |
| ≥2 | 50 (3.9) | 20 (3.7) | 11 (4.8) |
| Unknown | 400 (31.2) | 199 (36.7) | 90 (39.5) |
| Rai stage at diagnosis, n (%) | |||
| 0 | 356 (27.7) | 144 (26.6) | 56 (24.6) |
| I | 252 (19.6) | 105 (19.4) | 38 (16.7) |
| II | 120 (9.4) | 47 (8.7) | 19 (8.3) |
| III/IV | 208 (16.2) | 92 (17.0) | 43 (18.9) |
| Unknown | 347 (27.0) | 154 (28.4) | 72 (31.6) |
| Cytogenetic risk,∗n (%) | |||
| Low risk | 44 (3.4) | 16 (3.0) | 8 (3.5) |
| High risk | 284 (22.1) | 138 (25.5) | 65 (28.5) |
| Unknown | 955 (74.4) | 388 (71.6) | 155 (68.0) |
| Bulky disease,†n (%) | |||
| Presence | 93 (7.2) | 44 (8.1) | 21 (9.2) |
| Absence | 145 (11.3) | 66 (12.2) | 23 (10.1) |
| Unknown | 1,045 (81.4) | 432 (79.7) | 184 (80.7) |
| TP53, n (%) | |||
| Negative | 561 (43.7) | 218 (40.2) | 85 (37.3) |
| Positive | 122 (9.5) | 67 (12.4) | 36 (15.8) |
| Unknown | 600 (46.8) | 257 (47.4) | 107 (46.9) |
| IGHV, n (%) | |||
| Unmutated | 182 (14.2) | 77 (14.2) | 36 (15.8) |
| Mutated | 81 (6.3) | 25 (4.6) | 11 (4.8) |
| Unknown | 1020 (79.5) | 440 (81.2) | 181 (79.4) |
| Characteristics . | 2L . | 3L . | 4L . |
|---|---|---|---|
| N = 1283 . | N = 542 . | N = 228 . | |
| Age (y) at initiation of LOT, n (%) | |||
| <65 | 400 (31.2) | 145 (26.8) | 62 (27.2) |
| ≥65 | 883 (68.8) | 397 (73.2) | 166 (72.8) |
| Age (y) at initiation of LOT, median (IQR) | 70.0 (63.0-78.0) | 71.0 (64.0-79.0) | 72.0 (64.0-78.3) |
| Sex, n (%) | |||
| Female | 506 (39.4) | 230 (42.4) | 92 (40.4) |
| Race, n (%) | |||
| Black or African American | 99 (7.7) | 44 (8.1) | 21 (9.2) |
| Asian | 8 (0.6) | 6 (1.1) | 1 (0.4) |
| White | 997 (77.7) | 406 (74.9) | 173 (75.9) |
| Other race | 154 (12.0) | 69 (12.7) | 30 (13.2) |
| Unknown | 25 (1.9) | 17 (3.1) | 3 (1.3) |
| Ethnicity, n (%) | |||
| Hispanic | 107 (8.3) | 45 (8.3) | 24 (10.5) |
| Non-Hispanic | 1,134 (88.4) | 479 (88.4) | 200 (87.7) |
| Unknown | 42 (3.3) | 18 (3.3) | 4 (1.8) |
| Practice type, n (%) | |||
| Academic | 156 (12.2) | 76 (14.0) | 39 (17.1) |
| Community | 1,127 (87.8) | 466 (86.0) | 189 (82.9) |
| Year of initial diagnosis, n (%) | |||
| ≤2014 | 916 (71.4) | 413 (76.2) | 188 (82.5) |
| 2015-2019 | 349 (27.2) | 126 (23.2) | 39 (17.1) |
| ≥2020 | 18 (1.4) | 3 (0.6) | 1 (0.4) |
| Follow-up time from initial diagnosis (mo), median (IQR) | 106.0 (71.9-150.1) | 120.0 (85.3-160.4) | 128.2 (94.3-164.9) |
| Time from initial diagnosis to 2L initiation (mo), median (IQR) | 64.0 (34.7-102.9) | 61.0 (33.1-100.4) | 57.5 (32.6-102.2) |
| Follow-up time from 2L initiation (mo), median (IQR) | 38.0 (17.0-61.4) | 53.7 (34.6-73.1) | 61.8 (44.2-82.1) |
| ECOG performance status, n (%) | |||
| 0-1 | 833 (64.9) | 323 (59.6) | 127 (55.7) |
| ≥2 | 50 (3.9) | 20 (3.7) | 11 (4.8) |
| Unknown | 400 (31.2) | 199 (36.7) | 90 (39.5) |
| Rai stage at diagnosis, n (%) | |||
| 0 | 356 (27.7) | 144 (26.6) | 56 (24.6) |
| I | 252 (19.6) | 105 (19.4) | 38 (16.7) |
| II | 120 (9.4) | 47 (8.7) | 19 (8.3) |
| III/IV | 208 (16.2) | 92 (17.0) | 43 (18.9) |
