Key Points
Outcomes for older relapsed or refractory patients with AML remain poor, and novel approaches are needed.
Mitochondrial inhibition with devimistat added to chemotherapy did not improve remission rates or overall survival.
Visual Abstract
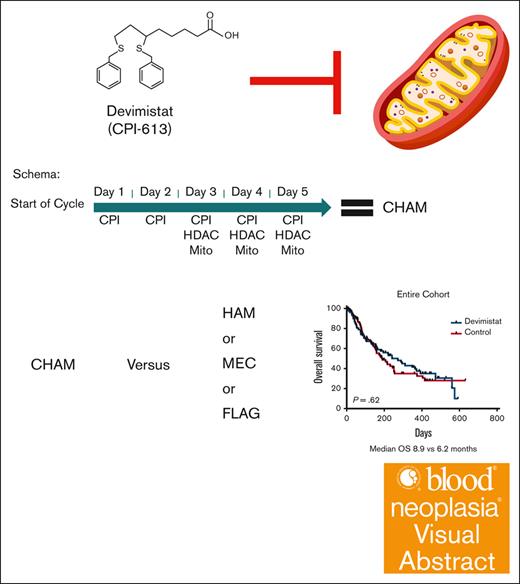
Acute myeloid leukemia (AML) is an aggressive cancer of the myeloid lineage. Outcomes in older patients are poor, with high rates of resistant and relapsed disease. Devimistat is a lipoic acid analog that inhibits mitochondrial metabolism. Devimistat combined with high-dose cytarabine and mitoxantrone resulted in promising phase 1 and 2 response rates especially in older patients. Therefore, the phase 3 ARMADA 2000 trial was conducted in patients aged ≥50 years with relapsed or refractory AML. The study randomized patients between devimistat combined with high-dose cytarabine and mitoxantrone (CHAM) or 1 of 3 control treatment regimens without devimistat: high-dose cytarabine and mitoxantrone; mitoxantrone, etoposide, and cytarabine; or fludarabine, cytarabine, and filgrastim. Overall, 265 patients consented to participate from 56 sites across 11 countries, and 200 patients were randomized, 98 patients to the devimistat arm and 102 patients to the control arm. The safety profile was consistent with high-dose cytarabine–based salvage regimens. There were 18 (9%) deaths on study (11 on CHAM and 7 on control). The study failed to meet its primary end point, with a complete remission (CR) rate of 20.4% in the devimistat arm compared with 21.6% in the control arm (P = .57). Overall survival was not statistically significantly different between the study arms, with a median of 8.9 months in the CHAM arm compared with 6.2 months in the control arm (P = .62). In conclusion, devimistat added to chemotherapy did not improve the CR rate or survival in patients aged ≥50 years with relapsed or refractory AML. This trial was registered at www.ClinicalTrials.gov as #NCT03504410.
Introduction
Acute myeloid leukemia (AML) is a hematologic malignancy characterized by the accumulation of clonal myeloid progenitor cells (“blasts”) in the blood or bone marrow. Malignant hematopoietic stem and myeloid progenitor cells proliferate uncontrollably and disrupt normal hematopoiesis, resulting in bone marrow failure and death.1 AML has a current incidence of ∼20 000 individuals in the United States, resulting in ∼11 000 deaths per year.2 Current treatment strategies for AML include cytotoxic chemotherapy with/without targeted treatments for remission induction with cytarabine and an anthracycline or hypomethylating agents combined with the B-cell lymphoma 2 protein inhibitor venetoclax.1,3 Responding patients receive either additional chemotherapy or allogeneic stem cell transplant (SCT) consolidation based on risk of relapse determined by cytogenetics and mutation profile. Many patients who achieve remission and complete consolidation therapy still have a guarded prognosis. This is driven by the high rates of relapse and difficulty achieving a second remission.4 There is no consensus treatment for relapsed or refractory disease, but many fit patients are treated with a high-dose cytarabine (HiDAC)–based salvage regimen.4 The goal of salvage therapy is to attain a second complete remission (CR) and, whenever possible, proceed to SCT. Stem cell transplantation is the only curative therapy in this setting, and outcomes are best when patients are in remission at the time of transplantation.1 Older age, typically defined as aged ≥60 years, is a poor prognostic factor for AML. Clinical trials show an estimated 5-year survival of <10% in such patients.5,6
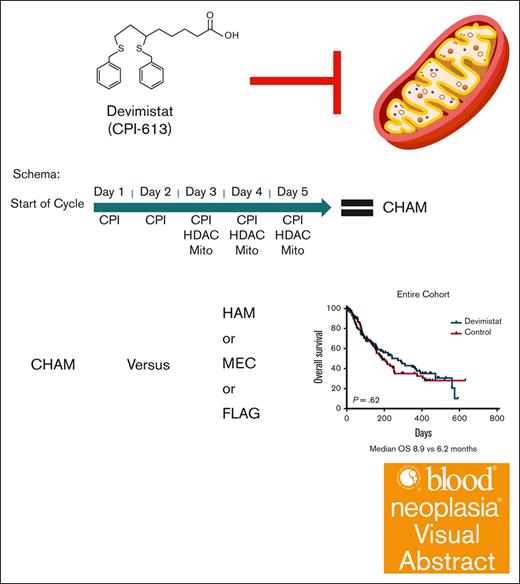
