Key Points
This all-oral quadruplet was well tolerated and had extended 22-month PFS in patients with relapsed myeloma.
Correlative studies identified predictive makers of response and identified alterations in immune subsets with this quadruplet.
Visual Abstract
Clarithromycin is a macrolide antibiotic with anti–multiple myeloma (MM) activity when combined with dexamethasone and immunomodulatory agents. This phase 1/2 study of clarithromycin, ixazomib, pomalidomide, and dexamethasone (ClIPd) assessed tolerability and efficacy in relapsed/refractory MM. The primary end points were the maximal tolerated and recommended phase 2 dose. Key secondary end points were the overall response rate (ORR) (≥partial response), disease control rate (DCR) (≥stable disease), progression-free survival (PFS), and overall survival (OS). All 4 medications were given at full dose for 6 cycles. Pomalidomide, ixazomib, and dexamethasone were given at reduced doses with full-dose clarithromycin in subsequent maintenance cycles until unacceptable toxicity or progression. Clarithromycin was withheld during weeks 1 to 2 of cycle 1 to facilitate correlative studies. A total of 28 patients were evaluable for response/survival. The ORR was 75%; DCR was 100%; 56% achieved ≥very good partial response (VGPR), whereas 14% achieved complete response (CR)/stringent CR. High-risk cytogenetics were not associated with ORR (Fisher exact test, P = 1) or ≥VGPR rates (Fisher exact test, P = .42). The median PFS was 22.2 months (95% confidence interval [CI], 13.3 to not reached [NR]). There was no difference in the median PFS between patients with del(17p) (26.8 months; 95% CI, 10.2 to NR) and those without (22.2 months; 95% CI, 13.3 to NR; log rank, P = .4). The median OS was NR. ClIPd combines convenient oral administration, a tolerable side effect profile, and long duration of disease control. This trial was registered at www.clinicaltrials.gov as #NCT02542657
Introduction
Despite therapeutic advances in the treatment of multiple myeloma (MM) that have led to prolonged survival across all patient populations, myeloma remains incurable and relapse is expected.1 Three drug combinations have demonstrated progression-free survival (PFS) and overall survival (OS) benefits in newly diagnosed and relapsed or refractory (R/R) MM (RRMM) populations.2-5 Quadruplet therapy developed before monoclonal antibodies led to deeper responses and PFS benefits.6 Monoclonal antibody–containing quadruplets are now being moved into earlier lines of therapy. Daratumumab, bortezomib, lenalidomide, and dexamethasone represent a new standard-of-care induction therapy for newly diagnosed patients.7-10 However, these regimens typically involve parenterally administered drugs, which may adversely affect quality of life.11 The move of monoclonal antibodies to front-line therapy highlights the need for effective and tolerable treatments in the R/R setting. The use of novel alternative approaches to maximize the utility of all oral treatment regimens may provide additional benefits to this patient population.
The third-generation immunomodulatory agent pomalidomide and the oral proteasome inhibitor ixazomib have both been studied in combination with dexamethasone. The former combination has a median reported PFS of 4.0 to 6.9 months,12-14 whereas an event-free survival of 11 months was reported with the latter combination.15 The combination of pomalidomide, ixazomib, and dexamethasone has been studied in 2 phase 2 trials, which reported overall response rates (ORRs) of 48% and 51.7% and median PFS times of 8.6 and 4.4 months.16,17
Clarithromycin is a macrolide antibiotic with potential immunomodulatory properties. However, its mechanism of action in this setting is unknown. It synergizes with thalidomide in vitro, potentially by suppressing interleukin-6 and tumor necrosis factor α.18 It increases dexamethasone exposure by decreasing its hepatic clearance.19 The use of clarithromycin in combination with lenalidomide and dexamethasone (Rd) in newly diagnosed myeloma led to high ORRs of 93% with very good partial response (VGPR) or better rates of 68% and a median PFS of 49 months.20 The ORR of clarithromycin, pomalidomide, and dexamethasone was 60%, and the median PFS and duration of response (DOR) were 7.7 months in RRMM.21 In this study, we report on the all-oral quadruplet regimen of Clarithromycin, Ixazomib, Pomalidomide, and Dexamethasone (ClIPd) in patients with RRMM.
Methods
Trial design and oversight
This phase 1b/2 study evaluated the combination of ClIPd in patients with RRMM. Patients were enrolled between December 2015 and March 2020 at participating sites within the University of California Hematologic Malignancies Consortium. The protocol and all amendments were approved by the institutional review board at the coordinating site and accepted through the University of California Reliance process22 at participating sites. All patients gave written informed consent. The study was conducted in accordance with the International Conference on Harmonization Good Clinical Practice guidelines, the principles originating from the Declaration of Helsinki, and study site–specific regulations.