| Unknown | 347 (27.0) | 154 (28.4) | 72 (31.6) |
| Cytogenetic risk,∗n (%) | |||
| Low risk | 44 (3.4) | 16 (3.0) | 8 (3.5) |
| High risk | 284 (22.1) | 138 (25.5) | 65 (28.5) |
| Unknown | 955 (74.4) | 388 (71.6) | 155 (68.0) |
| Bulky disease,†n (%) | |||
| Presence | 93 (7.2) | 44 (8.1) | 21 (9.2) |
| Absence | 145 (11.3) | 66 (12.2) | 23 (10.1) |
| Unknown | 1,045 (81.4) | 432 (79.7) | 184 (80.7) |
| TP53, n (%) | |||
| Negative | 561 (43.7) | 218 (40.2) | 85 (37.3) |
| Positive | 122 (9.5) | 67 (12.4) | 36 (15.8) |
| Unknown | 600 (46.8) | 257 (47.4) | 107 (46.9) |
| IGHV, n (%) | |||
| Unmutated | 182 (14.2) | 77 (14.2) | 36 (15.8) |
| Mutated | 81 (6.3) | 25 (4.6) | 11 (4.8) |
| Unknown | 1020 (79.5) | 440 (81.2) | 181 (79.4) |
ECOG, Eastern Cooperative Oncology Group; IGHV, immunoglobulin heavy-chain variable region gene.
High cytogenetic risk defined as at least 1 of: del(17p) and/or TP53 mutation and/or IGHV unmutated; low cytogenetic risk defined as none of: del(17p) and/or TP53 mutation and/or IGHV unmutated; and unknown defined as at least 1 unknown test result and none of: del(17p) and/or TP53 mutation and/or IGHV unmutated.
Bulky disease is reported as documented in the electronic health records by the treating physician.
Treatment patterns
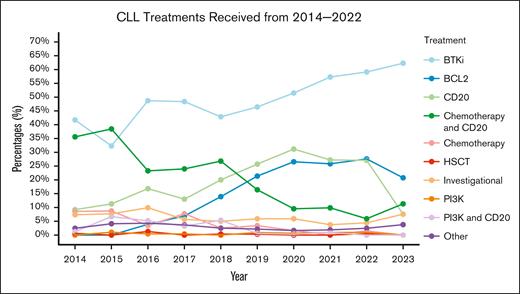
We found that CLL/SLL treatment patterns in the United States changed meaningfully from 2014 to 2023 (Figure 2). Namely, use of CIT (anti-CD20 with chemotherapy) in any ≥2L LOT decreased markedly, from 35.6% in 2014 to 11.3% in 2023, whereas use of BTKi and BCL2i therapies increased from 41.7% and 0.0% in 2014 to 62.3% and 20.8% in 2023, respectively. Use of investigational therapy remained relatively constant throughout the same time frame (7.4% in 2014 to 7.5% in 2023).
Treatment use line graph. Frequency of therapy receipt by year among patients treated in 2014 and thereafter in the rw setting. Percentages indicate person-LOTs in the given year, meaning that if a patient initiated 2L and 3L in 2015, both LOTs would be represented in 2015 as unique LOTs. If patients received BTKi and BCL2i in the same LOT, the patient is represented in both categories. If the patient received CIT in 1 LOT, the patient is represented only in “Chemotherapy and CD20”; this is also true of “PI3K and CD20.” HSCT: hematopoietic stem cell transplantation.
Treatment use line graph. Frequency of therapy receipt by year among patients treated in 2014 and thereafter in the rw setting. Percentages indicate person-LOTs in the given year, meaning that if a patient initiated 2L and 3L in 2015, both LOTs would be represented in 2015 as unique LOTs. If patients received BTKi and BCL2i in the same LOT, the patient is represented in both categories. If the patient received CIT in 1 LOT, the patient is represented only in “Chemotherapy and CD20”; this is also true of “PI3K and CD20.” HSCT: hematopoietic stem cell transplantation.