Devimistat (CPI-613) is an investigational new drug for the treatment of cancer. Devimistat is a non-redox active analog of lipoic acid, a required cofactor for key mitochondrial enzymes of the tricarboxylic acid cycle including pyruvate dehydrogenase and α-ketoglutarate dehydrogenase.6,7 Devimistat was shown to increase sensitivity of AML cells to chemotherapy and was active in combination with HiDAC and mitoxantrone (HAM) salvage therapy in a phase 1 study.8 In a pooled analysis of phase 1 and 2 data, devimistat in combination with HAM salvage therapy exhibited 52% CR and 10.4 months median overall survival (OS) in patients aged ≥60 years treated with the recommended phase 3 dose of 2000 mg/m2.9 Given the favorable safety profile of devimistat combined with HAM (CHAM) with the promising response results achieved in older patients with AML, a phase 3, randomized study (ARMADA 2000) was conducted.
Patients and methods
Study design
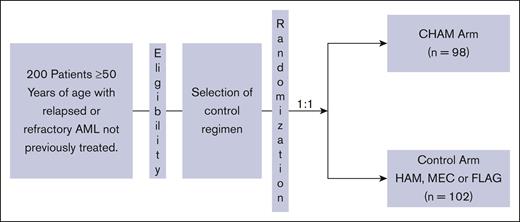
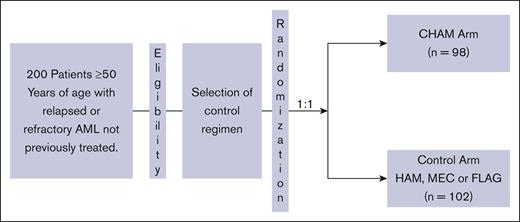
ARMADA 2000 was a phase 3, open-label, multicenter, randomized trial initially planned for ∼500 patients. The initial study design was a 1:1 randomization between the experimental arm of CHAM or the control arm of HAM in patients aged ≥ 60 years with relapsed or primary refractory AML not previously treated with cytotoxic chemotherapy. After concerns for slow accrual, the study was amended to decrease the age to ≥50 years and expand the control arm to 1 of 3 control treatments: control subgroup 1, HAM; control subgroup 2, mitoxantrone, etoposide, and cytarabine (MEC); control subgroup 3: fludarabine, cytarabine, and filgrastim (FLAG). The CHAM schedule is as follows for induction cycle 1 (14-day cycle): devimistat (2000 mg/m2 IV over 2 hours for 5 doses) once a day (QD), days 1 to 5. HiDAC was infused after completion of the devimistat infusion (1 g/m2 over 3 hours for 5 doses) every 12 hours starting on day 3 through day 5, followed by mitoxantrone after completion of the HiDAC infusion (6 mg/m2 over 15 minutes for 3 doses) QD after the first, third, and fifth doses of cytarabine starting on day 3. Patients could receive a second induction cycle (based on day-14 bone marrow aspirate/biopsy results). Induction cycle 2 was either identical to cycle 1 or an abbreviated cycle with devimistat (2000 mg/m2 for 3 doses) QD, days 1 to 3, followed by HiDAC (1 g/m2) after completion of devimistat infusion for 3 doses, every 12 hours starting on day 2 through day 3 followed by mitoxantrone (6 mg/m2) for 2 doses QD, after the first and third doses of cytarabine. Consolidation therapy (for responders to induction) used an abbreviated course as per induction cycles described above for up to 2 consolidation cycles. Patients must have maintained study eligibility criteria for organ function and performance status to be eligible for consolidation therapy. Maintenance therapy (for responders in the CHAM arm only) was devimistat (2500 mg/m2 for 5 doses) QD on days 1 to 5 (28-day cycle); continued until disease recurrence, availability of SCT, advent of intolerable side effects, or patient withdrawal of consent. There was no administration of HiDAC or mitoxantrone in maintenance therapy. Patients could move directly to maintenance therapy if not eligible for reinduction or consolidation therapy. The HAM control subgroup was treated identically except for the omission of devimistat and no maintenance therapy. The MEC subgroup was treated as per the following schedule: induction cycle 1 and 2 (2 14-day cycles) based on day-14 bone marrow aspirate/biopsy results; etoposide 80 mg/m2 over 60 minutes, 6 doses, day 1 through 6; cytarabine 1000 mg/m2 over 3 hours, 6 doses, day 1 through 6; and mitoxantrone 6 mg/m2 over 30 minutes, 6 doses, day 1 through 6. Up to 2 consolidation cycles (for responders) could be given. The FLAG subgroup was treated as per the following schedule without maintenance therapy. Induction cycle 1 and 2 (2 14-day cycles): induction cycle 2 is based on day-14 bone marrow aspirate/biopsy results. Fludarabine 30 mg/m2 per day over 30 minutes; 5 doses, days 1 through 5. Cytarabine 2 g/m2 over 4 hours; after fludarabine, 5 doses, days 1 through 5. Filgrastim 5 μg/kg per day subcutaneous or as per institutional guidelines, starting from day 1 through day 5, and can resume 7 days after completion of chemotherapy until absolute neutrophil count of ≥0.5 ×103/μL. Up to 2 consolidation cycles could be given.
