TO THE EDITOR:
Targeting metabolic pathways has emerged as a novel and innovative approach in cancer therapy.1-7 Among these pathways, glutamine metabolism has been shown to play a key role in cancer cell survival and proliferation by fueling biosynthesis, energy production, and redox balance.8-10 Glutaminase 1 (GLS1) and GLS2 form a critical enzymatic complex in glutamine metabolism, catalyzing the conversion of glutamine into glutamate. Inhibitors targeting GLS, such as the GLS1 inhibitor CB-839 (telaglenastat), have demonstrated significant potential in modulating cancer metabolism in lymphoma and other malignancies.8-15 However, clinical trials with CB-839 have shown suboptimal efficacy. This limited success likely stems from the absence of robust biomarkers capable of identifying tumors highly dependent on glutaminolysis. Therefore, identifying patients whose tumors rely on glutamine for growth and survival is essential for optimizing therapeutic outcomes.
1H-based magnetic resonance spectroscopy (1H MRS) is a noninvasive imaging technique that quantitatively assesses in vitro and in vivo metabolic activity of malignant cells and tumors across a wide range of malignancies in experimental models and patients.16 This technology holds significant potential for identifying responses to metabolic pathway inhibitors, including those targeting glutaminolysis, with promising perspectives for clinical translation.
Mantle cell lymphoma (MCL) remains an incurable disease with poor prognosis, particularly in aggressive variants.17-19 Although Bruton tyrosine kinase (BTK) inhibitors have shown promise in MCL therapy, upfront resistance and the eventual development of resistance in nearly all initially responsive patients pose significant clinical challenges.18,19 Therefore, there is a pressing need for novel treatment strategies for MCL and other malignancies, with targeting cellular metabolism emerging as a promising therapeutic frontier.
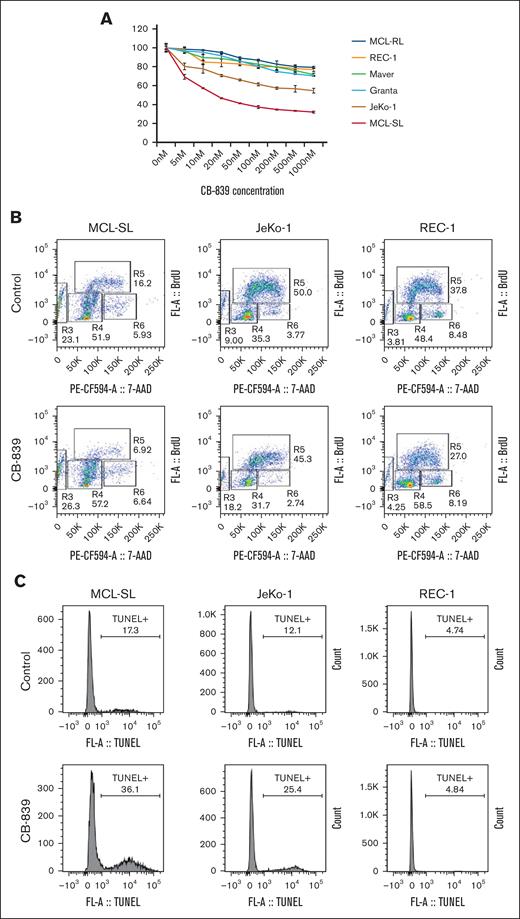
In this study, we used MCL as an index malignancy model to investigate glutamine dependency in cancer and the ability of 1H MRS to detect responses to glutaminolysis inhibition in vitro and in vivo. Using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) conversion assay (please see supplemental Materials and methods for details of the experimental approaches used throughout this study), we examined the effect of GLS inhibitor CB-839 (telaglenastat) on MCL cell growth (Figure 1A). The growth of MCL-SL cells was profoundly suppressed, and that of JeKo-1 cell growth was moderately inhibited. The remaining 4 MCL cell populations exhibited limited sensitivity to CB-839. Although CB-839 impaired cell cycle progression across all tested index MCL cell lines (Figure 1B), with a decrease in S-phase cells from >16% to <7% in MCL-SL cells, from 50% to ∼45% in JeKo-1 cells, and from >38% to 27% in the least sensitive REC-1 cells, it induced apoptotic cell death (Figure 1C) only in MCL-SL (∼36% vs >17%) and JeKo-1 (>25% vs ∼12%) but not in REC-1 cells (∼4.7% vs ∼4.8%).
