Key Points
Newly introduced CUB domain N-glycans reduce the binding of patient-derived anti-CUB antibodies.
Visual Abstract
In immune-mediated thrombotic thrombocytopenic purpura (iTTP), patients develop antibodies against ADAMTS13. Most patients exhibit inhibitory antispacer antibodies. Noninhibitory antibodies binding to the carboxy-terminal CUB domains have been suggested to enhance the clearance of ADAMTS13 in iTTP. Furthermore, anti-CUB antibodies induce an open conformation, which has been shown to be an important biomarker for disease severity and relapse risk. We explored whether the introduction of N-glycans in the CUB domains of ADAMTS13 can reduce the binding of pathogenic anti-CUB autoantibodies. The binding of a panel of anti-CUB monoclonal antibodies derived from patients with iTTP, to newly designed N-glycan modified ADAMTS13 CUB domain variants, was assessed by enzyme-linked immunosorbent assay. In addition, a subset of these variants was screened against plasma samples from patients with iTTP, which primarily contain antibodies directed toward the carboxy-terminal domains of ADAMTS13. Introduction of N-glycans at amino acid positions of 1251, 1255, and 1368 in the CUB1/2 domains of ADAMTS13 can effectively reduce the binding of 6 out of 7 anti-CUB antibodies derived from patients with iTTP. Reduced binding to CUB N-glycan variants was observed in 8 out of 9 patient samples. Binding was decreased from 81% to 47% for NGLY3+CUB-NGLY and 60% to 28% for 5ALA+CUB-NGLY variants. Collectively our findings show that the introduction of N-glycans within the CUB domain of ADAMTS13 can prevent the binding of anti-CUB antibodies in patients with iTTP. Based on these findings, we propose that CUB-NGLY modified ADAMTS13 variants can be used for improved treatment of patients with iTTP.
Introduction
Thrombotic thrombocytopenic purpura (TTP) is a rare hematological disorder with an annual incidence of 2 to 6 cases per million.1 In the majority of TTP patients, pathogenic autoantibodies develop against ADAMTS13 that prevent the processing of ultra large von Willebrand factor (VWF) multimers, thereby promoting the formation of platelet-rich thrombi in the microvasculature.2,3 This loss of activity is the result of direct inhibition of ADAMTS13 as well as immune-mediated clearance of the protease from the bloodstream.4-6 It has been well-established that 90% of immune TTP (iTTP) patients exhibit antibodies against a major epitope in the spacer domain, composed of residues 568, 592, 660, 661, and 665 of ADAMTS13, resulting in inhibition of ADAMTS13 activity.4,5,7-9 Introduction of alanine at these amino acid residues (5ALA) not only prevents the binding of antispacer domain antibodies, but also renders ADAMTS13 inactive.4,9 We have previously shown that introduction of an N-glycan (NGLY3) next to this epitope (employing a K608N substitution) was able to effectively shield ADAMTS13 from antispacer antibody binding, while simultaneously rescuing proteolytic activity.10,11 These findings suggest that N-glycan engineered ADAMTS13 could be a promising novel treatment for patients with iTTP.10,11
Several studies have shown that apart from the spacer domain, antibodies against the C-terminal domains of ADAMTS13 develop in 20% to 40% of patients with iTTP.4-6,12,13 These antibodies do not inhibit the activity of ADAMTS13, but instead enhance immune-mediated clearance of ADAMTS13, lowering its concentration in plasma and thereby indirectly its activity.6 Due to the antibody-mediated clearance, patients with iTTP present with very low ADAMTS13 levels compared to healthy controls. Recent studies speculate that ADAMTS13 clearance might be a major pathogenic mechanism of iTTP and a determinant of disease severity.14 Besides enhancing ADAMTS13 mediated clearance, anti-CUB (complement components C1r and C1s, sea urchin protein Uegf, and bone morphogenetic protein-1) domain antibodies have also been shown to induce conformational changes upon their binding to the CUB domains.15,16 Elegant studies have shown that binding of patient-derived anti-CUB domain antibodies promote the conversion of ADAMTS13 from a closed to an open conformation, which has been linked to the acute phase of iTTP.15-18 Furthermore, an open conformation of ADAMTS13 has been suggested as a biomarker for preclinical disease in patients at risk of relapse.15 Taken together, the anti-CUB domain antibodies might play a significant role in iTTP onset and development, and modification of only the spacer domain of ADAMTS13 might not be sufficient to fully prevent binding of pathogenic autoantibodies.
Therefore, we explore whether introduction of N-glycans within the CUB domains can prevent binding of anti-CUB antibodies. These CUB-NGLY mutants were then used together with spacer protective variants, like 5ALA or NGLY3, to study their involvement in avoiding the binding of antibodies present in a subset of patient samples with large proportion of antibodies that target the C-terminal domains of ADAMTS13. The results obtained show that these patients display reduced binding of anti-CUB antibodies, suggesting added benefit of combining the spacer protective mutants with the CUB-NGLY variants described in this study.
