Myelofibrosis (MF) is a myeloproliferative neoplasm characterized by constitutional symptoms, progressive cytopenias, and splenomegaly. Activating mutations in the JAK/STAT pathway and cytokine dysregulation driving bone marrow fibrosis and extramedullary hematopoiesis underlie the pathobiology of MF. Although multiple JAK inhibitors are currently approved and provide significant symptom improvement, these agents do not possess disease course modifying potential. Additionally, outcomes are poor for patients who fail JAK inhibitors, highlighting the need for novel mechanism-based therapies and innovative combination strategies. Selinexor, a novel Exportin 1 (XPO1) inhibitor that blocks nuclear export, increases nuclear localization and activity of p53 and other tumor suppressor pathways and decreases cytoplasmic activation of multiple proliferative and profibrotic pathways. Selinexor currently has approved indications in multiple myeloma and lymphoma, with broad potential applications in other malignancies, although it can be limited by toxicity in some settings. Selinexor has shown clinical activity and tolerability in MF, both as monotherapy and, particularly, in combination with ruxolitinib. The collective, early phase trial data support a phase 3 randomized, registration study of selinexor and ruxolitinib in patients with MF naïve to JAK inhibitor therapy. Further work is needed to elucidate the role of XPO1 inhibition as a potential disease-modifying strategy to improve outcomes in MF.
Background
Myelofibrosis (MF) is a myeloproliferative neoplasm (MPN) comprising 2 distinct entities, primary MF (PMF), which develops de novo, and secondary MF, which progresses from an antecedent BCR-ABL1–negative MPN such as essential thrombocythemia (ET) or polycythemia vera (PV).1 Classified as either post-ET/PV MF, secondary MF is characterized by histopathologic bone marrow features that are often indistinguishable from PMF.2 MF represents a clinically heterogeneous group of disorders that are typified by systemic symptoms, progressive cytopenias, hepatosplenomegaly, and cachexia.3
The wide range of MF clinical phenotypes is inherently related to the pathophysiology of these “classic” BCR-ABL1-negative MPNs. Clonal expansion of a single mutated hematopoietic stem cell (HSC) leads to hyperplasia of varying degrees in 1 or multiple hematopoietic cell lines: predominantly erythroid cells in PV, megakaryocytes in ET, and the deposition of reticulin and collagen fibers in PMF.4 Expansion of the malignant HSC is associated with an inflammatory milieu that results in an altered stromal microenvironment, which ultimately contributes to the aberrant trafficking of malignant CD34+ cells to extramedullary sites of hematopoiesis.5 This further hinders normal hematopoiesis, which can manifest as single or multiple lineage cytopenias. Founded on this imbalance of clonal expansion vs normal hematopoiesis, MF is often categorized into a myeloproliferative phenotype and a myelodepletive phenotype.6
Central to the pathogenesis of MPNs, and therefore MF, is constitutive activation of the JAK/STAT signaling pathway. The JAK2V617F somatic mutation, identified in 2005, is now well established as the driver mutation in ∼95% of PV, 50% to 60% of ET, and 50% to 60% of PMF cases.7 Driver mutations involving JAK2, MPL, and CALR lead to constitutive phosphorylation of STAT proteins, as well as activation of various downstream pathways including phosphatidylinositol 3-kinase, mitogen-activated protein kinase (MAPK), and NF-κB.4 Ultimately, this signaling cascade culminates in expression of an array of genes that govern the expression of cellular proliferation and production of inflammatory cytokines. Other subsequently identified somatic driver mutations include acquired subclonal mutations in epigenetic regulators (ASXL1, EZH2, TET2, and IDH1/2), the spliceosome (U2AF1, SRSF2, and SF3B1), and tumor suppressors (TP53), which further influence the biology and phenotype of MF.
While JAK2, CALR, and MPL driver mutations are involved in clonal expansion of the malignant HSC in MPNs, many downstream cell signaling pathways have been further implicated as fundamental mediators of bone marrow fibrosis, specifically transforming growth factor β (TGF-β) and NF-κB.8,9 Bone marrow fibroblasts, which deposit collagen and reticulin, are distinct from the malignant clone but are activated by inflammatory cytokines released by the clonal MPN cells.5 These fibrogenic cytokines are released, in large part, by activated megakaryocytes, underscoring megakaryocyte hyperplasia as fundamental to MF pathophysiology. TGF-β1 elaborated by malignant megakaryocytes has been shown to promote collagen synthesis and is implicated as a critical cytokine-mediating bone marrow fibrosis.10 Additionally, TGF-β1 upregulates osteoprotegerin expression and protein synthesis, which inhibits bone resorption and further promotes bone marrow fibrosis by decreasing matrix degradation.11 To better understand molecular mechanisms involved in megakaryocyte hyperplasia and bone marrow fibrosis, differences in megakaryocyte gene expression were investigated, identifying overexpression of immunophilin FKBP51 in MF megakaryocytes.9 FKBP51 overexpression leads to activation of NF-κB, which indirectly leads to greater production of TGF-β1.12 Although targeting driver mutations is an appealing therapeutic option to eliminate disease at the HSC source, recognition of TGF-β and NF-κB as vital downstream signaling pathways has identified additional targets for modulating bone marrow fibrosis.