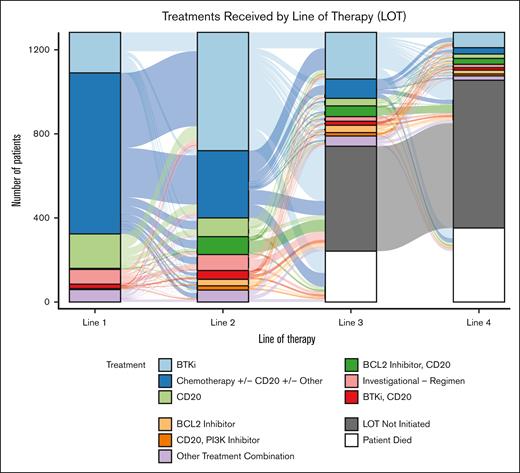
Over half (52.0%) of the study population received CIT with/without other therapy in 1L. Less than 1% of patients received a BCL2i with/without anti-CD20 in 1L, and 16.6% received a BTKi with/without anti-CD20. The most commonly prescribed 2L therapies were: ibrutinib (n = 431, 33.6%), bendamustine + rituximab (BR; n = 204, 15.9%), acalabrutinib (n = 118, 9.2%), investigational regimen (n = 76, 5.9%), rituximab (n = 70, 5.5%), and rituximab + venetoclax (n = 56, 4.4%; Table 2). The most commonly prescribed regimens in 3L and 4L are shown in Table 2 and Figure 3. The most common reason why patients who received BTKis in 2L discontinued therapy was toxicity (44.2% for ibrutinib, and 45% for acalabrutinib), whereas patients who received fixed-duration therapies most often completed therapy (59.4% for BR, and 68.2% for rituximab + venetoclax). Median rwTTD for patients on ibrutinib and acalabrutinib in 2L were 25.4 (95% confidence interval [CI]: 20.6-33.6) and 30.0 months (95% CI: 21.7 to not reached [NR]), respectively, and median rwTTD for patients on rituximab + venetoclax in 2L was 21.3 months (95% CI: 12.2-24.0). Median rwTTD for patients on BR and rituximab monotherapy in 2L were 4.0 (95% CI: 3.0-4.6) and 0.9 (95% CI: 0.7-1.4), respectively.
Most common treatment regimens by LOT
| Most common treatment regimens . | n (%) . |
|---|---|
| 2L (N = 1283) | |
| Ibrutinib | 431 (33.6) |
| Bendamustine, rituximab | 204 (15.9) |
| Acalabrutinib | 118 (9.2) |
| Investigational regimen | 76 (5.9) |
| Rituximab | 70 (5.5) |
| Rituximab, venetoclax | 56 (4.4) |
| Chlorambucil, obinutuzumab | 32 (2.5) |
| Venetoclax | 31 (2.4) |
| Obinutuzumab, venetoclax | 29 (2.3) |
| Cyclophosphamide, fludarabine, rituximab | 26 (2.0) |
| Other treatment regimens | 210 (16.4) |
| 3L (N = 542) | |
| Ibrutinib | 133 (24.5) |
| Acalabrutinib | 75 (13.8) |
| Bendamustine, rituximab | 47 (8.7) |
| Venetoclax | 35 (6.5) |
| Rituximab, venetoclax | 34 (6.3) |
| Rituximab | 24 (4.4) |
| Investigational regimen | 22 (4.1) |
| Obinutuzumab, venetoclax | 17 (3.1) |
| Idelalisib, rituximab | 15 (2.8) |
| Chlorambucil | 11 (2.0) |
| Other treatment regimens | 129 (23.8) |
| 4L (N = 228) | |
| Ibrutinib | 42 (18.4) |
| Acalabrutinib | 26 (11.4) |
| Rituximab, venetoclax | 22 (9.6) |
| Venetoclax | 18 (7.9) |
| Bendamustine, rituximab | 16 (7.0) |
| Investigational regimen | 16 (7.0) |
| Rituximab | 11 (4.8) |
| Idelalisib, rituximab | 9 (3.9) |
| Obinutuzumab | 8 (3.5) |
| Ibrutinib, rituximab | 7 (3.1) |
| Other treatment regimens | 53 (23.2) |
| Most common treatment regimens . | n (%) . |
|---|---|
| 2L (N = 1283) | |
| Ibrutinib | 431 (33.6) |
| Bendamustine, rituximab | 204 (15.9) |
| Acalabrutinib | 118 (9.2) |
| Investigational regimen | 76 (5.9) |
| Rituximab | 70 (5.5) |
| Rituximab, venetoclax | 56 (4.4) |
| Chlorambucil, obinutuzumab | 32 (2.5) |
| Venetoclax | 31 (2.4) |
| Obinutuzumab, venetoclax | 29 (2.3) |
| Cyclophosphamide, fludarabine, rituximab | 26 (2.0) |
| Other treatment regimens | 210 (16.4) |
| 3L (N = 542) | |
| Ibrutinib | 133 (24.5) |
| Acalabrutinib | 75 (13.8) |
| Bendamustine, rituximab | 47 (8.7) |
| Venetoclax | 35 (6.5) |
| Rituximab, venetoclax | 34 (6.3) |
| Rituximab | 24 (4.4) |
| Investigational regimen | 22 (4.1) |
| Obinutuzumab, venetoclax | 17 (3.1) |
| Idelalisib, rituximab | 15 (2.8) |
| Chlorambucil | 11 (2.0) |
| Other treatment regimens | 129 (23.8) |
| 4L (N = 228) | |
| Ibrutinib | 42 (18.4) |
| Acalabrutinib | 26 (11.4) |
| Rituximab, venetoclax | 22 (9.6) |
| Venetoclax | 18 (7.9) |
| Bendamustine, rituximab | 16 (7.0) |
| Investigational regimen | 16 (7.0) |
| Rituximab | 11 (4.8) |
| Idelalisib, rituximab | 9 (3.9) |
| Obinutuzumab | 8 (3.5) |
| Ibrutinib, rituximab | 7 (3.1) |
| Other treatment regimens | 53 (23.2) |
Treatments received by LOT. Sankey plot showing treatments received by the study population in 1L to 4L. Chemotherapy, chemotherapy + CD20 (CIT), and chemotherapy + CD20 + other regimens are combined into 1 category (chemotherapy w/wo CD20 w/wo other). All other therapies and combinations not stated explicitly are combined in the “other treatment combination” category. w/wo, with/without.
