Key Points
Short-term mortality after HCT was not excessive when compared with standard care for SCD.
Patients with SCD experienced fewer pain crises and improved patient-reported outcomes after HCT when compared with standard care.
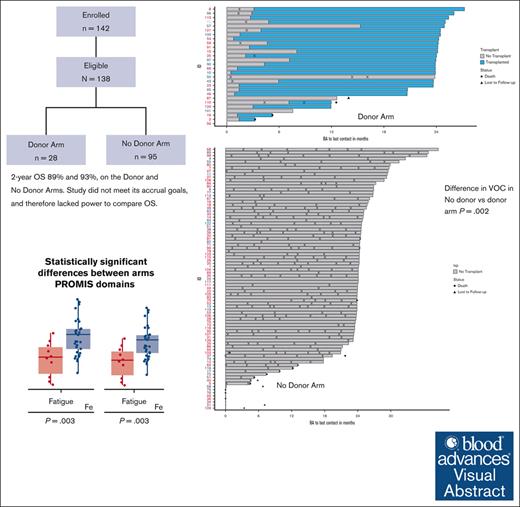
Visual Abstract
Disease-modifying therapies are standard of care (SOC) for sickle cell disease (SCD), but hematopoietic cell transplantation (HCT) has curative potential. We compared outcomes prospectively through 2 years after biologic assignment to a donor or no donor (SOC) arm based on the availability of an HLA-matched sibling or unrelated donor (BMT CTN 1503). A donor search was commenced after eligibility confirmation. The primary end point was a comparison of survival between the treatment arms 2 years after biologic assignment. Power calculations required 60 participants in the donor arm and 140 in the no donor arm to determine if early transplant-related mortality might be balanced by disease-related mortality over a longer period of follow-up. Secondary objectives were a comparison of the changes in SCD-related events, functional outcomes, and organ function. The data were analyzed according to the intent-to-treat principle. A total of 113 participants were enrolled with 28 in the donor arm and 85 in the no donor arm. The 2-year probabilities of survival were 89% and 93%, in the donor vs no donor arms. Vaso-occlusive pain (VOC) was less frequent in the donor arm in the second year after biologic assignment (P < .001). Based on PROMIS-57 surveys, there was a decrease in fatigue (P = .003) and an increase in the ability to participate in social roles and activities (P = .003) in the donor arm 2 years after biologic assignment. Differences in other secondary outcomes did not reach statistical significance. Barriers to accrual prevented an objective comparison of survival. Assignment to the donor arm led to improvements in VOC, fatigue, and social function. This trial was registered at www.clinicaltrials.gov as #NCT02766465.
Introduction
Sickle cell disease (SCD) is associated with significant morbidity, health care utilization, and impaired quality of life.1 Despite the availability of disease modifying therapies, adults with severe SCD still experience early mortality. In a retrospective cohort analysis of adults with SCD who survived to the age of 20 years from 2 comprehensive SCD centers, the median survival in persons with Hb SS and Hb SC was 48.0 and 54.7 years, respectively,2-4 representing a roughly 20- to 30-year decrease in life span when compared with Black Americans who do not have SCD. Investigations to identify curative therapies and to better define their risk vs benefit profiles have been conducted.5,6 Retrospective reports suggest excellent survival in patients younger than 13 years who were treated with HLA-matched sibling transplantation.2-4,7 The rationale for conducting the current phase 2 trial was to investigate whether hematopoietic cell transplantation (HCT) from an HLA-matched sibling or unrelated donor in adolescents and young adults with severe SCD might confer better survival when compared with standard of care (SOC). The HCT regimen employed in this trial was tested in a pilot trial that reported a 1-year survival of 91% and an event-free survival of 82%.8
We hypothesized that for participants who were assigned to the donor arm (biologic assignment based on donor availability) and who were expected to receive HCT, HCT-related mortality might exert an early negative impact on survival but that the mortality would plateau after transplant, followed by protection from disease-related mortality. In contrast, in the no donor arm, participants were expected to succumb over time to the cumulative effects of their disease with a mortality rate higher than that in the general population3,9
Methods
The Blood & Marrow Transplant Clinical Trials Network study BMT CTN 1503 (STRIDE2; NCT02766465) was a phase 2 multicenter trial of biologic assignment to treatment arms based on the availability of an HLA-matched sibling or HLA-matched unrelated donor (donor arm) or to a no donor arm to receive SOC treatment in adolescents and young adults (aged ≥15 and <41 years) with severe SCD. The trial opened for enrollment in December 2016 at 35 sites in the United States and was closed in August 2021 for slower than expected accrual after enrolling 138 of 200 participants (69% of target enrollment). Patients or legal guardians signed the informed consent forms. The trial was approved by an institutional review board at each participating site, or approval was delegated by a participating site to a single institutional review board at the National Marrow Donor Program.