JAKis
An improved understanding of the genetic framework and biology of MF has led to clinical development of therapeutics that target the JAK-STAT signaling pathway. The first approved JAK inhibitor (JAKi), ruxolitinib, received US Food and Drug Administration (FDA) approval in 2011 based on the Controlled Myelofibrosis Study with Oral JAK Inhibitor Treatment (COMFORT) 1 and 2 trials, which demonstrated significant spleen volume reduction (SVR), reduction in disease-related symptoms, and improved quality of life.13,14 In a long-term analysis of 5-year pooled data from the COMFORT 1 and 2 trials, ruxolitinib demonstrated improved overall survival (OS) of 5.3 years in International Prognostic Scoring System (IPSS) intermediate-2 and high-risk MF, compared with 3.8 years in the control group.15 Despite lack of disease modification in terms of histopathologic and molecular changes, several long-term updates in the post-FDA approval era of ruxolitinib have shown a significant survival advantage. The European Registry for Myeloproliferative Neoplasms: towards a better understanding of Epidemiology, Survival and Treatment (ERNEST) registry, which enrolled patients with MF from 13 centers across 5 European countries, provided an updated real-world analysis of >1000 patients, which demonstrated a median OS of 7.7 years in patients treated with ruxolitinib, compared with 3.4 years when treated with hydroxyurea alone.16 In a US-based real-world claims-based study, 1677 patients with intermediate- and high-risk MF were divided into 3 groups based on ruxolitinib exposure and FDA approval status at the time of diagnosis. The risk of mortality was significantly lower in the postapproval groups regardless of ruxolitinib exposure, with the lowest mortality in the postapproval group who were exposed to ruxolitinib.17
Fedratinib, which differs from ruxolitinib in its selective inhibition of JAK2 and FMS-like tyrosine kinase 3, was FDA approved based on favorable spleen and symptom improvement in the JAKi-naïve and previously treated populations, reported in the JAKARTA 1 and 2 trials, respectively. Fedratinib was effective in attaining spleen and symptom response in nearly a third of treated patients despite failure of prior ruxolitinib therapy, solidifying its commercial use as a second-line agent.18,19 Fedratinib was granted FDA approval in 2019 with a “black box warning” for Wernicke encephalopathy after an initial clinical hold was placed to investigate the development of neurologic symptoms.20
Nearly all patients with MF will develop anemia during their disease course, and approximately one-third of patients will develop therapy-limiting thrombocytopenia.21,22 In addition, 2 of the most common treatment-related adverse events of ruxolitinib and fedratinib are anemia and thrombocytopenia.13,20 This can make initiation of treatment difficult in patients with MF who are cytopenic and can often lead to dose modifications and interruption in therapy. Recently, momelotinib and pacritinib have been approved for use in patients with anemia and thrombocytopenia, respectively. Momelotinib is a selective inhibitor of JAK1, JAK2, and the bone morphogenic kinase ACVR1. Via inhibition of ACVR1 in preclinical animal models, momelotinib was shown to decrease expression of hepcidin in the liver, leading to release of iron from cellular stores and resultant improved erythropoiesis.23 Momelotinib was FDA approved in September 2023 for the treatment of intermediate- and high-risk MF in adults with anemia based on the landmark phase 3 MOMENTUM clinical trial, in which patients with MF previously treated with a JAKi and with anemia, defined by hemoglobin of <10 g/dL, were randomized to receive either momelotinib or danazol.24 The trial met its primary end point of improvement in MF-related total symptom score (TSS), but, more importantly, hemoglobin stabilization and rate of transfusion independence at week 24 in the momelotinib treatment group were statistically noninferior to that of the danazol group.24 Furthermore, although transfusion independence rates were noninferior at week 24 (30% in the momelotinib arm compared with 20% in the danazol arm), transfusion independence rates at week 48 were 57% in those who continued on momelotinib and 60% in those who crossed over from the danazol arm.25 Similarly, pacritinib, has received FDA approval for the treatment of intermediate and high-risk MF in patients with severe thrombocytopenia, defined as platelet count of <50 x 103/μL. Pacritinib is a selective inhibitor of JAK2 and IRAK1 pathways. Because JAK1 signaling inhibition has been implicated in impaired megakaryopoiesis and thrombopoiesis, sparing of JAK1 inhibition can minimize treatment-related thrombocytopenia.26 Pacritinib received accelerated FDA approval in February 2022 based on the phase 3 PERSIST-2 clinical trial, in which patients with MF and thrombocytopenia (defined by platelet count of <100 x 103/μL), were randomized to pacritinib 200 mg twice daily, pacritinib 400 mg once daily, or best available therapy.27 Spleen response was obtained in 29% of patients treated with pacritinib with platelet counts of <50 x 103/μL compared with only 3% in patients who received best available therapy, including ruxolitinib.27 More recent emerging data confirm that pacritinib is also a more potent ACVR1 inhibitor than momelotinib. In the PERSIST-2 trial, pacritinib was associated with a 37% transfusion independence rate in the cytopenic MF population.28
JAKi combination regimens
Although there are now 4 FDA-approved treatments for MF, further therapeutic options are unfortunately limited for patients that progress on, or cannot tolerate, JAKis. Other agents that target disease-relevant pathways outside of the JAK-STAT pathway are actively being evaluated in clinical trials. Navitoclax is an inhibitor of B-cell lymphoma 2 (Bcl-2)/Bcl-xL, antiapoptotic proteins that are increased as a consequence of JAK-STAT pathway activation.29 Preclinical studies evaluating dual inhibition of JAK2 and downstream Bcl-2/Bcl-xL have demonstrated synergistic activity in MF models.30 Clinically, the addition of navitoclax to ruxolitinib in patients who failed ruxolitinib monotherapy was explored in a phase 2 safety/efficacy trial and demonstrated a tolerable safety profile, a reduction in MF-associated symptoms and spleen volume, and improvement in bone marrow fibrosis.31 Initial results from a phase 3 placebo-controlled trial of navitoclax in combination with ruxolitinib vs ruxolitinib alone in patients with JAKi-naïve, intermediate-2/high-risk MF demonstrated SVR of ≥35% (SVR35) of 63.2% at 24 weeks in the navitoclax plus ruxolitinib group compared with 31.5% in the ruxolitinib plus placebo group, although with no significant difference in TSS.32 Grade 3 adverse events occurred in 85% of patients in the combination arm vs 70% of patients in the placebo arm, including increased rates of grade 3 thrombocytopenia (51% vs 15%), neutropenia (38% vs 4%), and anemia (46% vs 39%).
Pelabresib is an inhibitor of bromodomain and extraterminal (BET) proteins, which regulate transcription of many oncogenes and directly affect NF-κB activity.33 It is postulated that attenuation of the NF-κB pathway through BET inhibition may impart a disease-modifying effect in MF given the well-documented pivotal role of NF-κB in the proinflammatory state.34 Preclinical studies of pelabresib have shown that BET inhibition alone or in combination with JAK inhibition leads to potent reduction in inflammatory signaling, and is associated with significant disease modification in a murine model of MF.35 The phase 2 MANIFEST study included a cohort of 84 patients with JAKi-naïve MF who received combination therapy pelabresib plus ruxolitinib. Notable grade ≥3 toxicities included thrombocytopenia (12%) and anemia (35%). In efficacy analysis at 24 weeks, 68% of patients achieved SVR35 and 56% had a ≥50% reduction in TSS (TSS50). Additionally, of note, 28% of patients had reduction of marrow fibrosis by at least 1 grade, and 29.5% of patients attained at least 25% decrease in JAK2V617F variant allele frequency, suggesting the potential for disease modification.36 Pelabresib is being further evaluated in the phase 3 MANIFEST-2 trial, in which patients who are JAKi naïve are randomized to receive combination pelabresib plus ruxolitinib vs ruxolitinib plus placebo.37 The study includes patients with dynamic IPSS (DIPSS) intermediate-1 MF, a population of patients who have been historically excluded or underrepresented in prior randomized MF trials. Preliminary analysis demonstrated significantly greater SVR at week 24 in the pelabresib plus ruxolitinib treatment group compared with the ruxolitinib plus placebo treatment group, with SVR35 of 65.9% vs 35.2% (P ≤ .001). The pelabresib plus ruxolitinib arm also had a strong trend toward improved TSS50 at 24 weeks (P = .0545), which was evident across all TSS domains. The safety profile of combination pelabresib plus ruxolitinib was consistent with the phase 2 results, and no new concerning signal of toxicity was noted. Importantly, anemia was reported less frequently in the pelabresib plus ruxolitinib group, and a numerically higher proportion of patients achieved a hemoglobin response, with fewer patients requiring red blood cell transfusion.38 With completion of the full MANIFEST-2 analysis, authors anticipate definitive efficacy results of pelabresib combination therapy in patients who are JAKi naïve, as well as insights into treatment initiation at an earlier stage of disease.