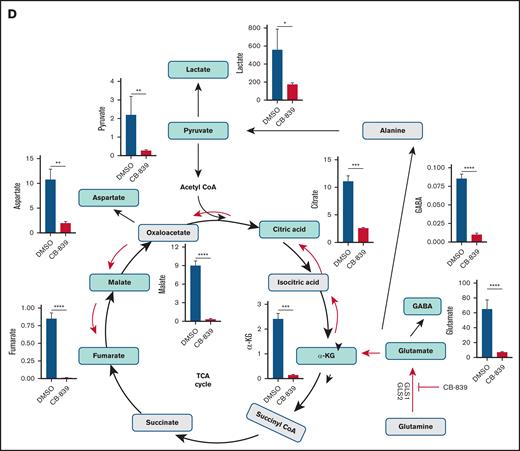
Next, we investigated the metabolic effects of CB-839 in the highly responsive MCL-SL cells (Figure 1D; supplemental Figure 1). CB-839 profoundly reduced glutamate concentrations and its direct derivatives, including gamma-aminobutyric acid (GABA),20-22 and α-ketoglutarate, and other downstream metabolites involved in the tricarboxylic acid cycle, as well as pyruvate and lactate. Consistently, CB-839 suppressed the messenger RNA expression of genes associated with multiple functional pathways, notably oxidative phosphorylation and, to a lesser extent, glycolysis (supplemental Figure 2). These inhibitory effects were corroborated by mitochondrial and glycolysis stress tests (supplemental Figure 3). Basal and maximal respiration were profoundly suppressed in CB-839–sensitive MCL-SL cells, with much less pronounced effects seen in the poorly responsive REC-1 cells. Glycolytic function was impaired exclusively in MCL-SL cells, with no significant changes observed in REC-1 cells.
We further evaluated the effects of CB-839 on the MCL cells using 1H MRS–based high-resolution imaging, a technique that was previously shown to detect metabolites involved in glycolysis and glutaminolysis: lactate and alanine,23 total choline, and taurine.24 In this study, we successfully visualized glutamine and its product, glutamate (supplemental Figure 4). CB-839 treatment resulted in a marked decrease in glutamate concentrations and an accumulation of glutamine across all 3 MCL cell populations tested, particularly in the highly sensitive MCL-SL cells. This confirmed the efficacy of CB-839 in inhibiting glutaminolysis in this lymphoid malignancy model. In contrast, total choline and taurine levels were not significantly affected by CB-839. Importantly, although lactate and alanine levels were significantly reduced in MCL-SL and JeKo-1 cells, no such changes were observed in the poorly responsive REC-1 cells. These findings suggest that lactate and alanine may serve as potential biomarkers of cellular dependency on glutaminolysis and the related cell growth impairment caused by its inhibition (Figure 1A-C).
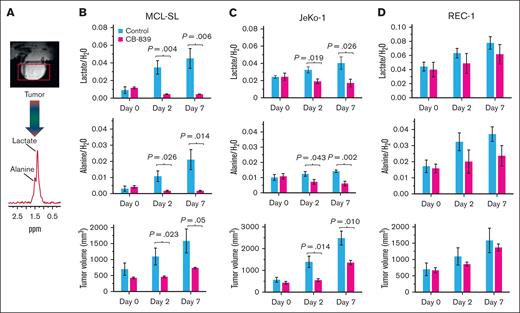
To further validate this hypothesis, we conducted an in vivo study using MCL xenotransplants that mimic the peripheral lymphadenopathy seen in patients with lymphoma. In this model, we examined the impact of CB-839 on intratumoral lactate and alanine concentrations, as detected by 1H MRS (Figure 2A), and correlated these findings with the inhibitor’s effects on tumor volumes. As shown in (Figure 2B-D, upper row), CB-839 significantly reduced lactate concentrations in MCL-SL and JeKo-1 tumors, whereas no such effect was observed in REC-1 tumors. Similar results were seen for alanine (Figure 2B-D, middle row). Both these metabolites exhibited a direct correlation with tumor volume, with CB-839 markedly suppressing tumor growth in MCL-SL and JeKo-1 but not in REC-1 (Figure 2B-D, lower row). These findings indicate that lactate and alanine can serve as biomarkers of effective glutaminolysis inhibition in vivo.
In vivo 1H MRS–detectable biomarkers of GLS inhibition vs tumor volume in MCL xenografts. The spectral peak areas of lactate (upper rows) and alanine (middle rows), normalized to the water signal, were measured by 1H MRS with an Hadamard Selective Multiquantum Coherence (HDMD-Sel-MQC) transfer pulse sequence. (A) A representative subcutaneous MCL xenograft and 1H MRS spectrum acquired with HDMD-Sel-MQC transfer pulse sequence on a 9.4T horizontal bore Bruker console. (B) MCL-SL tumor (n = 5 mice per control and CB-839–treated cohort). (C) JeKo-1 tumor (n = 5). (D) REC-1 tumor (n = 5). CB-839 was administered orally at 200 mg/kg, twice daily, with vehicle-treated controls included for each MCL tumor type.