Eligibility criteria
Key inclusion criteria were age of ≥50 years and histologically documented AML that is relapsed from, or refractory to, prior standard therapies. Refractory disease was defined as failure to achieve CR or CR with incomplete recovery after at least 1 cycle of any anthracycline-, cytarabine-, or fludarabine-containing induction regimen or persistence of disease on a nadir marrow biopsy after at least 1 cycle of any anthracycline-, cytarabine-, or fludarabine-containing induction regimen, or persistent disease after at least 2 cycles of a hypomethylating agent (azacitidine or decitabine) with or without venetoclax. Relapse was defined as the development of recurrent AML10 after CR or incomplete recovery has been achieved with prior chemotherapy or after disease progression on a hypomethylating agent with or without venetoclax. Eastern cooperative group performance score of 0 to 2 was required. Patients who had an allogeneic SCT in first remission were eligible as long as they were >6 months after transplant. Key exclusion criteria for the study were: patients who have already received cytotoxic chemotherapy for their relapsed or refractory AML. Patients with relapsed or refractory AML who were progressing or failing to respond to hypomethylating agents (decitabine or azacytidine) either alone or in combination with venetoclax were allowed to continue until the day before starting of CHAM or control treatment. Patients progressing on targeted therapies including FLT3 or IDH1/2 inhibitors and/or hydroxyurea and/or venetoclax were eligible. Hydrea, FLT3, IDH1/2, or B-cell lymphoma 2 protein inhibitors could be taken until the day before starting protocol therapy.
Study objectives
The primary objective of the study was to determine the efficacy of CHAM in terms of CR compared with control treatment. CR was determined as per standard response criteria for AML.10 The secondary objectives of the study were to determine OS and CR plus CR with partial hematologic recovery of CHAM vs control treatments. The OS and CR plus CR with partial hematologic recovery were determined as per standard response criteria for AML.10 Patient-reported outcomes were collected using the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire C30. Patient survival assessed 30-day and 60-day mortality after first dose of study-related treatment.
Statistical analyses
The experimental arm was compared with the control arm in terms of CR rate. The significance level was determined using an O’Brien-Fleming type Lan-DeMets boundary for efficacy equal to 0.023, which would have been reached if the difference in CR was >8.3%. This required 500 patients evaluable for response and would have taken 36 months after randomization. End point for the first interim analysis was CR. This was performed when 125 patients were evaluable for response after randomization. The significance level for efficacy was 0.0001 and would have been reached if the difference in CR was >26.7%. The significance level for futility was 0.48 and would have been reached if the difference in CR was <0.5%. After the initial interim analysis, another was requested when 200 patients were evaluable. Based on these interim analyses, if the CR difference was not sufficiently promising, consideration was given to stopping the trial. An independent data monitoring committee (IDMC) reviewed safety and efficacy periodically during the study.