Despite these recent advances in targeted therapies, current drug therapy for MF remains mostly palliative in nature, and the only potentially curative treatment modality is allogeneic HSC transplant.39 Unfortunately, survival outcomes after discontinuation of ruxolitinib are poor, with progression-free survival and OS of 6 months and 11 months, respectively.40 Furthermore, although symptom management and spleen reduction with JAKi are clinically meaningful, none of the currently approved treatment options have shown clear disease-modifying capability.
XPO1
Trafficking between the nuclear and cytoplasmic compartments is a highly regulated process mediated at the nuclear pore complex by a class of import and export receptors called karyopherins. Karyopherins bind to specific nuclear localization signals or nuclear export signals (NES) that are located in potential cargo proteins and are rich in hydrophobic residues such as leucine.41,42 The nuclear envelope provides a sequestered environment for DNA replication, RNA transcription, and cell cycle regulation, and most transcription factors and tumor suppressor proteins require nuclear retention to induce gene expression and downstream signaling.43 Mislocalization of nuclear regulatory proteins causes their functional inactivation. This may allow for the evasion of cell cycle arrest, apoptosis, or autophagy in response to DNA damage or metabolic stress.44 As such, aberrant nuclear export of tumor suppressor proteins can play an important role in malignant transformation and resistance to therapy, and it therefore represents a novel target for drug development.45
Exportin 1 (XPO1), also known as chromosome region maintenance 1, is a ubiquitous nuclear export protein in the karyopherin family that drives energy-dependent translocation of a variety of signaling and regulatory proteins of >40 kDa in size as well as various RNA species.46,47 The guanosine triphosphate–binding nuclear protein Ran facilities the export of cargo proteins through complexing with XPO1, inducing a conformational change and allowing binding to the NES domain on the cargo protein.48 Although XPO1 is fundamental to normal cellular function, overexpression or dysregulation of XPO1 in malignant cells leads to an increased export of >200 nuclear proteins and is associated with both poor prognosis and drug resistance.49-54 High expression of XPO1 is found in myeloid diseases such as acute myeloid leukemia and chronic myeloid leukemia; other hematologic malignancies including acute lymphoblastic leukemia and multiple myeloma (MM); and many solid tumor malignancies including ovarian cancer, pancreatic cancer, gastric cancer, sarcomas, melanoma, and cervical cancer.55-57 XPO1 has been shown to target multiple tumor suppressor proteins, growth regulators, and antiapoptotic proteins for nuclear export, including retinoblastoma, p53, adenomatous polyposis coli, IκB, p21, p27, FOXO, and topoisomerase, among others.43 It is also important to highlight the recognized nuclear transport–independent functions of XPO1, including regulating mitotic spindle nucleation and assembly, which may also contribute to dysregulated cell division.58 Therefore, XPO1 inhibition is an intriguing therapeutic target that has shown promising in vitro and in vivo activity across multiple tumor types.
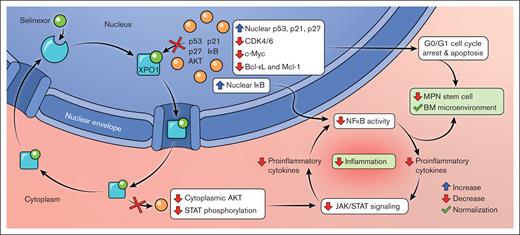
Selinexor (KP-330), along with its second generation analog eltanexor (KPT-8602), is a first-in-class orally bioavailable selective and reversible inhibitor of XPO1 that binds at the cysteine-528 residue within the NES-binding pocket in order to block nuclear export (Figure 1).43 Selinexor has shown efficacy in different tumor models through a variety of mechanisms corresponding to the broad range of XPO1 cargo proteins, including cell cycle arrest; decreased NF-κB signaling; and decreased phosphorylation and activation of STAT, Akt, and other growth pathways.41,42,59 Selinexor treatment of myeloma cells triggers upregulation of gene transcripts for p53 targets and drivers of apoptosis.60 In a murine prostate cancer model, XPO1 inhibition caused increased nuclear localization and degradation of cyclin D1, among other tumor suppressor proteins, which induced cell cycle arrest and reduced tumor proliferation.61 Fluorescent cell tracking in a variety of cancer cell lines exposed to selinexor similarly showed a protracted G1 and early S phase with decreased proliferation and subsequent cell death.62 Additionally, in a model of autosomal dominant polycystic kidney disease, XPO1 inhibition led to cell cycle arrest and decreased cyst growth through downregulation of CDK4.63 Upregulated XPO1 increases cytoplasmic export and degradation of the NF-κB inhibitor IκB, which increases NF-κB signaling and downstream transcriptional activation. Additionally, selinexor has been shown to increase nuclear accumulation of IκB and prevent its phosphorylation and proteasomal degradation, thereby attenuating NF-κB signaling in multiple cancer cell lines, including MM, non-small cell lung cancer, glioma, and sarcoma.60,64-68 Of note, multiple studies have shown synergy with selinexor and proteasome inhibitors in vitro in reducing IκB degradation.64,66-68
Mechanism of XPO1 inhibition in MF. The figure depicts the mechanism of action of selinexor, a novel XPO1 inhibitor, demonstrating the downstream effects on nuclear and cytoplasmic cell signaling pathways relevant to MF pathobiology. Figure created by Lilas Armstrong-Davies and The Mount Sinai Hospital.
Mechanism of XPO1 inhibition in MF. The figure depicts the mechanism of action of selinexor, a novel XPO1 inhibitor, demonstrating the downstream effects on nuclear and cytoplasmic cell signaling pathways relevant to MF pathobiology. Figure created by Lilas Armstrong-Davies and The Mount Sinai Hospital.