Methods
Patient samples selection
The study cohort consisted a subselection of 50 patient samples described previously,10 all of which were citrated plasma samples and have been obtained from the French TMA Registry (principal investigator: P.C. and A.V.). Selection occurred based on the following criteria: samples are obtained during the acute phase of iTTP, they exhibit high titers of anti-ADAMTS13 antibodies (>50 U/mL), and display ADAMTS13 activity of <10 IU/dL. Subsequent subselection of patient samples was made based on epitope mapping enzyme-linked immunosorbent assay (ELISA) data.10 If patients displayed <50% antibody binding to the MDTCS domains of ADAMTS13, patients were inferred to have significant levels of antibodies targeting the C-terminal domains (TSP2-CUB2) of ADAMTS13, and patient samples were included within the cohort. Of the initial 50 patient samples, 9 (18%) showed <50% binding to the MDTCS construct. Informed consent was obtained for each patient according to the Declaration of Helsinki. The study was approved by the ethics committee of Hospital Pitié-Salpêtrière and Hospital Saint-Antoine Assistance Publique. ADAMTS13 activity and anti-ADAMTS13 immunoglobulin G (IgG) titer were determined using the fluorescence resonance energy transfer substrate (FRETS)-VWF73 method (Peptide Institute Inc, Osaka, Japan) and the TECHNOZYM ADAMTS13-INHIBITOR ELISA (Technoclone, Vienna, Austria) respectively, as previously described.19
Production of ADAMTS13 variants and anti-ADAMTS13 antibodies
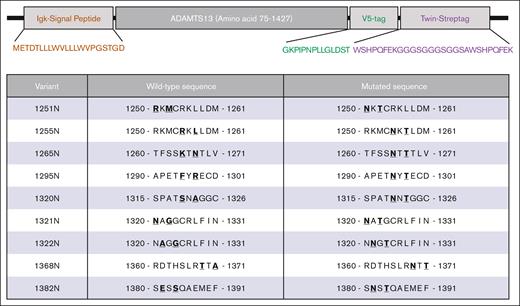
All ADAMTS13 variants were expressed from a plasmid cloning DNA vector, pcDNA3.1, containing a Kozak sequence (GCCGCCACC) and Igκ signal peptide (METDTLLLWVLLLWVPGSTGD) followed by the ADAMTS13 variant lacking the propeptide (amino acid 75-1427) followed by a V5 tag (GKPIPNPLLGLDST) for detection and a Twin-Strep-tag (WSHPQFEKGGGSGGGSGGSAWSHPQFEK) for purification (Figure 1; supplemental Methods). MDTCS was inserted into this vector using SgsI and XhoI restriction sites. CUB-NGLY variants were ordered as synthetic gene fragments and cloned using MreI and XhoI. Wild-type ADAMTS13, MDTCS,4,20 and CUB-NGLY combinations with 5ALA or NGLY3 were then generated by cloning the spacer fragment containing the 5ALA (R568A/F592A/R660A/Y661A/Y665A) or NGLY3 (K608N) mutations using SgsI and Esp3I. All constructs were sequenced before transfection. Constructs were expressed using the ExpiCHO system according to manufacturer instructions with minor adjustments. Cells were transfected using 0.5 μg of DNA per mL of culture. The high titer protocol was followed, where enhancer and feed were added after 24 hours and the cells were transferred from incubation at 37°C at 8% CO2 to 32°C at 5% CO2. On day 5, supernatant was collected after centrifugation for 30 minutes and 4000g. Ten millimolar benzamidine was added and aliquots were stored at −30°C. ADAMTS13 expression was confirmed by western blot using an anti-V5 antibody conjugated to horseradish peroxidase (HRP) for detection (supplemental Figure 1). Concentration was determined by ELISA as previously described.10,11 Patient-derived monoclonal antibodies were expressed in Expi293 cells using a pcDNA3.1 vector. Details regarding the expression constructs and production of the antibodies can be found in the supplemental Methods.
Overview of generated ADAMTS13 N-glycan variants. Top panel shows schematic overview of key elements of newly generated ADAMTS13 expression construct, containing Igκ signal peptide, ADAMTS13 lacking its propeptide, a V5-tag, and a Twin-Strep-tag. Lower panel shows amino acid modifications for each of the N-glycan variants of ADAMTS13. Mutant number indicates amino acid position of the asparagine substitution. At N+2 position, the amino acid has been mutated to threonine to complete N-x-S/T consensus sequence.
Overview of generated ADAMTS13 N-glycan variants. Top panel shows schematic overview of key elements of newly generated ADAMTS13 expression construct, containing Igκ signal peptide, ADAMTS13 lacking its propeptide, a V5-tag, and a Twin-Strep-tag. Lower panel shows amino acid modifications for each of the N-glycan variants of ADAMTS13. Mutant number indicates amino acid position of the asparagine substitution. At N+2 position, the amino acid has been mutated to threonine to complete N-x-S/T consensus sequence.