The eligibility and functional status criteria for enrollment are summarized in Table 1. Disease severity was confirmed by an eligibility review committee. Initially, this committee reviewed source documentation of disease severity for all protocol-specified severity criteria but was later restricted to review of the neurologic criteria only. HLA-typing and donor searches were performed only after confirmation of eligibility; an exception was made if a subject had an unsuccessful related donor search without an unrelated donor search before enrollment. After biologic assignment, participants were enrolled in the appropriate treatment arm (donor or no donor). With approval from the Data Safety and Monitoring Board (DSMB), confirmation that all eligibility criteria were met, including donor availability, was extended from 90 days to 180 days from enrollment to biologic assignment. The protocol and a summary of the amendments are available in the supplemental Materials.
Eligibility criteria
| Clinical severity . | Physical function . |
|---|---|
| Clinically significant neurologic event (stroke) or neurological deficit lasting >24 h. | Karnofsky/Lansky performance score ≥60; left ventricular ejection fraction >40% or left ventricular shortening fraction >26% by cardiac echocardiogram or multigated acquisition (MUGA) scan. |
| History of 2 or more episodes of acute chest syndrome (ACS) in the 2-year period preceding enrollment or referral, despite adequate supportive care measures, such as asthma therapy. | Pulse oximetry with baseline O2 saturation of ≥85% and lung diffusion test (DLCO) > 40%, corrected for hemoglobin. |
| An average of 3 or more pain crises per year in the 2-y period preceding enrollment or referral that required IV pain management in the outpatient or inpatient hospital setting. | Serum creatinine ≤1.5 × upper limit of normal (ULN) per local laboratory and either creatine clearance >70 mL/min using Cockcroft-Gault calculation or creatinine clearance >70 mL/min by 24-h urine or glomerular filtration rate (GFR) >70 mL/min per 1.73 m2 by radionuclide GFR; ALT and AST <5 × ULN. |
| Administration of regular red blood cell (RBC) transfusion therapy, defined as 8 or more transfusion events per year (in the 12 months before enrollment) to prevent vaso-occlusive clinical complications, such as pain, stroke, or ACS. | Serum conjugated bilirubin ≤2 × ULN for age per local laboratory (serum conjugated bilirubin >2 × ULN permitted if there is evidence of hyper hemolytic reaction after recent RBC transfusion or moderate direct hyperbilirubinemia (direct serum bilirubin <5 times ULN and not caused by underlying hepatic disease). |
| An echocardiographic finding of tricuspid valve regurgitant jet velocity ≥2.7 m/s. | |
| Ongoing high impact chronic pain on a majority of days per month for ≥6 months as defined by 1 or more of the following: chronic pain without contributory SCD complications OR mixed pain type in which chronic pain is occurring at sites unrelated to any sites associated with contributory SCD complications, such as leg ulcers and/or avascular necrosis. | |
| Participants assigned to the donor arm were required to meet the following additional criteria to proceed with HCT before start of conditioning: | |
| Liver MRI to document hepatic iron content if participant was currently receiving ≥8 packed RBC transfusions for 1 or more years or had received a cumulative total of ≥20 packed RBC transfusions. Participants with hepatic iron content ≥7 mg Fe/g liver dry weight by liver MRI were required to have a liver biopsy and histologic examination to document the absence of cirrhosis, bridging fibrosis, and active hepatitis. | |
| Lack of clinical or radiologic evidence of a recent neurologic event by cerebral MRI/MRA. | |
| Documentation of willingness to use approve contraception until discontinuation of all immunosuppressive medications. | |
| Clinical severity . | Physical function . |
|---|---|