Selinexor has similarly shown efficacy in abrogating growth pathways, including those downstream of JAK-STAT signaling. In a model of BCR-ABL1–positive chronic myeloid leukemia and acute lymphoblastic leukemia, XPO1 inhibition was shown to reduce phosphorylation and activation of STAT5, Akt, and MAPK; upregulate tumor suppressors such as p53 and PP2A; and increase sensitivity to imatinib in vitro.69,70 Selinexor similarly repressed STAT3 activation and binding to the survivin promoter in a breast cancer cell line and xenograft model and inhibited Akt activation and cyclin expression in liposarcoma cells.71,72 In chronic lymphocytic leukemia cells, selinexor inhibits phosphorylation of extracellular signal-regulated kinases (ERK) and Akt, which reduces c-Myc activation and subsequent cell cycling.73 Selinexor blocks mammalian target of rapamycin signaling and has potential synergy with everolimus in MM and lymphoma cells.74,75 Interestingly, blockade of XPO1 also reduces export of translational elongation factors such as eIF4E, reducing translation of oncogenes such as MYC, MCL1, and BCL2, potentially increasing sensitivity to the Bcl-2 inhibitor venetoclax.76
In summary, nuclear export blockade via selinexor modulates tumor growth and survival by increasing nuclear localization and activity of p53 and other tumor suppressors, downregulating NF-κB signaling, and decreasing activation and translation of proliferation pathways, which collectively trigger cell cycle arrest and apoptosis in preclinical tumor models.
Clinical data in other malignancies
As noted above, selinexor is currently under evaluation in preclinical and clinical models of multiple malignancies. Selinexor was FDA approved in 2019 for use in MM, both in combination with dexamethasone alone in relapsed and refractory disease in patients who have received at least 4 prior therapies and in combination with dexamethasone and bortezomib in patients who have received at least one prior therapy.77,78 Selinexor and other XPO1 targets continue to be studied in various combinations and in earlier lines of treatment in MM, as well as in the setting of chimeric antigen receptor T cells.79,80
Because of the ubiquitous nature of XPO1, selinexor also continues to be investigated in a multitude of different hematologic malignancies (Table 1). Selinexor gained its third FDA approval in 2020 in relapsed and refractory diffuse large B-cell lymphoma after being investigated as a single agent.81 Early phase 1/2 trials have been initiated in patients with acute myeloid leukemia, which have established selinexor as a safe and tolerable therapeutic option, although with varying efficacy.93 In another preclinical model–informed phase 1 trial of myelodysplastic syndrome with excess blasts, sequential selinexor and azacitidine demonstrated synergy with impressive overall response rates, including in a patient with TP53-mutated disease.84 Finally, although a phase 2 trial of selinexor for Richter transformation in the setting of chronic lymphocytic leukemia failed to show early efficacy, it remains a potential therapeutic option based on preclinical evidence and phase 1 data in combination with ibrutinib.85
Clinical trials of selinexor in other malignancies
| Malignancy . | Study population . | Clinical trial . | Intervention . | Efficacy outcomes . | Toxicity outcomes . | Reference . |
|---|---|---|---|---|---|---|
| MM | Relapsed/refractory (5 prior lines and triple-class refractory) | STORM trial (phase 2b) | Selinexor 80 mg twice weekly plus dexamethasone. | ORR 26%; OS 15.6 mo in patients with at least minimal response vs <2 mo in patients with PD. | Most common grade 3/4: thrombocytopenia (59%), anemia (44%), hyponatremia (22%), and neutropenia (21%). Most common nonhematologic grade 3/4: nausea (10%) and vomiting (3%). | 77 |
| MM | Relapsed/refractory (1-3 prior lines) | BOSTON trial (phase 3) | Bortezomib, dexamethasone, and selinexor, 100 mg weekly vs bortezomib and dexamethasone alone. | Median PFS 13.9 mo in selinexor arm vs 9.5 mo; ORR 76.4% in selinexor arm vs 62.3%; median OS not reached in selinexor arm. | Most common grade 3/4: thrombocytopenia (39% vs 17%), anemia (16% vs 10%), fatigue (13% vs 1%), and pneumonia (12% vs 10%). | 78 |
| DLBCL | Relapsed/refractory (≥2 lines or not a transplant candidate) | SADAL trial (phase 2) | Selinexor 60 mg twice weekly. | ORR 28%; CR 12%; PR 17%; median PFS 2.6 mo; median OS 9.1 mo; median OS was not reached in patients with PR or better. | Most common grade 3/4: thrombocytopenia (46%), neutropenia (24%), anemia (22%), and fatigue (11%). Most common nonhematologic TRAE were limited to grade 1/2. | 81 |
| NHL | Frontline therapy | Phase 1 | R-CHOP plus selinexor (escalating dose to 100 mg weekly), followed by weekly selinexor maintenance for 1 year. | ORR 100%; CR 90% | Recommended phase 2 dose, selinexor 60 mg weekly. Most common grade 3/4: neutropenia (33%), anemia (17%), fatigue (17%), thrombosis (17%), syncope (17%), hypertension (17%), and supraventricular tachycardia (17%). | 82 |
| AML | Frontline therapy (unfit for intensive therapy) | Phase 1/2 | Azacitidine, venetoclax, and selinexor, 60 mg weekly. | ORR 90% | Most common grade ≥ 3: thrombocytopenia (35%), neutropenia (35%), and infections (15%). | 83 |
| MDS-EB | Frontline therapy | Phase 1b/2 | Sequential azacitidine and selinexor (40 mg weekly, 40 mg twice weekly, or 60 mg weekly). | ORR 78.6%; CR 21%. Patients with mutated TP53: CR in 1 of 3; marrow CR in 2 of 3. | MTD and recommended phase 2 dose remain unknown. Most common TRAE (any grade): neutropenia (93%), anemia (87%), thrombocytopenia (80%), and fatigue (50%). | 84 |
| CLL or NHL | Relapsed/refractory | Phase 1 | Ibrutinib plus selinexor (escalating dose: 40 mg weekly, 30 mg twice weekly). | ORR 32% (CLL 44%, DLBCL 33%, and Richter transformation 11%). Median PFS 8.9 mo in CLL and 2.7 mo in NHL. Median OS 58.9 mo in CLL and 5.1 mo in NHL. | MTD selinexor 40 mg weekly with ibrutinib 420 mg daily. Most common grade ≥ 3: thrombocytopenia (24%), anemia (18%), and neutropenia (12%). | 85 |