N-glycan analysis using mass spectrometry
A total of 1.5 μg of CUB-NGLY mutants were purified using MagStrepXT beads. CUB-NGLY variants were deglycosylated overnight using PNGaseF (Thermo Scientific, A39245). For PNGaseF negative control samples, CUB-NGLY bound beads were incubated under the same conditions without PNGaseF. ADAMTS13 variants were reduced and alkylated. Afterwards, ADAMTS13 variants were digested overnight at 800 rpm using 100 ng of either thermolysin and chymotrypsin at 65°C and 37°C, respectively. Samples were supplemented to a final concentration of 1% trifluoroacetic acid and desalted using an Oasis PRiME HLB 96-well μElution Plate (Waters, 186008052). Peptides were separated using an UltiMate 3000 LC system. Mass spectrometry and subsequent data analysis using PEAKS was performed as previously described.10 Presence of the N-glycan can be detected by deamidation of the asparagine in the PNGaseF treated samples, while no peptide should be observed in the non-PNGaseF treated control samples. A detailed protocol of the methods can be found in the supplemental Methods.
Epitope mapping ELISA
Binding of monoclonal antibodies or antibodies from patient plasma was assessed as previously described.10,11 ELISA plates were coated using 1 μg/mL 3H9 (antimetalloprotease antibody) after which 100 μL of binding buffer (1% bovine serum albumin [BSA] in phosphate-buffered saline [PBS], pH 7.4) containing 5.85 nM of ADAMTS13 was added for 1 hour at 37°C. To take into account the influence of endogenous ADAMTS13 on the binding values, no ADAMTS13 was added to 1 well, to allow for background subtraction. Afterwards, patient samples were diluted in 100 μL of binding buffer and added to the plate for 1 hour at 37°C. Anti-IgG1, anti-IgG2, anti-IgG3, and anti-IgG4 antibodies were diluted 10 000-fold in binding buffer and mixed. Subsequently, 100 μL of this mix was added to the plate and incubated at 37°C for 1 hour. Signal was developed for 10 minutes using 3,3´,5,5´-tetramentylbenzidine (TMB) solution and quenched using 0.1M H2SO4. Absorption was measured at 450 nm wavelength using a SpectraMax plate reader. For the binding of monoclonal antibodies to wild-type ADAMTS13 (wtADAMTS13) and variants, instead of patient samples, monoclonal antibodies were diluted to 1 nM in 100 μL binding buffer. All other steps were identical. After background subtraction, all optical density values were interpolated to a II-1 antibody standard curve and subsequently represented as a percentage relative to wtADAMTS13 for that patient or monoclonal antibody. Values above the calibration curve were excluded, values below the calibration curve were set to 0%. Epitope mapping experiments were performed in triplicate for monoclonal antibodies. Limited volumes of patient samples allowed only for single measurements.
FRETS-VWF73 activity of ADAMTS13
Before performing FRETS-VWF73 activity, wtADAMTS13 and variants were purified using MagStrepXT beads. Overall, 150 μL of 5% bead suspension was added to a 1.5 mL Eppendorf and washed using Buffer W (100 mM tris(hydroxymethyl)aminomethane [Tris], 150 mM NaCl, pH8). Afterwards, 600 μL of ADAMTS13 containing expression medium was added to the beads together with 600 μL Buffer W. Beads were incubated at 37°C at 1200 rpm. After 30 minutes, beads were washed and ADAMTS13 was eluted using 100 μL Buffer W supplemented with 50 mM biotin. Concentration was determined by ELISA as previously described.10,11 FRETS-VWF73 activity was determined as follows. 0.5 nM ADAMTS13 in 100 μL of FRETS buffer (20 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], 20 mM Tris-HCl, 20 mM Bis-Tris, 25 mM CaCl2, 0.005% Tween20, pH 6 or pH 7.5) is incubated for 10 minutes at room temperature. Afterwards, 100 μL of 2 μM of FRETS-VWF73 substrate in FRETS buffer (pH 6 or pH 7.5) is added, diluting ADAMTS13 to 0.25 nM. The reaction was followed at 340/450 nm excitation/emission wavelength at 30 second intervals using a Tecan Infinite 200 for 15 minutes. Activity was expressed as the activity at pH 7.5 relative to pH 6.0 For activity in presence of monoclonal antibodies, 18 nM of antibody was added to 2.1 nM of ADAMTS13 in FRETS buffer pH 6. Afterwards, 100 μL of 8 μM of FRETS-VWF73 substrate in FRETS buffer pH6 was added, diluting ADAMTS13 and antibody concentrations to 1.05 and 9 nM respectively. In both assays, initial velocity was determined using GraphPad Prism 8.1.