Treatments received by LOT. Sankey plot showing treatments received by the study population in 1L to 4L. Chemotherapy, chemotherapy + CD20 (CIT), and chemotherapy + CD20 + other regimens are combined into 1 category (chemotherapy w/wo CD20 w/wo other). All other therapies and combinations not stated explicitly are combined in the “other treatment combination” category. w/wo, with/without.
Outcomes
Rw outcomes varied both by LOT and by therapy received within that LOT. Across endpoints, median times to event(s) were generally shorter with each subsequent LOT received both in the overall population and among patients receiving a given therapy in different LOTs. With a median follow-up time from 2L initiation of 38.0 months (IQR: 17.0-61.4), median rwTTNT from 2L, 3L, and 4L was 31.9 (95% CI: 28.5-36.0), 23.1 (95% CI: 19.2-27.4), and 15.0 months (95% CI: 11.5-21.8), respectively (supplemental Figure 1). Median rwPFS from 2L, 3L, and 4L was 33.8 (95% CI: 30.8-37.6), 24.5 (95% CI: 20.5-28.3), and 16.5 months (95% CI: 13.5-23.2), respectively (Figure 4).
Kaplan-Meier curve: rwPFS and rwOS by LOT. rwPFS (top) and rwOS (bottom) by LOT 2-4 among the study population and indexed to the initiation of the specified LOT.
Kaplan-Meier curve: rwPFS and rwOS by LOT. rwPFS (top) and rwOS (bottom) by LOT 2-4 among the study population and indexed to the initiation of the specified LOT.
Median rwTTNT from 2L, 3L, and 4L initiation among patients who received ibrutinib were 38.0 (95% CI: 32.3-45.2), 31.2 (95% CI: 25.5-40.8), and 30.2 months (95% CI: 21.8-51.9), respectively. Median rwTTNT from 2L, 3L, and 4L among patients who received acalabrutinib were 49.5 (95% CI: 30.0 to NR), 18.8 months (95% CI: 14.5 to NR), and NR (95% CI: 6.3 to NR), respectively. Median rwPFS from 2L, 3L, and 4L initiation among patients who received ibrutinib was 40.3 (95% CI: 33.3-47.9), 28.6 (95% CI: 24.6-37.0), and 30.6 months (95% CI: 15.0-68.1), respectively. Median rwPFS from 2L, 3L, and 4L among patients who received acalabrutinib were 32.1 (95% CI: 28.3 to NR), 19.6 (95% CI: 17.0 to NR), and 20.2 months (95% CI: 3.8 to NR), respectively.
Median rwDOR decreased over subsequent LOTs (33.4 months from 2L, 24.1 months from 3L, and 15.1 months from 4L), and varied by treatment received (39.0 months from 2L initiation among patients who received ibrutinib, 48.4 months from 2L initiation among patients who received acalabrutinib, and 52.8 months from 2L initiation among patients who received venetoclax + rituximab; supplemental Table 1). Median rwOS from 2L was >6.5 years (80.1 months; 95% CI: 73.4-85.3) for the study population (Figure 4). Median rwOS from 3L and 4L were 58.1 (95% CI: 52.6-72.0) and 51.9 months (95% CI: 44.9-70.5), respectively.
After adjusting for age, sex, race, practice type, CCI score, ECOG performance status, and cytogenetic risk, treatment with BR and fludarabine + cyclophosphamide + rituximab (FCR) resulted in statistically significantly shorter rwTTNT than with ibrutinib in 2L (supplemental Figure 2). Similarly, in the adjusted models, BR, FCR, and rituximab monotherapy were each significantly associated with shorter rwDOR in 2L than ibrutinib (supplemental Table 2). Finally, treatment with BR, rituximab monotherapy, or FCR in 2L were significantly associated with shorter rwPFS than ibrutinib in 2L, adjusting for the previously mentioned covariates (supplemental Table 3).