| Glioblastoma | Relapsed | Phase 2 | Selinexor 50 mg/m2 twice weekly, 60 mg twice weekly, or 80 mg weekly. | 6-month PFS 17.7% (selinexor 80 mg weekly), 10% (selinexor 50 mg/m2 twice weekly), and 7.7% (selinexor 60 mg twice weekly). Median OS 10.5 mo (selinexor 50 mg/m2 twice weekly), 8.5 mo (selinexor 60 mg twice weekly), 10.2 mo (selinexor 80 mg weekly); 28% of patients with reduction in tumor size. | Most common hematologic TRAE (any grade): thrombocytopenia (43%), neutropenia (26%), and anemia (17%). Most common nonhematologic TRAE (any grade): fatigue (60%), nausea (59%), decreased appetite (43%), vomiting (30%), and hyponatremia (20%). | 86 |
| Dedifferentiated liposarcoma | Relapsed/refractory (2-5 prior lines) | Phase 2/3 | Selinexor 60 mg twice weekly vs placebo. | PFS 2.8 mo in selinexor arm vs 2.1 mo; ORR 2.7% in selinexor arm vs 0%. No difference in OS at median 14.6 mo. | Most common grade 3/4: anemia (19%), hyponatremia (11%), asthenia (10%), and thrombocytopenia (10%). | 87 |
| Pancreatic adenocarcinoma | Frontline therapy (metastatic) | Phase 1b | Gemcitabine, nab-paclitaxel, and selinexor (escalating dose: 60 mg weekly, 80 mg weekly). | PR 40% SD 40% PD 20% | MTD selinexor 60 mg weekly. | 88 |
| Triple-negative breast cancer | Relapsed/refractory (metastatic) | Phase 2 | Selinexor 60 mg twice weekly. | No clinical benefit PD 70% SD 30% PR 0% CR 0% | Most common grade 3/4: thrombocytopenia (10%), fatigue (10%), and dyspnea (10%). | 89 |
| Metastatic castration-resistant prostate cancer | Refractory to abiraterone and/or enzalutamide | Phase 2 | Selinexor 60 mg twice weekly. | PFS not reported, study terminated early; PR 25%; SD 50%; unconfirmed PD 25%. | 21% of patients came off study because of unacceptable tolerability; most common TRAE (any grade): anorexia (86%), nausea (64%), weight loss (50%), fatigue (50%), and thrombocytopenia (50%). | 90 |
| Colorectal cancer | Relapsed/refractory (metastatic, unresectable) | SENTINEL (Phase 1) | mFOLFOX plus selinexor (escalating dose: 40 mg, 60 mg, 80 mg on days 1, 3, and 8 of 2-week cycles). | No relevant clinical activity observed. | MTD not reported; 100% of patients at selinexor 40 mg and 66% at selinexor 20 mg dose had DLTs/withdrew. | 91 |
| Endometrial cancer | Frontline therapy or first relapse with PR/CR to platinum-based chemotherapy | SIENDO (Phase 3) | Maintenance selinexor 80 mg weekly vs placebo. | Median PFS 5.7 mo in selinexor arm vs 3.8 mo; wild-type TP53 subgroup: median PFS 27.4 mo in selinexor arm vs 5.2 mo; PFS improvement remained significant in tumors that were MSS/pMMR. | Most common grade 3/4: neutropenia (18% vs 0%), nausea (12% vs 0%), and thrombocytopenia (9% vs 0%). | 92 |
| Malignancy . | Study population . | Clinical trial . | Intervention . | Efficacy outcomes . | Toxicity outcomes . | Reference . |
|---|---|---|---|---|---|---|
| MM | Relapsed/refractory (5 prior lines and triple-class refractory) | STORM trial (phase 2b) | Selinexor 80 mg twice weekly plus dexamethasone. | ORR 26%; OS 15.6 mo in patients with at least minimal response vs <2 mo in patients with PD. | Most common grade 3/4: thrombocytopenia (59%), anemia (44%), hyponatremia (22%), and neutropenia (21%). Most common nonhematologic grade 3/4: nausea (10%) and vomiting (3%). | 77 |
| MM | Relapsed/refractory (1-3 prior lines) | BOSTON trial (phase 3) | Bortezomib, dexamethasone, and selinexor, 100 mg weekly vs bortezomib and dexamethasone alone. | Median PFS 13.9 mo in selinexor arm vs 9.5 mo; ORR 76.4% in selinexor arm vs 62.3%; median OS not reached in selinexor arm. | Most common grade 3/4: thrombocytopenia (39% vs 17%), anemia (16% vs 10%), fatigue (13% vs 1%), and pneumonia (12% vs 10%). | 78 |
| DLBCL | Relapsed/refractory (≥2 lines or not a transplant candidate) | SADAL trial (phase 2) | Selinexor 60 mg twice weekly. | ORR 28%; CR 12%; PR 17%; median PFS 2.6 mo; median OS 9.1 mo; median OS was not reached in patients with PR or better. | Most common grade 3/4: thrombocytopenia (46%), neutropenia (24%), anemia (22%), and fatigue (11%). Most common nonhematologic TRAE were limited to grade 1/2. | 81 |
| NHL | Frontline therapy | Phase 1 | R-CHOP plus selinexor (escalating dose to 100 mg weekly), followed by weekly selinexor maintenance for 1 year. | ORR 100%; CR 90% | Recommended phase 2 dose, selinexor 60 mg weekly. Most common grade 3/4: neutropenia (33%), anemia (17%), fatigue (17%), thrombosis (17%), syncope (17%), hypertension (17%), and supraventricular tachycardia (17%). | 82 |
| AML | Frontline therapy (unfit for intensive therapy) | Phase 1/2 | Azacitidine, venetoclax, and selinexor, 60 mg weekly. | ORR 90% | Most common grade ≥ 3: thrombocytopenia (35%), neutropenia (35%), and infections (15%). | 83 |
| MDS-EB | Frontline therapy | Phase 1b/2 | Sequential azacitidine and selinexor (40 mg weekly, 40 mg twice weekly, or 60 mg weekly). | ORR 78.6%; CR 21%. Patients with mutated TP53: CR in 1 of 3; marrow CR in 2 of 3. | MTD and recommended phase 2 dose remain unknown. Most common TRAE (any grade): neutropenia (93%), anemia (87%), thrombocytopenia (80%), and fatigue (50%). | 84 |
| CLL or NHL | Relapsed/refractory | Phase 1 | Ibrutinib plus selinexor (escalating dose: 40 mg weekly, 30 mg twice weekly). | ORR 32% (CLL 44%, DLBCL 33%, and Richter transformation 11%). Median PFS 8.9 mo in CLL and 2.7 mo in NHL. Median OS 58.9 mo in CLL and 5.1 mo in NHL. | MTD selinexor 40 mg weekly with ibrutinib 420 mg daily. Most common grade ≥ 3: thrombocytopenia (24%), anemia (18%), and neutropenia (12%). | 85 |