Patients
Eligible patients were 18 years and older with RRMM who had received at least 1 previous line of therapy, including an immunomodulatory agent and a proteasome inhibitor. Key inclusion criteria included an Eastern Cooperative Oncology Group performance status of 0 to 2, an absolute neutrophil count of ≥1000/ mcL, a platelet count of ≥50 000/mcL, and an estimated creatinine clearance of ≥30 mL/min. Patients with previous pomalidomide or ixazomib exposure were excluded.
Study treatment
Treatment was administered in 28-day cycles. For cycles 1 to 6, 4 mg pomalidomide was administered on days 1 to 21, 4 mg ixazomib was administered on days 1, 8, and 15, and 40 mg dexamethasone (20 mg for patients older than 75) was given on days 1, 8, 15, and 22. During cycle 1, clarithromycin initiation was delayed by 2 weeks (days 15-21) to facilitate correlative studies but was administered on days 1 to 21 in subsequent cycles. Cycle 7 and beyond were considered maintenance, and pomalidomide, ixazomib, and dexamethasone doses were decreased to 2 mg, 2.3 mg, and 20 mg, respectively, on the same schedule. Clarithromycin was given on days 1 to 28 at the same dose throughout maintenance. The cycles were repeated until progression or intolerable toxicity. Dose level 1 was 250 mg by mouth twice daily of clarithromycin. To assess the immunologic effects of the combination of pomalidomide, ixazomib, and dexamethasone before clarithromycin exposure, blood samples were drawn at baseline and on day 15 after induction. Peripheral blood mononuclear cells were isolated and analyzed by flow cytometry.
End points and assessments
The phase 1b portion of the study included a 3+3 dose escalation to determine the maximum tolerated dose (MTD) and the recommended phase 2 dose (RP2D) of clarithromycin, in addition to the standard doses of pomalidomide, ixazomib, and dexamethasone. After the first 6 patients were enrolled, the protocol was amended to continue at dose level 1 given an acceptable toxicity profile and early efficacy data. Secondary end points were safety/tolerability and preliminary assessment of efficacy according to the International Myeloma Working Group criteria.23 The primary outcome of the phase 2 portion of the study was the combined rate of complete response (CR) and stringent CR (sCR).
Secondary end points were safety, ORR (CR, sCR, VGPR, partial response [PR]),23 disease control rate (DCR; ORR + stable disease [SD]), DOR, PFS, and OS.
Adverse events (AEs) were monitored continuously from informed consent through 28 days after last study treatment and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events Version 4.0.
Statistical analysis
The phase 1b portion used a standard 3+3 design to identify the MTD of clarithromycin and inform the RP2D. MTD was defined as the highest dose tested in which fewer than 33% of patients had a dose limiting toxicity (DLT). The phase 2 portion planned to enroll 40 patients in a Simon’s optimal 2-stage design to test the sCR rate based on the previous combination of clarithromycin, pomalidomide, and dexamethasone in which an sCR rate of 6% was reported.21 Assuming a type 1 error rate of 5% and 80% power, if an sCR rate of >6% was seen in the first 20 patients then an additional 20 patients could be accrued.
Correlative studies
Graphical methods were used to display the results of correlative studies. Longitudinal flow data were plotted as line graphs of the median values with interquartile ranges. Individual time points were plotted as median values with dots representing each patient. Samples for analysis of plasma cytokine levels and subset populations of T and natural killer (NK) cells, were collected at the indicated time points. To quantitatively evaluate the patterns over time, a mixed effects linear regression model was constructed using immune cell subsets and gated Mean Floureence Intensity (gMFI) as dependent variables. Time (as a categorical variable) and response to therapy were set as fixed effects, as was the interaction term, and patient was set as random effect. In these mixed effect linear regression models, none of the interaction terms were statistically significant, indicating that differences between responders and nonresponders, if they existed, tended to be constant over time. These interaction terms were dropped from the final models. To control for multiple testing, the Bonferroni method adjustment was used. Exploratory and descriptive analyses were also planned to identify patterns that would explain the treatment effects or that would merit further study with the goal of identifying a potential predictive biomarker.
Results
Patients and treatment
A total of 42 patients were screened, and 32 patients were enrolled. Accrual was halted in May of 2020 because of slow accrual to the trial after 32 patients had been enrolled (28 evaluable for efficacy outcomes). Of the 32 patients enrolled, 3 were unevaluable for toxicity, 4 were unevaluable for response and survival because of rapid disease progression during cycle 1 (n = 2), 1 withdrew consent before clarithromycin exposure, and 1 screen failure before administration of the study treatment. All patients who received any study treatment were included in analysis for toxicity, whereas 28 patients were assessed for efficacy. Patient baseline demographics and characteristics are summarized in Table 1. The median age at enrollment was 69. The median number of previous therapies was 2 (range, 1-5). Of the 26 patients with fluorescence in situ hybridization results available for analysis, high-risk cytogenetic abnormalities (del17p, t(4;14), t(14;16), and +1q) were present in 15 (57%). Of these, 6 (23%) had >1 high-risk cytogenetic features.