Subgroup analysis
A subgroup analysis was also performed examining characteristics, treatment patterns, and outcomes of patients who initiated 2L therapy in 2014 to 2019 (prepandemic group) or 2020 to 2022 (pandemic group). Of the study population, 936 (73.0%) patients initiated 2L therapy in 2014 to 2019, and 347 (27.0%) initiated 2L therapy in 2020 to 2022 (Table 3). Patient characteristics were similar between the 2 groups, with the exception of year of diagnosis (79.8% diagnosed in 2014 or earlier among the prepandemic group, and 48.7% in the pandemic group). Treatment patterns varied between the prepandemic and pandemic groups. Most common 2L therapies received among patients who initiated 2L in 2014 to 2019 were ibrutinib (n = 360, 38.5%), BR (n = 187, 20.0%), and rituximab (n = 58, 6.2%), whereas most common 2L therapies among patients who initiated 2L in 2020 to 2022 were acalabrutinib (n = 104, 30.0%), ibrutinib (n = 71, 20.5%), and rituximab + venetoclax (n = 29, 8.4%; supplemental Table 4).
Characteristics by subgroup
| Characteristics . | Prepandemic N = 936 . | Pandemic N = 347 . |
|---|---|---|
| Age (y) at initiation of 2L, n (%) | ||
| <65 | 489 (52.2) | 170 (49.0) |
| ≥65 | 447 (47.8) | 177 (51.0) |
| Sex, n (%) | ||
| Female | 364 (38.9) | 142 (40.9) |
| Race, n (%) | ||
| Black or African American | 68 (7.3) | 31 (8.9) |
| Asian | 6 (0.6) | 2 (0.6) |
| White | 744 (79.5) | 253 (72.9) |
| Other race | 102 (10.9) | 52 (15.0) |
| Unknown | 16 (1.7) | 9 (2.6) |
| Ethnicity, n (%) | ||
| Hispanic | 75 (8.0) | 32 (9.2) |
| Non-Hispanic | 831 (88.8) | 303 (87.3) |
| Unknown | 30 (3.2) | 12 (3.5) |
| Practice type, n (%) | ||
| Academic | 114 (12.2) | 42 (12.1) |
| Community | 822 (87.8) | 305 (87.9) |
| Year of initial diagnosis, n (%) | ||
| ≤2014 | 747 (79.8) | 169 (48.7) |
| 2015-2019 | 189 (20.2) | 160 (46.1) |
| ≥2020 | 0 (0.0) | 18 (5.2) |
| Follow-up time from initial diagnosis (mo), median (IQR) | 112.3 (77.4-155.7) | 91.9 (60.1-128.6) |
| Time from initial diagnosis to 2L initiation (mo), median (IQR) | 60.0 (33.5-98.1) | 73.0 (41.0-109.5) |
| Follow-up time from 2L initiation (mo), median (IQR) | 49.3 (28.9-69.1) | 18.1 (10.0-30.1) |
| ECOG performance status, n (%) | ||
| 0-1 | 636 (67.9) | 197 (56.8) |
| ≥2 | 33 (3.5) | 17 (4.9) |
| Unknown | 267 (28.5) | 133 (38.3) |
| Rai stage at diagnosis, n (%) | ||
| 0 | 241 (25.7) | 115 (33.1) |
| I | 200 (21.4) | 52 (15.0) |
| II | 91 (9.7) | 29 (8.4) |
| III/IV | 153 (16.3) | 55 (15.9) |
| Unknown | 251 (26.8) | 96 (27.7) |
| Cytogenetic risk,∗n (%) | ||
| Low risk | 30 (3.2) | 14 (4.0) |
| High risk | 184 (19.7) | 100 (28.8) |
| Unknown | 722 (77.1) | 233 (67.1) |
| Bulky disease,†n (%) | ||
| Presence | 70 (7.5) | 23 (6.6) |
| Absence | 113 (12.1) | 32 (9.2) |
| Unknown | 753 (80.4) | 292 (84.1) |
| TP53, n (%) | ||
| Negative | 370 (39.5) | 191 (55.0) |
| Positive | 79 (8.4) | 43 (12.4) |
| Unknown | 487 (52.0) | 113 (32.6) |
| IGHV, n (%) | ||
| Unmutated | 112 (12.0) | 70 (20.2) |
| Mutated | 53 (5.7) | 28 (8.1) |
| Unknown | 771 (82.4) | 249 (71.8) |
| Characteristics . | Prepandemic N = 936 . | Pandemic N = 347 . |
|---|---|---|
| Age (y) at initiation of 2L, n (%) | ||
| <65 | 489 (52.2) | 170 (49.0) |
| ≥65 | 447 (47.8) | 177 (51.0) |
| Sex, n (%) | ||
| Female | 364 (38.9) | 142 (40.9) |
| Race, n (%) | ||