| Glioblastoma | Relapsed | Phase 2 | Selinexor 50 mg/m2 twice weekly, 60 mg twice weekly, or 80 mg weekly. | 6-month PFS 17.7% (selinexor 80 mg weekly), 10% (selinexor 50 mg/m2 twice weekly), and 7.7% (selinexor 60 mg twice weekly). Median OS 10.5 mo (selinexor 50 mg/m2 twice weekly), 8.5 mo (selinexor 60 mg twice weekly), 10.2 mo (selinexor 80 mg weekly); 28% of patients with reduction in tumor size. | Most common hematologic TRAE (any grade): thrombocytopenia (43%), neutropenia (26%), and anemia (17%). Most common nonhematologic TRAE (any grade): fatigue (60%), nausea (59%), decreased appetite (43%), vomiting (30%), and hyponatremia (20%). | 86 |
| Dedifferentiated liposarcoma | Relapsed/refractory (2-5 prior lines) | Phase 2/3 | Selinexor 60 mg twice weekly vs placebo. | PFS 2.8 mo in selinexor arm vs 2.1 mo; ORR 2.7% in selinexor arm vs 0%. No difference in OS at median 14.6 mo. | Most common grade 3/4: anemia (19%), hyponatremia (11%), asthenia (10%), and thrombocytopenia (10%). | 87 |
| Pancreatic adenocarcinoma | Frontline therapy (metastatic) | Phase 1b | Gemcitabine, nab-paclitaxel, and selinexor (escalating dose: 60 mg weekly, 80 mg weekly). | PR 40% SD 40% PD 20% | MTD selinexor 60 mg weekly. | 88 |
| Triple-negative breast cancer | Relapsed/refractory (metastatic) | Phase 2 | Selinexor 60 mg twice weekly. | No clinical benefit PD 70% SD 30% PR 0% CR 0% | Most common grade 3/4: thrombocytopenia (10%), fatigue (10%), and dyspnea (10%). | 89 |
| Metastatic castration-resistant prostate cancer | Refractory to abiraterone and/or enzalutamide | Phase 2 | Selinexor 60 mg twice weekly. | PFS not reported, study terminated early; PR 25%; SD 50%; unconfirmed PD 25%. | 21% of patients came off study because of unacceptable tolerability; most common TRAE (any grade): anorexia (86%), nausea (64%), weight loss (50%), fatigue (50%), and thrombocytopenia (50%). | 90 |
| Colorectal cancer | Relapsed/refractory (metastatic, unresectable) | SENTINEL (Phase 1) | mFOLFOX plus selinexor (escalating dose: 40 mg, 60 mg, 80 mg on days 1, 3, and 8 of 2-week cycles). | No relevant clinical activity observed. | MTD not reported; 100% of patients at selinexor 40 mg and 66% at selinexor 20 mg dose had DLTs/withdrew. | 91 |
| Endometrial cancer | Frontline therapy or first relapse with PR/CR to platinum-based chemotherapy | SIENDO (Phase 3) | Maintenance selinexor 80 mg weekly vs placebo. | Median PFS 5.7 mo in selinexor arm vs 3.8 mo; wild-type TP53 subgroup: median PFS 27.4 mo in selinexor arm vs 5.2 mo; PFS improvement remained significant in tumors that were MSS/pMMR. | Most common grade 3/4: neutropenia (18% vs 0%), nausea (12% vs 0%), and thrombocytopenia (9% vs 0%). | 92 |
AML, acute myeloid leukemia; CLL, chronic lymphocytic leukemia; CR, complete response; DLBCL, diffuse large B-cell lymphoma; DLT, dose-limiting toxicity; MDS-EB, myelodysplastic syndrome with excess blasts,; MSS/pMRR, microsatellite stable/mismatch repair proficient; MTD, maximum tolerated dose; NHL, non-Hodgkin lymphoma; ORR, overall response rate; PD, progressive disease; PFS, progression-free survival; PR, partial response; R-CHOP, rituximab, cyclophosphamide, doxorubicin, Oncovin [vincristine], and prednisone; SD, stable disease; TRAE, treatment related adverse event.
Selinexor has been evaluated in early studies in a number of solid tumors, including glioblastoma, endometrial cancer, breast cancer, prostate cancer, colorectal cancer, pancreatic cancer, sarcoma, and melanoma, with mixed efficacy and tolerability (Table 1).86-91,94-96 Notably, it has been studied as a maintenance therapy after initial cytotoxic chemotherapy in the phase 3 SIENDO trial of advanced endometrial cancer.94 In a prespecified exploratory analysis stratified by TP53 mutational status, patients with wild-type TP53 had significantly improved outcomes with selinexor, highlighting the role of selinexor in modulating tumor suppressor pathways and its potential efficacy in malignant cells with intact p53 function.92,97,98
Multiple prior studies of selinexor have been curbed by significant dose-limiting toxicities. Some of the more frequent adverse events noted in patients receiving selinexor include gastrointestinal toxicities, thrombocytopenia, fatigue, and hyponatremia, especially in the twice weekly dosing studied in early clinical trials.41,99 Mechanistic studies revealed that dose-limiting cytopenias were related to an inhibitory effect on thrombopoietin signaling and resulting impairment in megakaryocyte maturation through inhibition of STAT3 phosphorylation.100 Toxicity could therefore be treated with thrombopoietin mimetics and other growth factors. Gastrointestinal and constitutional symptoms, including nausea, vomiting, and fatigue, are also frequent adverse events that were the most common indications for drug discontinuation in early studies.101 Because selinexor crosses the blood-brain barrier, these are thought to be centrally mediated, and use of prophylactic and centrally acting antiemetics can significantly mitigate the effects of nausea and anorexia.99 Additionally, certain central nervous system–mediated toxicities, such as gastrointestinal side effects, may be attenuated with eltanexor, which does not cross the blood-brain barrier.102 Although initially limiting its use in clinical practice, adaptation of lower twice weekly doses, once weekly dosing, and aggressive supportive care with antiemetics and growth factor support has increased selinexor tolerability.103
Selinexor in MF