The study obtained institutional review board approval at all study sites before enrolling any patient at those sites. This study was conducted in accordance with the Declaration of Helsinki.
Results
Patient characteristics
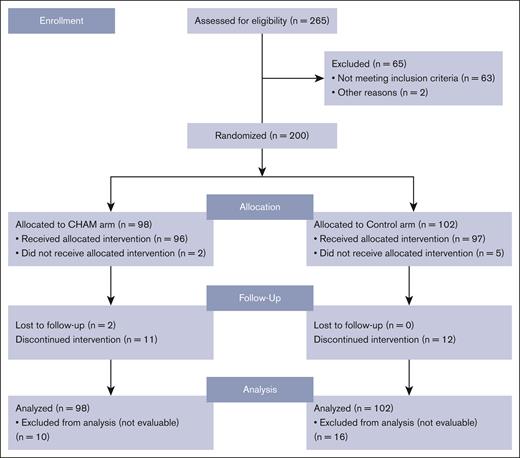
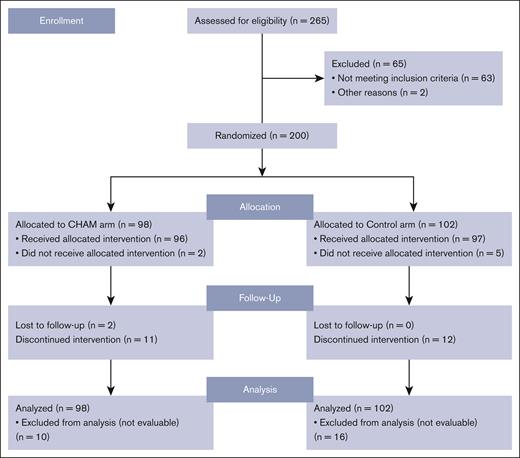
Between November 2018 and October 2021, 200 patients were randomized from 52 sites across 11 countries into the study before it was closed secondary to a lack of difference in the CR rates as discussed below in the efficacy section. The study schema is outlined in Figure 1. Of the enrolled patients, 98 were randomized to the CHAM group and 102 to the control group (see CONSORT diagram; Figure 2). The baseline patient demographics and clinical characteristics are listed in Table 1. Overall, mean ± standard deviation age of the 200 patients enrolled in both (CHAM and control) groups was 64.9 ± 7.15 years. The study enrolled more males (121 [60.5%]) than females (79 [39.5%]) and the proportion of patients by sex was similar (<5% difference) between the 2 treatment groups. The CHAM arm enrolled 61 (62.2%) males and 37 (37.8%) females, and the control arms enrolled 60 (58.8%) males and 42 (41.2%) females. Key demographics were well matched in both arms. Patients with relapsed disease made up 52% and 51% of the CHAM and control arms, respectively, whereas refractory patients made up 48% and 49%. Median time from initial AML diagnosis was shorter in the CHAM arm at 7.2 months compared with 8.7 for the control arm. Performance status was well matched between arms, with 89 (90.8%) patients in the CHAM arm and 93 (91.2%) patients in the control arm with an Eastern Cooperative Oncology Group performance status of 0 to 1, and 9 (9.2%) patients in the CHAM group and 9 (8.8%) patients in the control group with a performance status of 2. Likewise, European LeukemiaNet 2017 risk categories were well matched, with <5% difference in each category between the study arms. Secondary to budget constraints there was only limited molecular data available for 60 of 200 patients (27 in the CHAM arm, and 33 in the control arm). Among the subset in which data were available, there were no statistically significant differences in the rates of mutations in FLT3 (7 in each arm), NPM1 (8 in CHAM, 16 in control), DNMT3a (2 in CHAM, 9 in control), TET2 (4 in CHAM, 3 in control), IDH1/2 (2 in CHAM, 6 in control), and TP53 (4 in each arm). The most common first-line AML treatment was 7 days of infusional cytarabine and 3 days of bolus anthracycline (7+3) for both arms (57.1% and 56.9%, respectively), followed by hypomethylating agent with/without venetoclax (30.6% and 26.5%, respectively). This differed slightly by disease status, with relapsed patients receiving 7+3 regimen 51% of the time vs 61% for refractory patients; hypomethylating agent with/without venetoclax in 26% of relapsed patients vs 33% refractory patients, and HiDAC in 23% relapsed patients vs only 6% in refractory patients. Of 97 patients in the control arm who received treatment, 70 were treated with HAM, 11 with MEC, and 16 with FLAG. Overall, the number of patients receiving a second round of induction treatment was similar in both arms with 12 (12.5%) patients in the CHAM arm and 11 (11.3) patients in the control arm. A summary of all therapy received by arm is shown in supplemental Table 1.