| Black or African American | 68 (7.3) | 31 (8.9) |
| Asian | 6 (0.6) | 2 (0.6) |
| White | 744 (79.5) | 253 (72.9) |
| Other race | 102 (10.9) | 52 (15.0) |
| Unknown | 16 (1.7) | 9 (2.6) |
| Ethnicity, n (%) | ||
| Hispanic | 75 (8.0) | 32 (9.2) |
| Non-Hispanic | 831 (88.8) | 303 (87.3) |
| Unknown | 30 (3.2) | 12 (3.5) |
| Practice type, n (%) | ||
| Academic | 114 (12.2) | 42 (12.1) |
| Community | 822 (87.8) | 305 (87.9) |
| Year of initial diagnosis, n (%) | ||
| ≤2014 | 747 (79.8) | 169 (48.7) |
| 2015-2019 | 189 (20.2) | 160 (46.1) |
| ≥2020 | 0 (0.0) | 18 (5.2) |
| Follow-up time from initial diagnosis (mo), median (IQR) | 112.3 (77.4-155.7) | 91.9 (60.1-128.6) |
| Time from initial diagnosis to 2L initiation (mo), median (IQR) | 60.0 (33.5-98.1) | 73.0 (41.0-109.5) |
| Follow-up time from 2L initiation (mo), median (IQR) | 49.3 (28.9-69.1) | 18.1 (10.0-30.1) |
| ECOG performance status, n (%) | ||
| 0-1 | 636 (67.9) | 197 (56.8) |
| ≥2 | 33 (3.5) | 17 (4.9) |
| Unknown | 267 (28.5) | 133 (38.3) |
| Rai stage at diagnosis, n (%) | ||
| 0 | 241 (25.7) | 115 (33.1) |
| I | 200 (21.4) | 52 (15.0) |
| II | 91 (9.7) | 29 (8.4) |
| III/IV | 153 (16.3) | 55 (15.9) |
| Unknown | 251 (26.8) | 96 (27.7) |
| Cytogenetic risk,∗n (%) | ||
| Low risk | 30 (3.2) | 14 (4.0) |
| High risk | 184 (19.7) | 100 (28.8) |
| Unknown | 722 (77.1) | 233 (67.1) |
| Bulky disease,†n (%) | ||
| Presence | 70 (7.5) | 23 (6.6) |
| Absence | 113 (12.1) | 32 (9.2) |
| Unknown | 753 (80.4) | 292 (84.1) |
| TP53, n (%) | ||
| Negative | 370 (39.5) | 191 (55.0) |
| Positive | 79 (8.4) | 43 (12.4) |
| Unknown | 487 (52.0) | 113 (32.6) |
| IGHV, n (%) | ||
| Unmutated | 112 (12.0) | 70 (20.2) |
| Mutated | 53 (5.7) | 28 (8.1) |
| Unknown | 771 (82.4) | 249 (71.8) |
High cytogenetic risk defined as at least 1 of: del(17p) and/or TP53 mutation and/or IGHV unmutated; low cytogenetic risk defined as none of: del(17p) and/or TP53 mutation and/or IGHV unmutated; and unknown defined as at least 1 unknown test result and none of: del(17p) and/or TP53 mutation and/or IGHV unmutated.
Bulky disease is reported as documented in the electronic health records by the treating physician.
The prepandemic group experienced 12-month rwTTNT and rwPFS rates of 75% (95% CI: 72-78) and 76% (95% CI: 73-79) from 2L initiation, respectively, with a median follow-up time from 2L initiation of 49.3 months (IQR: 28.9-69.1; supplemental Table 5). Twelve-month rwTTNT and rwPFS rates from 2L initiation for the pandemic group were 76% (95% CI: 72-81) and 79% (95% CI: 74-83), respectively, with a median follow-up time from 2L initiation of 18.1 months (IQR: 10.0-30.1).
Discussion
We sought to characterize the contemporary ≥2L CLL population and examine rw treatment patterns and outcomes among this cohort. At the time of the initiation of this study, the existing literature focused predominantly on treatment patterns from diagnosis through 1L therapy, which highlighted the need for updated rw evidence related to treatment patterns and outcomes of patients who receive later LOTs in the United States. Furthermore, given the continued innovation in CLL treatment and the increase in therapeutic options for patients with R/R disease, this study sought to fill a gap in the literature regarding rw treatment patterns in later LOTs and outcomes for patients receiving targeted agents.