Safety
One patient in the initial dosing cohort had a DLT, which led to the enrollment of 3 additional subjects at the starting 250 mg twice daily treatment dose, none of whom had a DLT. However, once the first 6 patients were enrolled, the safety committee assessed both the toxicities and outcomes of patients and given the early responses and safety signals, opted to forgo dose level 2. The protocol was amended, and dose level 1 was declared the RP2D. Overall, 28 (97%) patients experienced at least 1 AE, whereas 17 (59%) had at least 1 grade 3 to 4 AE (Table 2). Grade 3 to 4 hematologic AEs were seen in 8 (26%) patients of which 5 (16%) had neutropenia, and 13 (45%) experienced grade 3 to 4 nonhematologic AEs. Grade 3 to 4 infections were the most common nonhematologic AE, seen in 4 (13%) patients, with upper respiratory tract infections being the most common. Grade 3 neuropathy was seen in 1 (3%) patient. Hyperglycemia was seen at any level in 4 (14%) patients; muscle weakness was seen in 6 (21%) patients; insomnia was seen in 10 (34%) patients, and mood disturbance was seen in 6 (21%). No venous thrombosis was seen. Serious AEs were reported 19 times of which only 2 (grade 3 skin infection and grade 2 upper respiratory tract infection) were considered possibly related to ClIPd.
Efficacy
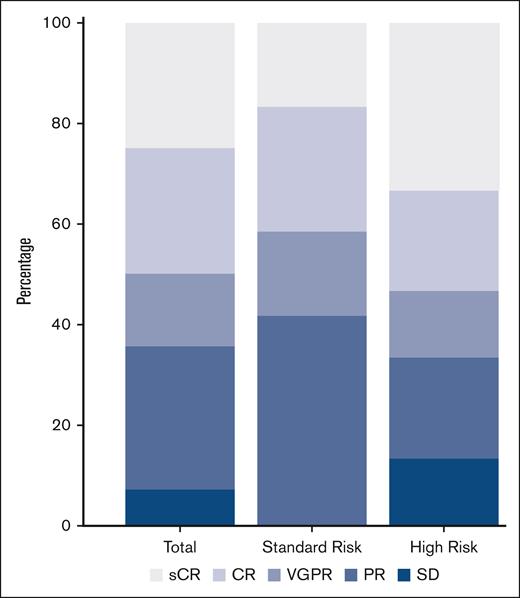
The ORR was 75% with a DCR of 100%; 13 patients (46%) achieved ≥VGPR, and 6 (21%) patients achieved a CR/sCR with 4 (14%) achieving sCR. High-risk cytogenetic abnormalities were not associated with inferior ORR (odds ratio [OR],1.32; 95% confidence interval [CI], 0.14-12.35) or with ≥VGPR rates (OR, 2.87; 95% CI, 0.49-19.82 (Figure 1).
Best responses to pomalidomide, ixazomib, clarithromycin, and dexamethasone. Proportion of responses to ClIPd therapy in the total cohort and stratified by cytogenetic risk. High-risk cytogenetics were considered +1q (either gain or amplification), del(17p), t(4;14) or t(14;16).
Best responses to pomalidomide, ixazomib, clarithromycin, and dexamethasone. Proportion of responses to ClIPd therapy in the total cohort and stratified by cytogenetic risk. High-risk cytogenetics were considered +1q (either gain or amplification), del(17p), t(4;14) or t(14;16).