Given the broad utility of selinexor in multiple tumor types, as well as its ability to reduce NF-κB and downstream JAK-STAT signaling, selinexor has been evaluated in MF murine models, both as monotherapy and in combination with ruxolitinib. Yan et al performed an initial short-hairpin RNA screen of JAK2-mutated cell lines and found that transcripts involved in nuclear-cytoplasmic transport such as RAN and RANBP2 were critical for cellular proliferation and survival.104 Selinexor was found to reduce viability of JAK2 mutated cells in vitro. Notably, selinexor inhibited MF cells ex vivo and increased nuclear localization of p53, but it did not significantly affect growth of wild-type JAK2 cord blood stem cells. When given in conjunction with ruxolitinib, there was a synergistic increase in apoptosis in MF cells compared with cord blood stem cells. The group similarly tested the efficacy of selinexor in a mouse model of post-PV MF and found that although mice treated with ruxolitinib alone acquired resistance to JAK2 inhibitors, treatment with selinexor caused significant reduction in spleen size and white blood cell count, with most pronounced improvement in the group receiving selinexor/ruxolitinib combination therapy.104 Maloof et al have also demonstrated single-agent activity of selinexor, as well as synergistic and additive activity in combination with other MF-directed therapies such as ruxolitinib, pacritinib, momelotinib, pelabresib, and navitoclax when tested in distinct driver mutation cell lines.105 In the HEL cell line, selinexor plus ruxolitinib led to degradation of XPO1 protein as well as enhanced reduction of the prosurvival protein myeloid cell leukemia-1 and increased cleavage of poly-(adenosine 5'-diphosphate-ribose) polymerase. The in vitro antiproliferative activity was associated with downregulation of key gene sets involved in proliferation (MYC), cell senescence (CDKN1A/B), and cell cycle arrest (cyclin D1/A2/B1). Selinexor activity was also observed in a ruxolitinib-resistant HEL subclone at clinically achievable doses.105
Selinexor has also demonstrated effective and selective killing of CD34+ MPN cells harboring JAK2V617F and TP53 mutations.106 Expression levels of TP53 and both the downstream intrinsic and extrinsic apoptotic pathway proteins were increased with reduced expression of prosurvival Bcl-xL and c-Myc and increased cell cycle arrest. In in vitro combination studies, selinexor plus ruxolitinib or the HDM2 antagonist HDM201 led to enhanced and selective depletion of CD34+ MF cells and reduced colony formation. This intriguing data further support the ongoing evaluation of combination selinexor and ruxolitinib, as well as potential future non-JAKi–based combination therapy hinged on both p53-dependent and -independent mechanisms.
In clinical evaluation for MF, selinexor was initially studied as monotherapy at 60 or 80 mg weekly in a phase 2 single-arm study of patients with MF who progressed on, or were intolerant to, a JAKi.107 In an early analysis of 12 patients, 56% of patients had attained SVR of ≥25% and 22% attained SVR35 at 24 weeks of treatment. Five of 8 evaluable patients had reduced symptom score at time of best response. One patient was noted to have had reduction in bone marrow fibrosis from grade 3 to grade 1 at 72 weeks of therapy, suggesting possible disease modification with selinexor therapy. Common grade 3/4 toxicities included fatigue (33%), anemia (33%), and thrombocytopenia (17%). Ten patients required dose reduction because of toxicity, and 6 patients discontinued treatment, including 1 death due to sepsis from a liver abscess.107
Promising results of this selinexor monotherapy study provide rationale for the use of selinexor in other MF subpopulations. For example, patients with thrombocytopenia, who often receive suboptimal doses of JAKi given the on-target side effect of thrombocytopenia with JAK inhibition. A phase 2 study is currently ongoing evaluating selinexor monotherapy for patients with JAKi-naïve MF with a platelet count of 50 × 103 to 99 × 103/μL (NCT05980806, XPORT-MF-044).108 In this 2 arm, sequential trial design, patients in arm 1 will receive selinexor 60 mg weekly, and in arm 2 will receive selinexor 40 mg weekly. Patients who achieve SVR of <10% after 12 weeks or SVR35 after 24 weeks of selinexor monotherapy will have the option to add ruxolitinib or pacritinib to their treatment, based on platelet count. The primary end point is SVR35 at 24 weeks and secondary end points include TSS50 at week 24, anemia response at week 24, OS, and overall response rate. This trial is currently enrolling with planned enrollment of 29 patients to each arm, with an additional 30 patients that can be enrolled independently for the optional expansion arms.108
Selinexor is also currently under clinical evaluation in combination with ruxolitinib in patients with JAKi-naïve disease. In a phase 1 dose escalation study of patients with MF with DIPSS intermediate or high-risk disease, selinexor was administered once weekly at 40 mg or 60 mg in combination with ruxolitinib, per standard of care.109 In a preliminary analysis of 24 patients (10 patients at 40 mg and 14 patients at 60 mg), grade 3/4 toxicities included anemia (37.5%), thrombocytopenia (20.8%), neutropenia (12.5%), and atrial fibrillation (12.5%), with 2 patients (8.3%) discontinuing therapy because of toxicity. Notably, nausea and vomiting were limited to grade 1 events in patients receiving a prophylactic antiemetic, with only 1 patient who did not receive prophylaxis experiencing grade 3 nausea. Additionally, patients demonstrated a median weight increase of 3.3 kg at 24 weeks. At 24 weeks, SVR35 was achieved in 40% of patients at the 40 mg dose and in 79% of patients at the 60 mg dose, with TSS50 in 10% and 58% of patients, respectively. Importantly, 5 of 13 (38%) of patients with available data at 24 weeks showed ≥20% reduction in JAK2/CALR/MPL driver mutation variant allele frequency, and the majority of patients showed decreased levels of inflammatory cytokines, supporting a possible role in disease modification.109 Interestingly, reduction in interleukin-18 levels specifically correlated with SVR and TSS response at week 24 (R2 = 0.29 and 0.28, respectively). It is important to note in this trial the relative stability in the median hemoglobin and platelet counts and the improvement in meaningful weight gain across treated patients, suggesting gastrointestinal toxicity was not occurring to a degree that compromised nutrition.
Table 2 provides a list of active and planned clinical trials evaluating selinexor in all myeloid malignancies.