Our results are comparable with those from other rw studies. The demographic characteristics of our study population, including race (78% White) and sex (61% male), were broadly consistent with existing literature.4,5 Median age at diagnosis was 64 years in our population, which is marginally younger than median age results in other rw studies.4-6 This is possibly because of the requirement of 2L initiation in our study inclusion criteria, thereby selecting for younger patients who were more fit to receive subsequent LOTs or who, because of diagnosis at a younger age, had greater time to receive 1L and subsequent LOTs. Additionally, some studies show that patients diagnosed with CLL/SLL at a younger age (≤55 years) are more likely to have documented adverse prognostic factors and, therefore, are likely to require more LOTs through the course of their CLL.7
The broad use of the watch-and-wait paradigm for most patients is supported by our data, because the median ages at diagnosis and 1L initiation were 64 and 67 years, respectively. Additionally, results of the treatment pattern analysis in our study highlight the evolving landscape of CLL treatment among patients who received ≥2L LOTs during the study period. Patients most often received CIT, BTKis, or anti-CD20 antibodies in 1L, representing the treatment paradigm consistent with CLL treatment guidelines at the time of diagnosis (71.4% diagnosed in 2014 or earlier).5 Our analysis confirmed widespread uptake of targeted agents in the United States over the study period, as demonstrated by the marked increase in use of BTKi and BCL2i therapies from 2014 to 2023. Over the same time period, our results show a meaningful decrease in use of CIT. These trends are comparable with results from other rw studies, which demonstrate greater use of BTKis and other targeted therapies in the United States in recent years.8 Among patients who initiated 2L therapy in 2020 or later in our study (pandemic group), BTKi- and BCL2i-based therapies were the most common therapies used (acalabrutinib: n = 104, 30.0%; ibrutinib: n = 71, 20.5%). This is again consistent with the changing treatment paradigm as a result of the approval of targeted agents, beginning in 2014, although could also potentially reflect hesitation about using more immunosuppressive CIT regimens during the pandemic.
Our study demonstrates that use of targeted agents results in improved health outcomes for patients who received ≥2L LOTs relative to CIT, as shown by longer median rwTTNT, rwPFS, and rwOS for patients who received BTKi and BCL2i therapies than those who received BR and other historically used regimens. This mirrors results from clinical trials, which have previously demonstrated the efficacy of targeted agents, namely ibrutinib, acalabrutinib, and venetoclax, for improving outcomes for patients with R/R CLL.9-11 This finding has also been demonstrated in rw studies in which treatment with targeted therapies resulted in longer treatment-free survival when compared with treatment with FCR or BR with a similar trend in OS.12
Additionally, our study confirms the observation of shorter median times to event across subsequent LOTs for key endpoints.3 In the CLL CONNECT registry, median event-free survival in 2L and 3L was 19.0 and 13.0 months, respectively, and median OS from 2L and 3L initiation was 63.0 and 38.0 months, respectively. Although these results demonstrate shorter median times to events than our results, likely because of differences between inclusion criteria and time frames for the studies, the trend of deterioration of key outcomes over subsequent LOTs is clear and highlights the continued unmet need in this population. The significant rate of premature treatment discontinuation because of toxicity for patients who received BTKis highlights the higher rate of toxicities for this class of therapies in the rw setting compared with the rates reported in clinical trials, and further underscores the unmet need among this cohort.13
Finally, although treatment patterns analyzed by 2L initiation year confirmed the uptake of targeted agents in recent years, additional follow-up time is needed to determine whether patients who initiated 2L in 2020 to 2022 experienced better health outcomes than patients who initiated 2L between 2014 and 2019. Notably, for patients who initiated 2L therapy in 2014 to 2019, ibrutinib was the most commonly prescribed 2L regimen (38.5%, n = 360/936). For patients who initiated 2L in 2020 to 2022, acalabrutinib was more frequently prescribed (acalabrutinib: 30.0%, n = 104/347; ibrutinib: 20.5%, n = 71/347).
A strength of this study is the contemporaneous and longitudinal nature of the data source, which allows the exploration of current treatment patterns and outcomes of interest by LOT. The database contains relevant diagnostic, clinical, and outcome data associated with a patient’s cancer treatment journey, which allowed the evaluation of the evolution of standard of care and treatment use over time. Additionally, the COTA provider network represents diverse treatment settings including both community and academic practice settings, across the United States. The limitations of this study are primarily related to the retrospective nature inherent to rw data. Similar to other rw data sources, this dataset may incompletely capture details about a patient’s care delivered by facilities outside the COTA network or care not explicitly documented in the electronic health records. For example, missingness of molecular marker status may be because of lack of testing and/or lack of reporting of test results. It is not possible to differentiate these reasons for missingness within the dataset. Additionally, although COTA abstracts only regimens prescribed for antineoplastic intent, there may be instances in which regimens (i.e., rituximab monotherapy) prescribed for noncancer treatment (i.e., autoimmune cytopenias) may be captured when prescribing intent is not clear.