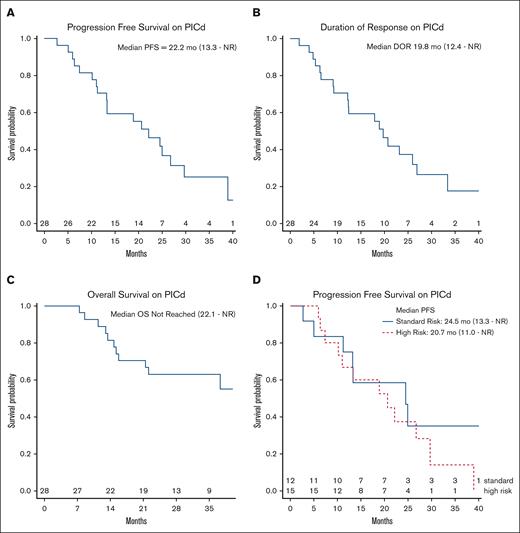
The median PFS was 22.2 months (95% CI, 13.3 to not reached [NR]), whereas the median DOR was 19.8 months (95% CI, 12.4 to NR) (Figure 2A-B). At a median follow-up of 35.6 months (95% CI, 28-54), the median OS was NR (95% CI, 22.1 to NR) with a 3-year OS rate of 63% (95% CI, 47-84) (Figure 2C). The median PFS was similar in patients with or without high-risk cytogenetics (20.7 months; 95% CI, 11 to NR vs 24.5; 95% CI, 13.3 to NR, respectively; log rank P = .4; Figure 2D). There was no difference in the median PFS between patients with or without del(17p) (26.8 months; 95% CI, 10.2 to NR vs 22.2 months; 95% CI, 13.3 to NR, respectively; log rank P = .8). Patients with +1q had a numerically shorter median PFS of 17.0 months (95% CI, 10.2 to NR) as opposed to 25 months (95% CI, 13.3 to NR) in those without +1q, but this did not reach statistical significance (log rank P = .3).
Survival analysis of patients treated with ClIPd. (A) PFS, (B) DOR, (C) OS, and (D) PFS stratified by high-risk cytogenetics. High-risk cytogenetics were considered +1q (either gain or amplification), del(17p), t(4;14), or t(14;16). mo, months.
Survival analysis of patients treated with ClIPd. (A) PFS, (B) DOR, (C) OS, and (D) PFS stratified by high-risk cytogenetics. High-risk cytogenetics were considered +1q (either gain or amplification), del(17p), t(4;14), or t(14;16). mo, months.
Correlatives
Alterations in immune populations associated with pomalidomide, ixazomib, and dexamethasone
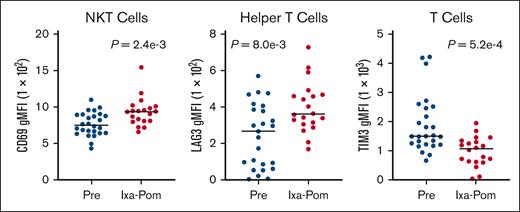
The results demonstrated that therapy was associated with increased expression of CD69 on NK T cells (gMFI P = 2.4e−3; Figure 3) and Lymphocyte Activation Gene 3 (LAG3) on helper T cells (LAG3 gMFI P = 8.0e−3; Figure 3) and with decreased expression of T cell immunoglobulin and mucin domain-containing protein 3 (TIM3) on T cells (gMFI P = 5.2e−4; Figure 3). Therapy was also associated with an increase in the frequency of CD56Bright CD16Dim and CD56Bright CD16negative NK Cells (P = 2.4e−3 and 2.3e−3, respectively; supplemental Figure 1) and central memory cytotoxic T cells (P = 6.2e−4; Figure 4). Therapy led to a decrease in peripheral B cells (P = 7.8e−3; supplemental Figure 2) and a decrease in effector cytotoxic T cells (P = 4.0e−3; Figure 4).
Alteration of immune effector cell subsets after initiation of pomalidomide, ixazomib, dexamethasone. Peripheral blood samples taken at day 1 (before treatment), and day 15 (after 2 weeks of pomalidomide, ixazomib, and dexamethasone) were analyzed by flow cytometry to identify changes in the immune subsets associated with treatment. The CD69 expression on NK-T cells was increased (left plot), LAG3 expression was increased on helper T cells (center plot), and TIM3 expression decreased on T cells (right plot). gMFI, gated mean fluorescence intensity; Ixa-Pom, ixazomib, pomalidomide.
Alteration of immune effector cell subsets after initiation of pomalidomide, ixazomib, dexamethasone. Peripheral blood samples taken at day 1 (before treatment), and day 15 (after 2 weeks of pomalidomide, ixazomib, and dexamethasone) were analyzed by flow cytometry to identify changes in the immune subsets associated with treatment. The CD69 expression on NK-T cells was increased (left plot), LAG3 expression was increased on helper T cells (center plot), and TIM3 expression decreased on T cells (right plot). gMFI, gated mean fluorescence intensity; Ixa-Pom, ixazomib, pomalidomide.
Alterations in central memory and effector cytotoxic T cells after initiation of pomalidomide, ixazomib, dexamethasone. Peripheral blood samples taken at day 1 (before treatment), and day 15 (after 2 weeks of pomalidomide, ixazomib, and dexamethasone) were analyzed by flow cytometry to identify changes in the immune subsets associated with treatment. Relative percentages of central memory cytotoxic T cells were increased, whereas the effector cytotoxic T-cell percentage fell after initiation of therapy. Ixa-Pom, ixazomib, pomalidomide.