Active and planned clinical trials evaluating selinexor in myeloid malignancies
| Study population . | Interventions . | Phase . | Primary outcome(s) . | NCT no. . |
|---|---|---|---|---|
| JAKi-naïve MF | Selinexor plus ruxolitinib vs placebo plus ruxolitinib | 1/3 | Phase 1: MTD Phase 3: SVR35 | 04562389 |
| MF, R/R, or intolerant to JAKi | Selinexor monotherapy | 2 | Change in spleen volume | 03627403 |
| MF, JAKi-naïve with moderate thrombocytopenia (50 × 109/L-100 × 109/L) | Selinexor monotherapy | 2 | SVR35 | 05980806 |
| MF, R/R, or intolerant to JAKi | Selinexor monotherapy vs clinicians’ choice | 2 | SVR35 | 04562870 |
| AML, treatment-naïve | Selinexor plus induction/consolidation chemotherapy | 2 | OS | 02835222 |
| AML, treatment-naïve, not candidate for intensive chemotherapy | Selinexor plus azacitidine and venetoclax | 2 | CRc (CR/CRh/CRi) | 05736965 |
| AML, R/R DLBCL, R/R | Selinexor plus venetoclax | 1b | MTD and ORR | 03955783 |
| Pediatric and young adults (aged 2-30 y) with R/R AML | Selinexor and venetoclax plus salvage chemotherapy | 1 | MTD | 04898894 |
| Pediatric patients (aged 1-21 y) with R/R AML and ALL | Selinexor monotherapy | 1 | Safety and tolerability | 02091245 |
| Study population . | Interventions . | Phase . | Primary outcome(s) . | NCT no. . |
|---|---|---|---|---|
| JAKi-naïve MF | Selinexor plus ruxolitinib vs placebo plus ruxolitinib | 1/3 | Phase 1: MTD Phase 3: SVR35 | 04562389 |
| MF, R/R, or intolerant to JAKi | Selinexor monotherapy | 2 | Change in spleen volume | 03627403 |
| MF, JAKi-naïve with moderate thrombocytopenia (50 × 109/L-100 × 109/L) | Selinexor monotherapy | 2 | SVR35 | 05980806 |
| MF, R/R, or intolerant to JAKi | Selinexor monotherapy vs clinicians’ choice | 2 | SVR35 | 04562870 |
| AML, treatment-naïve | Selinexor plus induction/consolidation chemotherapy | 2 | OS | 02835222 |
| AML, treatment-naïve, not candidate for intensive chemotherapy | Selinexor plus azacitidine and venetoclax | 2 | CRc (CR/CRh/CRi) | 05736965 |
| AML, R/R DLBCL, R/R | Selinexor plus venetoclax | 1b | MTD and ORR | 03955783 |
| Pediatric and young adults (aged 2-30 y) with R/R AML | Selinexor and venetoclax plus salvage chemotherapy | 1 | MTD | 04898894 |
| Pediatric patients (aged 1-21 y) with R/R AML and ALL | Selinexor monotherapy | 1 | Safety and tolerability | 02091245 |
ALL, acute lymphoid leukemia; AML, acute myeloid leukemia; DLBCL, diffuse large B-cell lymphoma; CR, complete response; CRc, composite complete response; CRh, complete response with partial hematologic recovery; Cri, complete response with incomplete hematologic recovery; MTD, maximal tolerated dose; NCT, National Clinical Trial; ORR, overall response rate; R/R, relapsed and refractory.
Upcoming phase 3 study
Given the promising phase 1 efficacy noted above, an international phase 3 randomized trial (XPORT-MF-034) is currently enrolling, evaluating selinexor and ruxolitinib combination therapy for patients with JAKi-naïve MF (ClinicalTrials.gov identifier: NCT04562389).110 Eligible patients include adults with DIPSS intermediate-1, intermediate-2, or high-risk MF with splenomegaly, defined by spleen volume of ≥450 cm3 on imaging, as well as the presence of active symptoms, defined by at least 2 symptoms with a score of ≥3 or a TSS of ≥10. Exclusion criteria include platelet count of <100 × 103/μL, >10% blasts in peripheral blood or bone marrow, or prior therapy with a JAKi or XPO1 inhibitor. Patients receive ruxolitinib twice daily and are randomized 2:1 to receive selinexor 60 mg weekly vs placebo, with randomization stratified by DIPSS risk score, spleen volume, and baseline platelet count. The primary end points are SVR35 and TSS50 at 24 weeks, with a key secondary end point of anemia response at week 24, all defined by International Working Group Myeloproliferative Neoplasms Research and Treatment and European Leukemia Network criteria.
Conclusion
Targeting of XPO1 offers a novel approach to the treatment of a broad array of malignancies. Recently emerging preclinical and early clinical data in MF support the global phase 3 trial of selinexor as the first-in-class XPO1 inhibitor, used in combination with ruxolitinib for patients with JAKi-naïve MF. At present, there are no validated clinical or laboratory biomarkers to guide therapeutic decision-making with available JAKis or combination regimens, including selinexor. Within the context of prospective studies, it will be important to evaluate and identify potential molecularly defined patient subsets that may be more likely to benefit from combination therapy with a BET inhibitor, Bcl-2/Bcl-xL inhibitor, or XPO1 inhibitor, while balancing potential increased toxicity. XPO1 alterations (expression level or mutation status) may be of value in developing a predictive biomarker-informed treatment algorithm. Through nuclear transport inhibition, multiple relevant disease related pathways are disturbed, resulting in the net effect of enhanced p53-dependent and -independent apoptosis and cell cycle arrest in CD34+ MPN stem cells. This MPN stem cell–depleting effect may ultimately prove to have disease modifying outcomes. It will be incumbent on randomized phase 3 trials to validate the biologic activity of this agent with outcome measures that are clinically meaningful, such as progression-free survival and OS.
Authorship
Contribution: M.M., Z.M.A., J.W., P.V., and J.M. contributed to the literature review, organization/writing, and editing of this manuscript.
Conflict-of-interest disclosure: P.V. has received consulting fees from AbbVie, Blueprint Medicines, Cogent Biosciences, Incyte, CTI BioPharma Corp (now Sobi), Daiichi Sankyo, GlaxoSmithKline, Karyopharm, Novartis, Pfizer, Genentech, Servier, Stemline, and MorphoSys. J.M. receives research funding paid to the institution from Incyte, Novartis, Bristol Myers Squibb, CTI BioPharma/Sobi, AbbVie, Karyopharm, Kartos, Geron, and PharmaEssentia; and receives consulting fees from Incyte, Novartis, MorphoSys, Karyopharm, Kartos, Geron, PharmaEssentia, Bristol Myers Squibb, AbbVie, Roche, Merck, GlaxoSmithKline, CTI/Sobi, Galecto, and Pfizer. The remaining authors declare no competing financial interests.
Correspondence: John Mascarenhas, Hematology Oncology, Icahn School of Medicine at Mount Sinai, 1 Gustave L Levy Place, Box 1079, New York, NY 10029; email: john.mascarenhas@mssm.edu.
References
Author notes
For any questions, please contact the corresponding author, John Mascarenhas (john.mascarenhas@mssm.edu).