Conformation ELISA
ELISA plates were coated overnight using 5 μg/mL 6A6.17 The plate was blocked for 2 hour using 2% BSA in PBS. Subsequently, 0.3 μg/mL of purified ADAMTS13 or CUB-NGLY variants were incubated with or without 2.5 μg/mL 17G2 at 37°C for 30 minutes. All samples were added to the ELISA plate and incubated at 37°C for 1 hour. Biotinylated 19H4 antibody (1.5 μg/mL) was used to detect ADAMTS13 and incubated for 1 hour at 37°C. Afterwards, Streptavidin-HRP was added (1:10 000) for 1 hour at 37°C. Signal was developed for 20 minutes using TMB solution and quenched using 0.1M H2SO4. Absorption was measured at 450 nm wavelength using a SpectraMax plate reader. All optical density values were then expressed as a conformational index, which is the ratio of ADAMTS13 without 17G2 over ADAMTS13 with 17G2.
VWF-multimer activity assay
Prewarmed ADAMTS13 and variants were incubated with VWF multimers and urea at final concentrations of 1.9 nM ADAMTS13, 40 nM VWF, and 1.5M urea. After 10 minutes of incubation at 37°C and the reaction was stopped by addition of 1:1 volume-to-volume ratio of sample buffer (5 mM Tris, 0.5 mM Na2EDTA, 1% sodium dodecyl sulfate, 0.25 mM Bromophenolblue, 50% glycerol, pH 6.9). Samples were then heated to 60°C for 20 minutes. Multimers were separated on a 0.9% agarose gel using 75V at 4°C for 6 hours. Blotting was performed by overnight capillary transfer onto a polyvinylidene fluoride membrane. Membrane was blocked using 10% BSA in PBS-Tween (0.05%) for 1 hour and stained using VWF-HRP (1:1000) in 5% BSA in PBS-Tween (0.05%). Membrane was washed 3 times using PBS-Tween (0.05%) and 3 times with PBS. Multimers were visualized using SuperSignal Chemiluminescent substrate for 5 minutes and measured using the Amersham ImageQuant 800 with automatic exposure.
Flow assays
Human umbilical vein endothelial cells were cultured as previously described.10 Ibidi μ-slides were coated with 50 μg/mL collagen and seeded with 100 000 cells. Fresh EGM-18 medium was supplemented daily. Flow assays were performed on the fourth day of confluency. Flow assays were performed at a shear stress of 0.25 N/m2. Cells were stimulated using M199 medium supplemented with 100 μM of histamine for 10 minutes. After VWF release, M199 medium containing isolated platelets (160 × 109/L) was added for 5 minutes. Platelet isolation protocol is in the supplemental Methods. After 5 minutes, M199 medium containing wtADAMTS13 or CUB-NGLY variants was flown over the cells for 10 minutes (n = 2). As a negative control, M199 medium containing no ADAMTS13 was used. Every 10 seconds images were taken using a Zeiss Axio Observer Z1 microscope. Platelets were counted using FIJI and platelet numbers were expressed as a percentage of time point 0, which was set as the moment that ADAMTS13 reached the channel.
Statistical analysis
Statistical analysis was done using GraphPad Prism 9.1.1. Description of statistical analysis per figure can be found in the figure legend.
Results
Rational design of CUB-NGLY variants of ADAMTS13
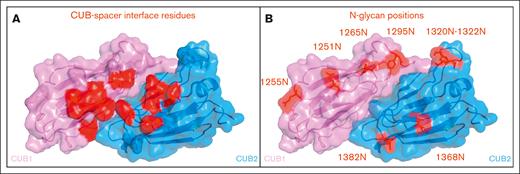
We explored whether introduction of N-glycans in the CUB domains of ADAMTS13 can prevent the binding of pathogenic autoantibodies that develop in patients with iTTP. A panel of N-glycan modified CUB domain variants was prepared based on 2 main criteria: first, the N-glycans were preferably introduced away from the modeled spacer-CUB interface, to minimize the chance of conformational alterations within this interface (Figure 2A).21 Second, the amino acid side chain had to be facing outward, as mutating an inward facing side chain could potentially affect protein stability and/or the efficacy of N-glycan attachment.21 Based on these criteria, 4 positions in the CUB1 (residues 1251, 1255, 1265, and 1295), and 5 positions in the CUB2 domain (residues 1320, 1321, 1322, 1368, and 1382), were converted to an asparagine (Figure 2B). To adhere to the N-glycan consensus sequence (N-X-S/T), for each converted asparagine the N+2 residues were substituted for a threonine, due to higher N-glycan attachment rates.22,23 Next to the single N-glycan variants, also combination variants, 1251N+1368N and 1255N+1368N, were generated, consisting of a CUB1 N-glycan (1251N or 1255N) and a CUB2 N-glycan (1368N).