Alterations in central memory and effector cytotoxic T cells after initiation of pomalidomide, ixazomib, dexamethasone. Peripheral blood samples taken at day 1 (before treatment), and day 15 (after 2 weeks of pomalidomide, ixazomib, and dexamethasone) were analyzed by flow cytometry to identify changes in the immune subsets associated with treatment. Relative percentages of central memory cytotoxic T cells were increased, whereas the effector cytotoxic T-cell percentage fell after initiation of therapy. Ixa-Pom, ixazomib, pomalidomide.
Alterations in immune populations associated with clarithromycin
Clarithromycin was initiated on day 15 of cycle 1. When compared with cycle 1 day 15 (before clarithromycin), cycle 2 day 1 (after clarithromycin) was associated with increased T cell expression of TIM-3 (P = 5.0e−3; supplemental Figure 3). The postclarithromycin samples also had a higher frequency of Natural Killer Group 2 member A (NKG2A)-expressing CD56bright CD16negative NK cells (P = 8.2e−3) and decreased frequencies of regulatory T cells (P = 8.8e−3) and CD56bright CD16Dim NK cells (P = 3.4e−3) (Figure 5).
Effects of clarithromycin on NK and NK T-cell expression patterns in patients treated with pomalidomide, ixazomib, dexamethasone. Peripheral blood samples taken on day 15 (after 2 weeks of pomalidomide, ixazomib, dexamethasone) and on cycle 2 day 1 (after the final week of pomalidomide, ixazomib, dexamethasone in combination with 2 weeks of clarithromycin) were analyzed by flow cytometry and compared to identify potential immunomodulatory effects of clarithromycin. Postclarithromycin samples showed increased percentages of NKG2A+, CD56 Bright, CD16 negative NK cells (left) and a decrease in the percentage of CD56 Bright, CD16 Dim NK T cells (right).
Effects of clarithromycin on NK and NK T-cell expression patterns in patients treated with pomalidomide, ixazomib, dexamethasone. Peripheral blood samples taken on day 15 (after 2 weeks of pomalidomide, ixazomib, dexamethasone) and on cycle 2 day 1 (after the final week of pomalidomide, ixazomib, dexamethasone in combination with 2 weeks of clarithromycin) were analyzed by flow cytometry and compared to identify potential immunomodulatory effects of clarithromycin. Postclarithromycin samples showed increased percentages of NKG2A+, CD56 Bright, CD16 negative NK cells (left) and a decrease in the percentage of CD56 Bright, CD16 Dim NK T cells (right).
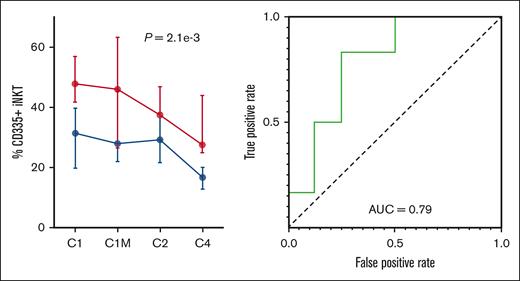
Baseline CD335+ iNK-T cells predicted the response to the combination of pomalidomide, ixazomib, clarithromycin, and dexamethasone. Baseline peripheral blood samples (cycle 1 day 1, before treatment) of patients who had at least a VGPR or better (left curve, blue points) were compared with those who had a PR or SD (left curve, red points). Across all time points, deeper responders had lower CD335+ iNK T cells. The c-statistics for the AUC was 0.79, which indicates that baseline CD335+ iNK T cells were predictive of response to treatment with pomalidomide, ixazomib, clarithromycin, and dexamethasone. AUC, area under the receiver operator curve.
Baseline CD335+ iNK-T cells predicted the response to the combination of pomalidomide, ixazomib, clarithromycin, and dexamethasone. Baseline peripheral blood samples (cycle 1 day 1, before treatment) of patients who had at least a VGPR or better (left curve, blue points) were compared with those who had a PR or SD (left curve, red points). Across all time points, deeper responders had lower CD335+ iNK T cells. The c-statistics for the AUC was 0.79, which indicates that baseline CD335+ iNK T cells were predictive of response to treatment with pomalidomide, ixazomib, clarithromycin, and dexamethasone. AUC, area under the receiver operator curve.
Low baseline expression of CD335 on iNKT predicts response to therapy
To identify immunophenotypes that could predict response to therapy, the flow cytometry data were parsed into 2 groups. Patients who experienced an sCR, CR, or VGPR were compared with those who experienced either SD or a PR. Across all time points, the individuals with a low frequency of CD335+ invatiate natural killer T (iNKT) cells responded better to therapy. To evaluate the ability of CD335-expressing iNKT cells to classify sCR, CR, or VGPR from SD or PR, receiver operator characteristic curves were constructed and the area under the curves (AUCs) were calculated. This analysis revealed that the percentage of CD335-expressing iNKT cells could predict response to therapy (AUC = 0.79 at baseline) (Figure 6).