Safety
There were 18 deaths on study (11 CHAM and 7 control). A total of 93 (96.9%) patients in the CHAM group and 94 (96.9%) patients in the control group experienced treatment-emergent adverse events (TEAEs) during the study. Similar number of occurrences were observed in the most frequently reported TEAEs in both arms: anemia (33 [34.4%] in the CHAM group and 35 [36.1%] in the control group), and febrile neutropenia (35 [36.5%] in the CHAM group and 32 [33.0%] in the control group). The CHAM group incurred higher observance in diarrhea (43 [44.8%] vs 22 [22.7%]) and nausea (31 [32.3%] vs 25 [25.8%]) consistent with previous studies.8,11 The control group experienced higher incidence of hypokalemia (36 [37.1%] vs 28 [29.2%]). In terms of grade ≥3 TEAEs, CHAM had higher rates of acute kidney injury and neutropenia whereas the control arm had higher rates of tumor lysis syndrome. A list of all grade ≥3 toxicities occurring in ≥5% of the patients is listed in Table 2. At the time of the study closure, a similar number of patients from each arm died: 52 in the CHAM arm vs 55 in the control arm (P ≥ .999). Likewise, the rate of death attributed to progressive disease was not significantly different between arms, with 28 patients in the CHAM arm vs 31 in the control arm (P = .8769).
Efficacy
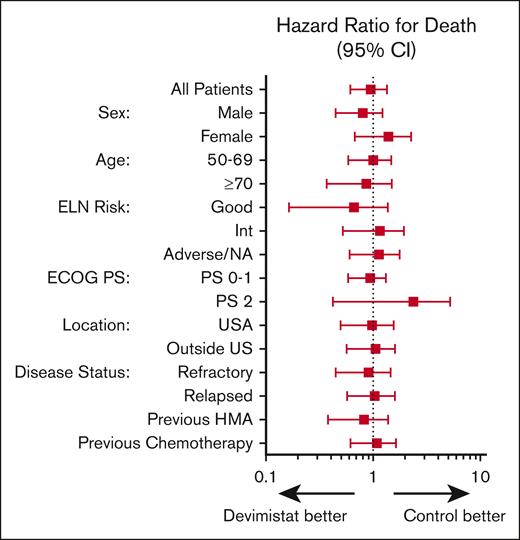
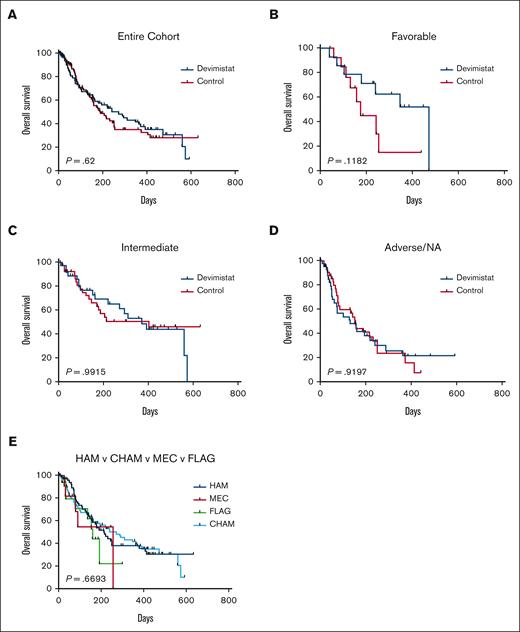
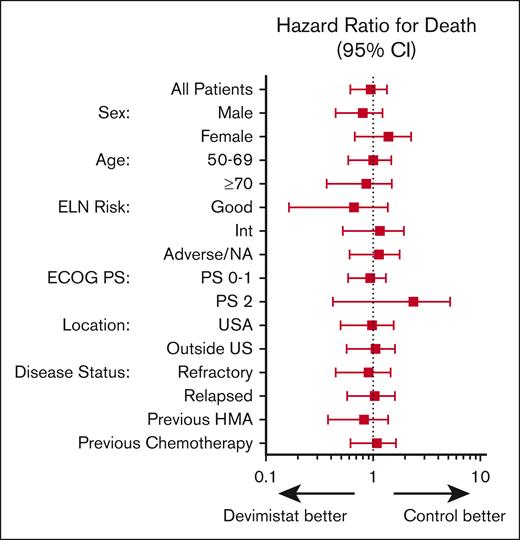
An initial planned interim analysis was conducted after 125 patients were evaluable for a response. The IDMC recommended continuing the study without change but requested another interim analysis when 200 patients were evaluable. At this analysis the study did not meet the primary objective to demonstrate the efficacy of CHAM in terms of rate of CR in comparison with the control arm because the overall CR rate was not statistically significantly different (20 [20.4%] vs 22 [21.6%]). The IDMC recommended the study be terminated secondary to lack of sufficiently promising results in the CHAM arm. The best response analysis results of the CHAM and control groups are summarized in Table 3. Minimal residual disease assessment was not mandated by the study, and data were available only for a single patient in the control arm. The method of minimal residual disease assessment was not disclosed. In terms of the key secondary end point of OS, there were 96 patients in each