Regarding endpoints of interest, in our study, median rwPFS and rwTTNT were similar; however, because of the nature of rw clinical practice, progression documentation in the rw setting does not always strictly adhere to International Workshop on CLL guidelines. Therefore, it is possible that progression documentation may have a higher rate of missingness compared with treatment data used in calculating rwTTNT. As such, some rw studies choose to emphasize rwTTNT as a proxy for rwPFS, because it is assumed that a physician would not initiate a subsequent therapy unless progression or disease relapse is observed.14 Although our results for these endpoints are similar and therefore the impact of rw documentation on rwPFS is likely small, we report both estimates because of the limitation of rw progression documentation.
Overall, our data help to better understand the rapidly evolving treatment paradigm for ≥2L CLL in the rw setting. Although there has been steady uptake of targeted therapies across LOTs, unmet need persists for patients with CLL/SLL who receive later LOTs. Our study demonstrates deterioration in outcomes, including rwPFS and rwOS, as patients advance to later LOTs, even after the approvals of targeted therapies, and highlights the meaningful proportion of patients who discontinue targeted agents because of toxicity. As such, our data highlight the persistent unmet need for new and effective treatment options to improve health outcomes for patients with CLL who have received ≥2L LOTs.
Acknowledgments
Financial support for this study was provided by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ. M.S.D. acknowledges funding support from the National Institutes of Health (1R01CA266298-02A1).
Authorship
Contribution: M.S.D., E.d.N., J.P., L.L.F., C.K.W., and M.S. provided scientific supervision; J.A. conducted the formal analysis; E.d.N. was responsible for study conceptualization and project supervision; C.M.Z. was responsible for project administration and drafting the manuscript; and all authors were responsible for the study methodology and reviewing and editing the manuscript.
Conflict-of-interest disclosure: M.S.D. reports royalties or licenses from UpToDate; consulting fees from AbbVie, Bristol Myers Squibb, Janssen, Secura Bio, Ascentage Pharma, Eli Lilly, Merck, Takeda, Adaptive Biotechnologies, Genentech, MEI Pharma, TG Therapeutics, AstraZeneca, Genmab, Nuvalent, KeyQuest Health, Gerson Lehrman Group, Guidepoint Global, and BeiGene; payment or honoraria from Curio Science LLC, Med-IQ, DAVA Oncology, PeerView, Medical Education Specialists, PlatformQ Health, Plexus Communications, Continuing Education Alliance, Aptitude Health, IntrinsiQ, Bio Ascend, Research to Practice, Talem Health LLC, Physicians’ Education Resource, Trinity Health LLC, Clinical Care Options, Axis Medical, and Projects in Knowledge; participation on a data safety monitoring board or advisory board for German CLL Study Group; leadership or fiduciary role as the cochair of the medical advisory board for the CLL Society; and institutional grants or contracts (to Dana-Farber Cancer Institute) from Novartis, MEI Pharma, and Ascentage Pharma. J.A., C.M.Z., L.L.F., and C.K.W. report current employment and equity ownership in COTA, Inc.. E.d.N. reports current employment in Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, and equity ownership in Merck & Co., Inc., Rahway, NJ. J.P., S.L., M.Z.H.F., and S.R.G. report current employment by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, and equity ownership in Merck & Co., Inc., Rahway, NJ. M.S. reports grants or contracts from Mustang Bio, Genentech, AbbVie, BeiGene, AstraZeneca, Genmab, MorphoSys/Incyte, and Vincerx; consulting fees from AbbVie, Genentech, AstraZeneca, Genmab, Janssen, BeiGene, Bristol Myers Squibb, MorphoSys/Incyte, Kite Pharma, Eli Lilly, Fate Therapeutics, Nurix, and Merck; support for attending meetings and/or travel from Clinical Education Alliance; participation on a data safety monitoring board or advisory board for AbbVie, Genentech, AstraZeneca, Genmab, Janssen, BeiGene, Bristol Myers Squibb, MorphoSys/Incyte, Kite Pharma, Eli Lilly, Fate Therapeutics, Nurix, and Merck; and stock or stock options in Koi Biotherapeutics.
Correspondence: Matthew S. Davids, Department of Medical Oncology, Dana-Farber Cancer Institute, 450 Brookline Ave, Boston, MA 02215; email: matthew_davids@dfci.harvard.edu.
References
Author notes
The data underlying this article were provided by COTA, Inc. and cannot be shared because of privacy reasons; summary-level data are provided throughout the manuscript and in the accompanying tables and figures.
The full-text version of this article contains a data supplement.