Discussion
We present the safety and efficacy of a novel all-oral combination of ClIPd in RRMM. Overall, ClIPd was well tolerated with grade 3 to 4 toxicities being principally confined to cytopenias. The ORR was 75% with a DCR of 100%, paired with a median PFS of almost 2 years, indicating good clinical efficacy and tolerability in this cohort of patients with 54% harboring high-risk cytogenetic abnormalities.
The treatment of RRMM has become increasingly complex with numerous options available. In general, 3-drug combinations have outperformed 2-drug combinations by demonstrating prolonged PFS and in some cases OS.3-5 Pomalidomide is a common component of regimens in the R/R space with most patients receiving an initial induction regimen that relies on extended exposure to lenalidomide.13 In the ELOQUENT-2 study in which elotuzumab-pomalidomide-dexamethasone was compared with pomalidomide-dexamethasone (pom-dex) with 90% and 82.5%, respectively. of patients refractory to lenalidomide.5,24 The ORR was 79% vs 66% in the 2 arms, corresponding to an initial median PFS of 19.4 vs 14.9 months24 and a long-term OS benefit (hazard ratio, 0.59; 95% CI, 0.37-0.93).5 The combination of daratumumab, pomalidomide, and dexamethasone (DPD) has been reported in the phase 1 to 2 EQUULEUS25 study and in the phase 3 APOLLO study.4 The EQUULEUS study participants were all previously exposed to lenalidomide, and 20% were refractory to lenalidomide or thalidomide. The study reported an ORR of 60% with a median DOR that was not estimable and a median PFS of 8.8 months. The APOLLO study randomized patients to DPD vs pom-dex, with 79% and 80%, respectively, of patients refractory to lenalidomide. The ORRs were 69% and 46% with a longer median PFS of 12.4 vs 6.9 months in the DPD vs pom-dex arms (hazard ratio, 0.63; 95% CI, 0.47-0.85). Both the Multiple Myeloma Research Consortium (MMRC) and the Alliance for Clinical Trials in Oncology reported phase 1 to 2 studies of the all-oral triplet pomalidomide, ixazomib, and dexamethasone (PID).16,17 Among the 25 patients treated at the RP2D in the MMRC study, the ORR was 48% and the DCR was 76%, leading to a median DOR and PFS of 9.2 and 8.6 months, respectively.17 The Alliance trial reported an ORR of 52% with an additional 2 patients achieving a minor response for a clinical benefit rate of 59%. The median DOR and PFS were 16.8 months and 4.4 months. More recently, based on the results of the GRIFFIN9 and PERSEUS10 trials, daratumumab, lenalidomide, bortezomib and dexamehasone has become the standard of care for newly diagnosed mutiple myeloma. In addition, recent approvals of ciltacabtagene autoleucel after 1 previous line of therapy, idecabtagene vicleucel after 2 previous lines of therapy, and bispecific monoclonal antibodies after 4 previous lines of therapy continue to alter the treatment landscape in the R/R setting.26-30 Despite these ongoing advances, however, myeloma remains incurable. Novel and well-tolerated approaches continue to be needed.
Clarithromycin has been used for the treatment of newly diagnosed and RRMM. In the long-term follow-up of a phase 2 study of newly diagnosed patients treated with clarithromycin (500 mg twice daily), lenalidomide, and dexamethasone (BiRd), investigators reported an ORR of 93% with responses deepening during longer follow-up times.20 The median PFS, including the 53% of patients who proceeded to an autologous stem cell transplant, was 49 months. A randomized controlled trial comparing Rd to BiRd in older adults and patients unfit for autologous stem cell transplant reported similar ORRs in the 2 arms (76% vs 71%, respectively) but deeper responses in the BiRd arm (≥VGPR, 46% vs 53% and ≥CR rate of 14% vs 23%). However, the median PFS was 29 months in the Rd vs 23 months in the BiRd arms, corresponding to a hazard ratio of 1.29 (95% CI, 0.92-1.82). The investigators observed a higher percentage of deaths caused by AEs in the BiRd arm (25%) than in the Rd arm (15%).31 In the relapsed setting, clarithromycin (500 mg twice daily), pomalidomide, and dexamethasone (ClaPd) had an ORR of 60% with a ≥CR rate of 6%. The median DOR and PFS were 9.3 and 7.7 months, respectively.