Visual representation of CUB1/2 domains. (A) Location of hypothesized CUB residues involved in its interaction with the spacer domain in closed conformation. (B) Locations of newly introduced N-glycans within the CUB1 and CUB2 domains.
Visual representation of CUB1/2 domains. (A) Location of hypothesized CUB residues involved in its interaction with the spacer domain in closed conformation. (B) Locations of newly introduced N-glycans within the CUB1 and CUB2 domains.
Functional characterization of CUB-NGLY variants
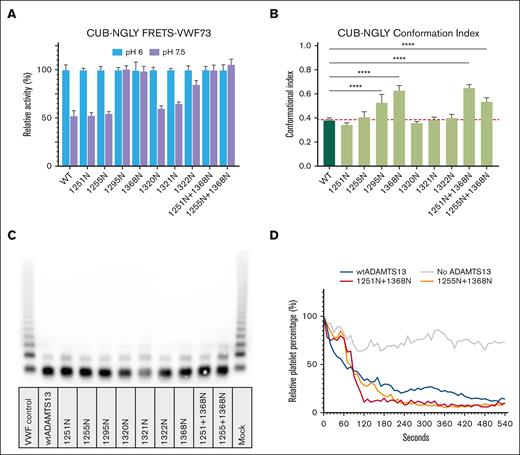
All CUB N-glycan variants, with the exception of 1265N and 1382N, were expressed at similar levels to wtADAMTS13; 1265N and 1382N were excluded from further analysis due to their limited expression. Activity of all remaining variants was assessed using the FRETS-VWF73 substrate at both pH 6 and pH 7.5 (Figure 3A). All variants displayed activity toward the FRETS-VWF73 substrate. CUB-NGLY variants 1295N, 1368N, 1251N+1368N, and 1255N+1368N showed similar activity at both pH 6 as well as at pH 7.5, suggesting these CUB-NGLY variants are present in an open conformation. To confirm these findings, we employed an ELISA where ADAMTS13 is captured using 6A6, which detects a cryptic epitope in the metalloprotease which is only available if ADAMTS13 is present in an open conformation.17 As a control, ADAMTS13 was incubated with monoclonal antibody 17G2, which converts ADAMTS13 from a closed to an open conformation.18 The conformational index was then calculated by division of the amount of binding of ADAMTS13 to 6A6 in the absence of 17G2 over the amount of binding in presence of 17G2. This analysis showed that the conformational index of 1295N, 1368N, 1251N+1368N, and 1255N+1368N was significantly higher when compared to wtADAMTS13 (Figure 3B). These findings confirm that the introduction of N-glycans at these positions alter the conformation of ADAMTS13. CUB-NGLY 1251N, 1555N, 1320N, 1321N, and 1322N are present in a closed conformation, similar to wtADAMTS13. The activity of the CUB-NGLY variants was also assessed by monitoring processing of VWF multimers. All CUB-NGLY variants were able to process VWF multimers under static conditions (Figure 3C). The data revealed that 1321N and 1255+1368N were slightly less efficient in processing VWF multimers when compared to wtADAMTS13. Lastly the ability of wtADAMTS13, 1251N+1368N, and 1255N+1368N to cleave VWF under flow was assessed. All variants were able to effectively cleave VWF multimers under flow (Figure 3D). Representative images at timepoints 0, 2, and 9 minutes can be found in supplemental Figure 2.
Functional characterization of CUB-NGLY variants. (A) FRETS-VWF73 activity (mean ± standard deviation, n = 3) of CUB-NGLY variants at pH 6 and pH 7.5. All activity values are relative to the activity of the variant at pH 6. (B) Conformation index of CUB-NGLY variants. Index is ratio of ADAMTS13 bound to the ELISA in the absence of 17G2 (an activating antibody) divided by amount of ADAMTS13 in the presence of 17G2 (n = 3). Significance was determined using ordinary 1-way analysis of variance using a Dunnett multiple comparison test, with a single pooled variance. ∗∗∗∗P < 0.0001. (C) VWF-multimer assay of wtADAMTS13 and CUB-NGLY variants. (D) Activity of wtADAMTS13 and CUB-NGLY variants under flow (n = 2). Platelet number percentage is relative to the number of platelets counted in the first frame.
Functional characterization of CUB-NGLY variants. (A) FRETS-VWF73 activity (mean ± standard deviation, n = 3) of CUB-NGLY variants at pH 6 and pH 7.5. All activity values are relative to the activity of the variant at pH 6. (B) Conformation index of CUB-NGLY variants. Index is ratio of ADAMTS13 bound to the ELISA in the absence of 17G2 (an activating antibody) divided by amount of ADAMTS13 in the presence of 17G2 (n = 3). Significance was determined using ordinary 1-way analysis of variance using a Dunnett multiple comparison test, with a single pooled variance. ∗∗∗∗P < 0.0001. (C) VWF-multimer assay of wtADAMTS13 and CUB-NGLY variants. (D) Activity of wtADAMTS13 and CUB-NGLY variants under flow (n = 2). Platelet number percentage is relative to the number of platelets counted in the first frame.