In vivo 1H MRS–detectable biomarkers of GLS inhibition vs tumor volume in MCL xenografts. The spectral peak areas of lactate (upper rows) and alanine (middle rows), normalized to the water signal, were measured by 1H MRS with an Hadamard Selective Multiquantum Coherence (HDMD-Sel-MQC) transfer pulse sequence. (A) A representative subcutaneous MCL xenograft and 1H MRS spectrum acquired with HDMD-Sel-MQC transfer pulse sequence on a 9.4T horizontal bore Bruker console. (B) MCL-SL tumor (n = 5 mice per control and CB-839–treated cohort). (C) JeKo-1 tumor (n = 5). (D) REC-1 tumor (n = 5). CB-839 was administered orally at 200 mg/kg, twice daily, with vehicle-treated controls included for each MCL tumor type.
In this study, we evaluated 6 patient-derived MCL cell line populations and identified MCL-SL as highly sensitive and JeKo-1 as moderately sensitive to the GLS1 inhibitor CB-839 (telaglenastat). Interestingly, MCL-SL showed no response, and JeKo-1 exhibited only a poor response to BTK inhibition, whereas MCL-RL and REC-1 were sensitive to BTK inhibitors.24 This suggests that targeting glutaminolysis may be particularly effective in the BTK inhibition–resistant MCL. Furthermore, we demonstrated that GLS inhibition significantly disrupts cellular metabolism, primarily affecting the tricarboxylic acid cycle at multiple levels. Finally, we demonstrated that the effectiveness of GLS inhibition can be accurately assessed in vitro and in vivo using 1H MRS–based imaging with 2 index metabolites, alanine and lactate, serving as biomarkers of inhibition-mediated suppression of MCL tumor growth.
From a translational perspective, our findings suggest that 1H MRS–detectable metabolites such as lactate and alanine may serve as early and reliable biomarkers of GLS inhibition in a clinical setting. This approach may not only permit real-time monitoring of therapeutic responses but also facilitate the early identification of patients with MCL most likely to benefit from such therapy. Given the pathogenic role of glutaminolysis across various cancer types, evaluating responses to GLS inhibition using 1H MRS–based imaging may also prove beneficial in non-MCL malignancies.
Our previous study24 demonstrated that impairment of lactate and alanine synthesis also occurred in response to BTK inhibition in sensitive MCL tumors, affecting their growth. This observation, combined with our current finding that a significant reduction in lactate and alanine concentrations indicates effective glutaminolysis inhibition, suggests that these key metabolites may serve as biomarkers of tumor growth inhibition, regardless of the type of targeted therapy used. If this premise proves correct, 1H MRS–based imaging may be applicable for monitoring tumor responses to a broad spectrum of inhibitors targeting oncogenic kinases and metabolic enzymes in various types of cancer.
Animal protocols were approved by the institutional animal care and use committee at the University of Pennsylvania.
Acknowledgments: This work was supported in part by grants from the National Cancer Institute (1R01CA250102, 1R01CA228457, 1R01CA268601, and R21CA280523) and funds from the Fox Chase Cancer Center Institute for Cancer Research.
Contribution: K.N., P.K.G., J.B., D.S.N., A.A.S., and M.A.W. designed research; K.N., P.K.G., J.B., S.W., A.A.S., N.S., S.R., D.R., and S.O. performed research; K.N., P.K.G., J.B., D.S.N., C.L., A.A.S., F.A.-M., D.S.N., and M.A.W. analyzed data; and K.N. and M.A.W. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mariusz A. Wasik, Department of Pathology and Laboratory Medicine, Fox Chase Cancer Center, 333 Cottman Ave, Philadelphia, PA 19111-2497; email: mariusz.wasik@fccc.edu; and Kavindra Nath, University of Pennsylvania, 423 Curie Blvd, Philadelphia, PA 19104-6069; email: kavindra.nath@pennmedicine.upenn.edu.
References
Author notes
Primary data are available on request from the corresponding authors, Mariusz A. Wasik (mariusz.wasik@fccc.edu) and Kavindra Nath (kavindra.nath@pennmedicine.upenn.edu).
The full-text version of this article contains a data supplement.