When compared with trials of PID and ClaPd, the ORR and PFS of ClIPd of 75% and 22 months, respectively, are encouraging. However, there are some notable differences in the patient population that must be considered, which highlight the challenges with cross-trial comparisons. When compared with the ClaPd trial, our patient population had less previous treatments with a median of 2 previous therapies as opposed to 5, whereas our patient population had slightly higher rates of del(17p) at 33%. When compared with the MMRC population, the ClIPd population had a median of 2 previous lines of therapy, but the MMRC population had less del(17p) (16%) and +1q (16%). The Alliance trial population had a median of 3.5 previous therapies, and 63% had del(17p), +1q, t(14;16), or t(14;20). Thus, some of the improvements in PFS seen in the current trial may be related to a less pretreated population when compared with previous trials of all-oral triplets. However, the PFS of nearly 2 years is substantial in any RRMM population, and the ClIPd population was uniformly refractory to lenalidomide with high rates of previous stem cell transplants, proteasome inhibitor exposure. and high-risk cytogenetics. We also used a lower dose of clarithromycin than was given in previous trials. The initial trial was designed to increase the dose from 250 mg by mouth twice daily to 500 mg by mouth twice daily. After the first 6 patients were reviewed with high response rates and good tolerance, the protocol was amended to proceed with a dose of 250 mg by mouth twice daily for the phase 2 portion of the trial.
The toxicity profile of ClIPd is notable for a paucity of high-grade nonhematologic toxicities. The most common were infections, as has been seen in previous trials. This may be related to increased dexamethasone exposure and decreased clearance caused by drug-drug interactions through the CYP450 system.19 In line with this hypothesis, we saw higher rates of grade 1 to 2 hyperglycemia, generalized weakness, cataracts, and mood disturbances/insomnia, which may have been related to steroid exposure. Peripheral neuropathy was seen in more than half of patients, however, no grade 4 neuropathy was seen. Although infectious complications were seen, no infectious deaths were reported in contrast with the concerns raised in the randomized trial of BiRd vs Rd. This may be because of the lower dose of clarithromycin in the ClIPd regiment than in the BiRd regiment. Overall, the toxicity profile was in line with previous studies containing the various components of ClIPd and were tolerable, and many patients remained on treatment for extended periods.
To better elucidate the mechanism of clarithromycin on MM, we used comprehensive flow cytometric analysis to assess changes in the immune subsets after 2-week exposure to PID and again after 2 weeks of full exposure to ClIPd. This analysis revealed significant modulation of immune cell populations. NK cells are a subtype of cytotoxic lymphocytes that belong to the innate immune system and that recognize and can kill malignantly transformed cells. It is therefore notable that ClIPd increased the frequency of CD56Bright CD16Dim and CD56Bright CD16negative NK cells. CD56bright, CD16 negative NK cells are efficient cytokine producers and are frequently considered to be the precursor cells of CD56dim, CD16bright NK cells, which have considerably higher cytotoxic activity.32 The NK population that was altered by ClIPd therapy, the CD56bright CD16dim subpopulation of NK cells, is an intermediate population between these 2 extremes; they are efficient cytokine producers and effective at target cell killing. Given the known anticancer properties of NK cells, we can speculate that the ability of ClIPd to increase CD56bright CD16dim NK cells is beneficial in MM.
It is also noteworthy that ClIPd induced a decrease in peripheral B cells, a known effect of immunomodulatory agents and proteasome inhibitors, and may be a surrogate for ClIPd’s ability to inhibit MM (P = 7.8e−3). Indeed, previous studies in R/R non-Hodgkin lymphoma have demonstrated that the loss of peripheral B cells corresponds to response to therapy.33,34
ClIPd strongly increased the frequency of central memory cytotoxic T cells (P = 6.2e−4) but also modestly decreased the frequency of effector cytotoxic T cells (P = 4.0e−3). Based on the limited nature of this analysis, it is difficult to interpret the impact of these results. However, a possible explanation is that the decrease in effector cytotoxic T cells in the peripheral blood may represent a shift in the location of these cells from the periphery into tissues. It is also noteworthy that in animal models of cancer, central memory T cells were found to have more potent antitumor capacity when compared with effector memory T cells and that peripheral central memory T cells have been associated with increased cancer survival in humans.35 With regard to MM, additional mechanistic studies will be needed to understand the ramifications of these findings.