arm for whom data were available. Patients enrolled in the CHAM arm had a numerical OS advantage, but this was not statistically significant (median, 8.9 months vs 6.2 months; P = .62; Figure 3A). There was a median follow-up time of 4.9 months at the time of study termination. There was no significant difference in the number of patients who went on to receive an SCT, 16 patients (16.3%) in the CHAM arm and 14 patients (13.7%) in the control arm (P = .3033). A post-hoc analysis for hypothesis generation was completed to see whether any subgroup may have benefited. OS was not significantly different in any of the European LeukemiaNet 2017 risk groups (Figure 3B-D). In the control arm, there was no significant difference in survival based on salvage regimen (Figure 3E) although the study was not powered to directly assess this. Additionally, there was no benefit to the addition of devimistat for either primary refractory or relapsed patients (Figure 4). Indeed, there was no subgroup analyzed that demonstrated a significant benefit of the CHAM arm over the control arm (Figure 4).
OS of patients on ARMADA 2000. (A) OS by arm for the entire cohort for cases for which data are available. (B) OS for European LeukemiaNet 2017 (ELN 2017) favorable risk disease. (C) OS for ELN 2017 intermediate risk disease. (D) OS for ELN 2017 adverse risk disease and those patients for whom risk category was unknown. (E) OS by control salvage regimen chosen.
OS of patients on ARMADA 2000. (A) OS by arm for the entire cohort for cases for which data are available. (B) OS for European LeukemiaNet 2017 (ELN 2017) favorable risk disease. (C) OS for ELN 2017 intermediate risk disease. (D) OS for ELN 2017 adverse risk disease and those patients for whom risk category was unknown. (E) OS by control salvage regimen chosen.
Discussion
The outcomes for patients aged >50 years with relapsed or refractory AML are poor, and novel therapies are needed. Devimistat demonstrated a promising signal in phase 1 and 2 trials when combined with HAM, specifically in older patients with AML.9 This signal led to this phase 3, multicenter, open-label randomized study of devimistat in combination with CHAM vs a control salvage regimen of either HAM, MEC, or FLAG. When 200 patients were evaluable for response, the IDMC recommended that the trial be stopped because of lack of sufficiently promising CR rate in the CHAM arm.