Assessment of N-glycan attachment using mass spectrometry
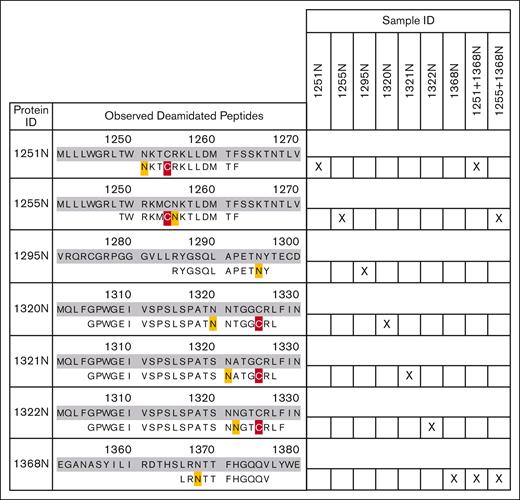
N-glycan attachment for the CUB-NGLY variants was analyzed by mass spectrometry after PNGaseF processing (more detailed description in supplemental Methods). PNGaseF removal of an N-glycan results in the deamidation of asparagine, yielding a measurable +0.98 Dalton mass shift. Peptides were generated using either chymotrypsin or thermolysin, to ensure coverage of all N-glycan sites. For all generated CUB-NGLY variants, a deamidation event was observed on the respective asparagine after PNGaseF treatment (Figure 4; supplemental Figure 3; supplemental Table 1), whereas no deamidation was observed on this location in the control samples which had not been PNGaseF treated (supplemental Figure 4).
Observed deamidated peptides for each CUB-NGLY variant. Identified peptides are aligned with the amino acid sequences of the respective mutant. Deamidated residues are marked yellow and carboxymethylated residues are marked red. Right panel shows the CUB-NGLY variants for which the peptide was observed.
Observed deamidated peptides for each CUB-NGLY variant. Identified peptides are aligned with the amino acid sequences of the respective mutant. Deamidated residues are marked yellow and carboxymethylated residues are marked red. Right panel shows the CUB-NGLY variants for which the peptide was observed.
Binding of patient-derived monoclonal antibodies to ADAMTS13 CUB-NGLY variants
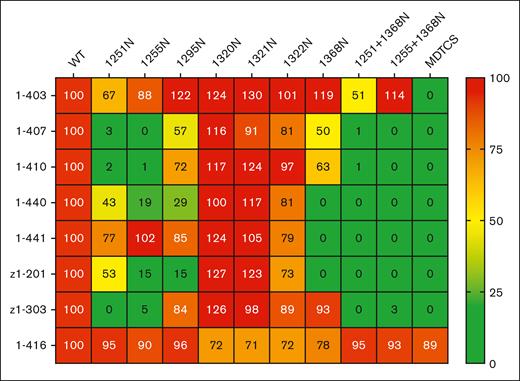
Antibody responses against ADAMTS13 during an iTTP episode are largely polyclonal in nature, with the majority of antibodies targeting the spacer domain.5,6,8,9,13,24,25 To directly study the effect of the N-glycans on anti-CUB antibodies, we generated 7, previously published, monoclonal antibodies derived from patients with iTTP, which target the CUB domains of ADAMTS13.26,27 In addition, 1 antispacer antibody (1-416) was taken along as a control. None of the anti-CUB domain antibodies were able to inhibit ADAMTS13 activity toward the FRETS-VWF73 substrate, with antibody 1-440 increasing activity to 142%, whereas the antispacer antibody (1-416) completely abolished activity (supplemental Figure 5). Binding of the 7 anti-CUB and 1 antispacer (1-416) antibodies against wtADAMTS13, MDTCS, and the CUB-NGLY variants (Figure 5). None of the 7 anti-CUB antibodies were able to bind to the MDTCS construct, whereas the antispacer antibody 1-416 bound MDTCS and wtADAMTS13 with the same efficacy, showing that the antibodies target the carboxy-terminal TSP2-CUB2 domains. In addition, antibody 1-416 effectively bound all CUB-NGLY variants indicating that similar amounts of CUB-NGLY molecules were captured on the ELISA plate for each variant. Analysis of CUB-NGLY variants 1251N and 1255N revealed strongly reduced reactivity to antibodies 1-407, 1-410, and z1-303. Binding of antibodies 1-403, 1-440, and z1-201 to 1251N was slightly reduced. Binding of 1-440 and z1-201 to 1255N and 1295N was reduced to 15% to 30% when compared to wtADAMTS13. CUB-NGLY variant 1368N displayed strongly reduced binding of 1-440, 1-441, and z1-201. Interestingly, 1368N was the only CUB-NGLY variant which displayed reduced binding of 1-441. Antibody 1-403 bound efficiently to all CUB-NGLY variants; only a slightly reduced binding to 1251N was observed. Combination mutants 1251N+1368N and 1255N+1368N had the expected combined effect of the separate mutants, where binding of 1-407, 1-410, 1-440, 1-441, z1-201, and z1-303 was almost completely abolished for both 1251N+1368N and 1255N+1368N. Combining the 1251N mutant with 1368N resulted in a modestly reduced binding of 1-403.