Following pomalidomide-ixazomib–based induction, NK-T cells increased the expression of CD69, a marker of immune cell activation, (P = 2.4e−3). LAG-3 is an inhibitory receptor expressed by activated immune cells, mainly activated T cells.36 ClIPd induced contradictory expression patterns of inhibitory proteins. LAG-3 on helper T cells was modestly increased (P = 8.0e−3). This may be an indication of the immunostimulatory capacity of this therapy, which eventually leads to LAG-3 upregulation. However, because LAG-3 sends an inhibitory signal, its expression by immune cells in the tumor microenvironment has been associated with immune escape and poor prognosis, however, this is primarily in patients being treated with checkpoint inhibitors.37,38 Thus, the implication of increased LAG-3 expression observed in our study is difficult to interpret. Simultaneously, the expression of the inhibitory coreceptor TIM-3 was dramatically downregulated on T cells (P = 5.2e−4). In solid tumors, increased TIM-3 expression has been associated with more advanced tumor stage and worse survival,38 and in MM, TIM-3 expression is strongly elevated when compared with healthy controls.39 Although the TIM-3 pathway is highly complex, its inhibitory functions are becoming more apparent as is its use as a marker of T cell exhaustion.38 Taken together, we can speculate that a modest increase in LAG-3 accompanied by a strong downregulation of TIM-3 may represent an overall more activated and less exhausted T cell phenotype, which would favor the development of an anticancer immune response.
CD335 (also called natural cytotoxicity triggering receptor, NCR1) is an inhibitory coreceptor expressed by various immune cells. The finding that decreased CD335 expression on iNKT predicts response to therapy is noteworthy (AUC = 0.79) and is supported by a recent trial of lenalidomide and blinatumomab for R/R non-Hodgkin lymphoma.
Regarding clarithromycin’s potential immunomodulating properties, the immunoprofiling performed in this study did support the hypothesis that the entire regimen augments the immune response, and it did not support the hypothesis that clarithromycin primarily induced additional cellular cytotoxicity.
In summary, this clinical study suggests that the therapeutic benefit of ClIPd is achieved, at least partially, through immunomodulation as demonstrated by increased the expression of CD69 on NK T cells, expression of LAG3 on helper T cells, frequency of central memory cytotoxic T cells, the frequencies of NKG2A expressing CD56Bright CD16Negative NK cells, CD56Bright CD16Dim NK cells, and CD56Bright CD16Negative NK cells. This therapy also decreased TIM3 expression on T cells. These alterations in immunoprofile were noted after 14 days of pomalidomide, ixazomib, and dexamethasone with little additional change noted after the addition of clarithromycin. Although these correlative studies cannot rule out clinically relevant immunomodulation by clarithromycin not captured by our ancillary immune monitoring, the extended durations of response seen in this study when compared with PID alone may be because of the altered drug metabolism that leads to increased drug exposure.
In conclusion, ClIPd is an all-oral quadruplet regimen that is relatively well tolerated and offers convenient dosing with relatively high rates and durable responses and is feasible for patients in whom parenteral therapy is not feasible or preferred.
Acknowledgment
The trial was supported by Millenium-Takeda. Correlative studies were supported by Celgene.
Authorship
Contribution: A.S.R. enrolled patients, analyzed clinical data, interpreted data, and drafted the manuscript; C.C. designed the trial, enrolled patients, interpreted data, and edited the manuscript; E.A.B., M.J.W., and P.K. enrolled patients, interpreted data, and edited the manuscript; E.M. and G.L. analyzed correlative data, interpreted data, and edited the manuscript; J.T. designed the trial, enrolled patients, analyzed correlative data, interpreted data, and edited the manuscript; and K.A. and S.H. analyzed correlative data and interpreted data.
Conflict-of-interest disclosure: A.S.R. reports receiving research funding to the institution from Kangpu Pharmaceuticals, Takeda, Pfizer, and Biomea and serving as a consultant for Sanofi, Pfizer, and Bristol Myers Squibb. C.C. reports receiving research funding from Bristol Myers Squibb, J&J, Pfizer, Takeda, Poseida, and Ionis and serving as a consultant for Bristol Myers Squibb, Genentech, J&J, Karyopharm, Pfizer, and Takeda. E.A.B. reports serving as a speaker for BeiGene, Astra Zeneca, Seagen, Incyte/MorphoSys, and Genmab/AbbVie and serving as a consultant for Caribou Biosciences, Genentech, MorphoSys, BeiGene, ADC Therapeutics, and AstraZeneca. J.T. reports receiving research funding from Bristol Myers Squibb, Genentech, ADC Therapeutics, Regeneron, AbbVie, and Pharmacyclics. M.J.W. reports serving on an advisory board for Jazz, Bristol Myers Squibb, Pfizer, and Gilead/Kite. P.K. reports serving as a speaker for BeiGene and Janssen. The remaining authors declare no competing financial interests.
Correspondence: Aaron S. Rosenberg, Division of Malignant Hematology, Cellular Therapy & Transplantation, University of California Davis School of Medicine, 4501 X St, Ste 3016, Sacramento, CA 95817; email: asrosenberg@ucdavis.edu.
References
Author notes
Data are available on request from the corresponding author, Aaron S. Rosenberg (asrosenberg@ucdavis.edu).
The full-text version of this article contains a data supplement.