The outcome is disappointing, with CR rates in both arms of only ∼20%. This is lower than previous reports for HAM, MEC, or FLAG in the salvage setting from single- or multi-institution retrospective analyses12,13 but remarkably similar to a previous randomized, multi-institution phase 3 study using these regimens.14 This discrepancy highlights the need for randomized, prospective, multi-institution studies when evaluating a novel therapeutic approach. Despite the disappointing CR rates, the OS in the control arm at 6.2 months is similar to that of previous phase 3 studies using HiDAC salvage regimens in relapsed/refractory AML.15,16 Indeed, a systemic review of outcomes for conventional chemotherapy in relapsed/refractory AML found median survivals that ranged from 6.2 to 8.7 months, consistent with these results.17 This study adds an additional data set to the outcomes of older patients treated with cytotoxic salvage regimens. The control arm performing on the lower end of these estimates likely reflects the fact that patients in this trial were older with a median age of 65 years and all patients were aged >50 years. The CHAM median OS of 8.9 months was numerically superior than that of the control arm and similar to what has been reported for targeted therapies in the relapsed setting 18 but less than the combined phase 1 and 2 experience in older patients for which a median OS of 10.4 months was reported.9 The CR rate in the previous studies was also higher at 52% of older patients treated with 2000 mg/m2 of devimistat. Some important differences to note between those studies are the fact that the cytarabine dose was significantly lower in this study in which 1 g/m2 was used compared with 1.5 to 3 g/m2 in the previous study. The lower dose of cytarabine was chosen after discussions with key opinion leaders who expressed concern that for patients aged ≥60 years, most investigators would not be comfortable dosing the cytarabine over 1 g/m2. Additionally, a day-14 marrow aspirate/biopsy was routinely performed, and ∼40% of older patients received a second induction cycle in the previous studies whereas only 12.5% of patients who received CHAM received a second induction cycle in this study. This was the result of the fact that many participating institutions were not comfortable using a second induction cycle with a HiDAC salvage regimen and so opted out of performing a day-14 marrow aspirate/biopsy for patients enrolled at their site. Finally, the trial was designed before the US Food and Drug Administration approval of hypomethylating agents and venetoclax for the treatment of patients with AML, and this likely contributed to the slow accrual that necessitated the changes in eligibility age and control chemotherapy options.
Apart from these potentially confounding factors, targeting metabolic pathways in AML has so far yielded no definitive clinical benefit.19-21 This may be a result of the complexity and plasticity of AML cell metabolism. Indeed, a recent study demonstrated that devimistat induced an increased reliance on gluconeogenesis and alternative pathways to generate the key carbon metabolite, acetyl-coenzyme A.22 These salvage pathways may allow for AML cells to circumvent the inhibition of devimistat and lead to resistance to therapy. Future strategies exploring metabolic inhibition in AML will need to use a combination of inhibitors to limit AML cell metabolic escape and resistance. Additional studies are needed to determine the most promising strategies.
Acknowledgment
This study was supported by Cornerstone Pharmaceuticals.
Authorship
Contribution: T.S.P., B.L.P., S.L., and J.C. designed the study and interpreted data; T.S.P. wrote the manuscript; and all other authors recruited patients and edited the manuscript.
Conflict-of-interest disclosure: S.L. was the CEO of Cornerstone Pharmaceuticals. T.S.P. and B.L.P. were paid consultants of Cornerstone Pharmaceuticals; and T.S.P. formerly served as the Chief Medical Officer. T.S.P. has received research funding from Cornerstone Pharmaceuticals in the past, which owns the licensing rights to CPI-613 and is currently developing it for use in oncology patients. R.A.L. has acted as a consultant or adviser to Amgen, Ariad/Takeda, Astellas, Celgene/Bristol Myers Squibb, CVS/Caremark, Epizyme, Immunogen, Jazz, Kling, Medpace, MorphoSys, Novartis, and Servier; and has received clinical research support to his institution from Astellas, Celgene, Cellectis, Daiichi Sankyo, Forty Seven/Gilead, Novartis, and Rafael Pharmaceuticals; and royalties from UpToDate. M.F. has acted as a consultant or adviser to Zentalis Pharmaceuticals; has received honoraria from Medscape; has received clinical research support to his institution from Bellicum Pharmaceuticals; and has ownership interest in Novartis and Sandoz. The remaining authors declare no competing financial interests.
The current affiliation of P.P. is Servier Pharmaceuticals, Boston, MA.
The current affiliation of M.F. is Novartis Pharmaceuticals, East Hanover, NJ.
Correspondence: Timothy S. Pardee, Internal Medicine, Section on Hematology/Oncology, Atrium Health Wake Forest Baptist, 1 Medical Center Blvd, Winston-Salem, NC 27157; email: tspardee@wakehealth.edu.
References
Author notes
Data are available on request from the corresponding author, Timothy S. Pardee (tspardee@wakehealth.edu).
The full-text version of this article contains a data supplement.