Heat map of reactivity of monoclonal anti-CUB antibodies with wtADAMTS13 and CUB-NGLY variants (n = 3). All binding percentages are relative to wtADAMTS13 binding.
Heat map of reactivity of monoclonal anti-CUB antibodies with wtADAMTS13 and CUB-NGLY variants (n = 3). All binding percentages are relative to wtADAMTS13 binding.
Binding of patient antibodies to ADAMTS13 CUB-NGLY variants
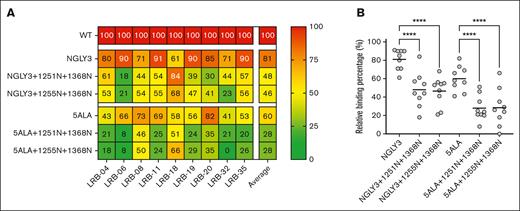
While the monoclonal patient-derived antibodies showed that the CUB-NGLY variants of ADAMTS13 can effectively reduce the binding of the anti-CUB antibodies, still some questions remained toward the general applicability of this approach in the broader iTTP population, as all the monoclonal antibodies tested are derived from a single patient with iTTP. Therefore, the CUB-NGLY variants were also screened against a panel of plasma samples of 9 patients with iTTP, which previously displayed <50% binding against the MDTCS domains of ADAMTS13 when compared to wtADAMTS13, while simultaneously displaying high binding against NGLY3 (full-length ADAMTS13 containing an N-glycan on position 608), a spacer protective mutant (supplemental Figure 6A-C). Together this suggests that much of their binding to ADAMTS13 is owing to antibodies targeting the C-terminal domains.10 CUB-NGLY reactivity to these patient samples was assessed by ELISA. Either 5ALA or NGLY3 was used to eliminate the binding of any anti-spacer antibodies that could also be present in these patient samples. CUB-NGLY combination mutants 1251N+1368N and 1255N+1368N were cloned into the 5ALA and NGLY3 constructs and used to assess the effect of these mutations on the binding of anti-CUB antibodies. Reactivity was then expressed as a heat map with binding to wild-type ADAMTS13 set as 100% (Figure 6A). Compared with wild-type ADAMTS13, all patients still display average binding levels of 60% to 5ALA and 81% against NGLY3. Upon introduction of the CUB-NGLY combinations, we observed a pronounced decrease in antibody binding for 8 of 9 patients both for the 5ALA combinations as well as the NGLY3 combinations. Only patient LRB-18 did not show a decrease in binding to the CUB-NGLY variants (Figure 6A). For 5ALA a significant decrease in binding from 60% to 28% for both 5ALA+1251N+1368N and 5ALA+1255N+1368N was observed (Figure 6B). NGLY3+1251N+1368N reduced binding from 81% to 48%. NGLY3+1255N+1368N displayed similar effectiveness, reducing binding to 46% instead. Taken together, this data show that 8 of 9 (89%) patients with iTTP with anti–C-terminal antibodies share a common CUB domain epitope on ADAMTS13 and that introducing N-glycans within this epitope can effectively reduce the binding of pathogenic autoantibodies.
CUB-NGLY variants reduce the binding of nonspacer targeting antibodies. (A) Heat map of reactivity of plasma from patients with iTTP with wtADAMTS13 and CUB-NGLY variants (n = 1). (B) ELISA binding result of patient antibodies against spacer protective mutants (NGLY3 and 5ALA) ± CUB-NGLY variants. All binding is relative to wtADAMTS13. Significance was determined using an ordinary 1-way analysis of variance using a Tukey multiple comparison test, with a single pooled variance. ∗∗∗∗P < 0.0001.
CUB-NGLY variants reduce the binding of nonspacer targeting antibodies. (A) Heat map of reactivity of plasma from patients with iTTP with wtADAMTS13 and CUB-NGLY variants (n = 1). (B) ELISA binding result of patient antibodies against spacer protective mutants (NGLY3 and 5ALA) ± CUB-NGLY variants. All binding is relative to wtADAMTS13. Significance was determined using an ordinary 1-way analysis of variance using a Tukey multiple comparison test, with a single pooled variance. ∗∗∗∗P < 0.0001.
Discussion
In patients with iTTP, pathogenic autoantibodies inhibit the activity of ADAMTS13 and have also been suggested to enhance clearance of ADAMTS13 from the circulation.14,28 Previously, we have shown that introduction of an N-glycan in the spacer domain of ADAMTS13 prevents the binding of antispacer domain antibodies to ADAMTS13.10,11 It is well-established that antibodies targeting the carboxy-terminal TSP2-CUB2 domains develop in a subset of patients with iTTP.4-6,12,13 Here, we introduced N-glycans within the CUB1 and CUB2 domains of ADAMTS13 and determined whether a panel of patient-derived monoclonal antibodies as well as 9 samples from patients with iTTP were capable of binding to these newly generated CUB-NGLY variants.
Functional characterization of the CUB-NGLY variants revealed that CUB-NGLY 1295N, 1368N, 1251N+1368, and 1255N+1368N are in an open conformation. Conversely, CUB-NGLY 1251N, 1255N, 1320N, 1321N, and 1322N are primarily in a closed conformation. The closed conformation of ADAMTS13 is maintained by interactions between residues in the CUB1/2 domain and spacer domain.29 Our findings suggest that attachment of N-glycans at residues 1295 and 1368 interfere with spacer-CUB domain interactions.
CUB-NGLY variants of ADAMTS13 were able to efficiently reduce binding of 6 out of 7 patient-derived monoclonal anti-CUB antibodies.26,27 Interestingly, binding of 1-403 could not be fully abolished using either single or double N-glycan mutants. Binding of 1-403 to 1251N was slightly reduced when compared to wild-type ADAMTS13; a further decrease in binding was observed upon introduction of an N-glycan at position 1368. These findings suggest that the binding site of 1-403 differs from that of the other patient-derived antibodies. We also analyzed 9 patient samples containing anti-TSP2-CUB2 domain antibodies for their ability to bind to the CUB-NGLY variants described in this study. To perform this analysis, we combined the CUB-NGLY variants with variants in which the major autoantibody binding site in the spacer domain was modified. For this we used a 5ALA variant in which the residues 568, 592, 660, 661, and 665 were replaced by an alanine as well as the NGLY3 variant in which an N-glycan was introduced at K608.7,10,11 We observed that addition of the CUB-NGLY combination mutations had a beneficial effect for 8 of 9 patients with iTTP, with an average reduction in antibody binding of 33% for 1251N+1368N and 34% for 1255N+1368N (Figure 6A-B). The decrease in antibody binding is similar for both 5ALA as well as NGLY3 combination mutants, showing that the reduced antibody binding is caused by the CUB-NGLY variants.
Taken together, this shows that the CUB-NGLY variants prevent binding of autoantibodies to an epitope located within the CUB domains that is distinct from the spacer epitope. These observations show that the CUB-NGLY variants 1251N+1368N and 1255N+1368N are capable of shielding ADAMTS13 from the binding of anti-CUB domain antibodies that develop in patients with iTTP. It should be noted that introduction of N-glycans in the CUB domains does not completely abolish binding in all patient samples. This indicates that additional binding sites for autoantibodies are present on ADAMTS13 that are not shielded by the N-glycan variants that have been described in this study. It has been proposed that anti-CUB domain antibodies may promote the clearance of ADAMTS13. Therefore, it will be important to investigate whether autoantibody-mediated clearance is affected by N-glycan shielding of ADAMTS13 in future studies. Overall, taking into account that 20% to 40% of patients develop C-terminal targeting antibodies,4-6,12,13 creation of N-glycan shielded variants preventing the binding of both spacer as well as CUB domain directed antibodies could be a beneficial strategy for quickly restoring and maintaining functional levels of ADAMTS13 in patients with iTTP.
Acknowledgments
The authors thank Tom Arfman for helpful discussions and critical review of the manuscript.
This work was supported by funding from Landsteiner Foundation for Blood Transfusion Research (LSBR 2026).
Authorship
Contribution: T.P., N.S., J.F.d.S., P.K., and P.L. designed and performed experiments; T.P., N.S., and J.V. designed the constructs; T.P., N.S., J.F.d.S., P.K., P.L., and J.V. analyzed and interpreted the data; B.S.J., A.V., and P.C. provided the patient samples; K.V. provided monoclonal antibodies that were used in this study; and T.P. and J.V. wrote the first draft of the manuscript.
Conflict-of-interest disclosure: J.V. and T.P. are listed as inventors on a patent application related to the work described in this manuscript. P.C. is a member of the clinical advisory board for Alexion, Sanofi, Takeda, and Janssen. A.V. is a member of the advisory boards for Sanofi, Takeda, and LFB Biomedicaments. The remaining authors declare no competing financial interests.
Correspondence: Jan Voorberg, Department of Molecular Hematology, Sanquin-Academic Medical Center Landsteiner Laboratory, Plesmanlaan 125, 1066 CX Amsterdam, The Netherlands; email: j.voorberg@sanquin.nl.
References
Author notes
Original data are available on request from the corresponding author, Jan Voorberg (j.voorberg@sanquin.nl).
The full-text version of this article contains a data supplement.