| Clinically significant neurologic event (stroke) or neurological deficit lasting >24 h. | Karnofsky/Lansky performance score ≥60; left ventricular ejection fraction >40% or left ventricular shortening fraction >26% by cardiac echocardiogram or multigated acquisition (MUGA) scan. |
| History of 2 or more episodes of acute chest syndrome (ACS) in the 2-year period preceding enrollment or referral, despite adequate supportive care measures, such as asthma therapy. | Pulse oximetry with baseline O2 saturation of ≥85% and lung diffusion test (DLCO) > 40%, corrected for hemoglobin. |
| An average of 3 or more pain crises per year in the 2-y period preceding enrollment or referral that required IV pain management in the outpatient or inpatient hospital setting. | Serum creatinine ≤1.5 × upper limit of normal (ULN) per local laboratory and either creatine clearance >70 mL/min using Cockcroft-Gault calculation or creatinine clearance >70 mL/min by 24-h urine or glomerular filtration rate (GFR) >70 mL/min per 1.73 m2 by radionuclide GFR; ALT and AST <5 × ULN. |
| Administration of regular red blood cell (RBC) transfusion therapy, defined as 8 or more transfusion events per year (in the 12 months before enrollment) to prevent vaso-occlusive clinical complications, such as pain, stroke, or ACS. | Serum conjugated bilirubin ≤2 × ULN for age per local laboratory (serum conjugated bilirubin >2 × ULN permitted if there is evidence of hyper hemolytic reaction after recent RBC transfusion or moderate direct hyperbilirubinemia (direct serum bilirubin <5 times ULN and not caused by underlying hepatic disease). |
| An echocardiographic finding of tricuspid valve regurgitant jet velocity ≥2.7 m/s. | |
| Ongoing high impact chronic pain on a majority of days per month for ≥6 months as defined by 1 or more of the following: chronic pain without contributory SCD complications OR mixed pain type in which chronic pain is occurring at sites unrelated to any sites associated with contributory SCD complications, such as leg ulcers and/or avascular necrosis. | |
| Participants assigned to the donor arm were required to meet the following additional criteria to proceed with HCT before start of conditioning: | |
| Liver MRI to document hepatic iron content if participant was currently receiving ≥8 packed RBC transfusions for 1 or more years or had received a cumulative total of ≥20 packed RBC transfusions. Participants with hepatic iron content ≥7 mg Fe/g liver dry weight by liver MRI were required to have a liver biopsy and histologic examination to document the absence of cirrhosis, bridging fibrosis, and active hepatitis. | |
| Lack of clinical or radiologic evidence of a recent neurologic event by cerebral MRI/MRA. | |
| Documentation of willingness to use approve contraception until discontinuation of all immunosuppressive medications. | |
Individuals with a previous HLA typing of both related and unrelated potential donors were not eligible for this study. Those who only had previous HLA typing performed on family members, without identifying a matched related donor and with no extension of HLA-typing to potential unrelated donors, were eligible for this study.
MRA, magnetic resonance angiogram; MRI, magenetic resonance imaging.
HCT conditioning and GVHD prophylaxis regimen
Based on a pilot trial for HCT,6 participants assigned to the donor arm were intended to receive a bone marrow graft and a myeloablative conditioning regimen of busulfan (12.8 mg/kg), fludarabine (105 mg/m2), and rabbit antithymocyte globulin (6 mg/kg). Graft-versus-host disease (GVHD) prophylaxis included tacrolimus and methotrexate (supplemental Table 1; regimen A). An amendment permitted 2 additional conditioning regimens (B and C) with alternate GVHD prophylaxis and graft (supplemental Table 1).10,11 Regimen B was an alternative for HLA-matched sibling transplantation and included alemtuzumab and low-dose total body irradiation (TBI), peripheral blood graft, and sirolimus for GVHD prophylaxis. Regimen C consisted of alemtuzumab, fludarabine, and melphalan, a marrow graft, and tacrolimus and methotrexate for GVHD prophylaxis.
Primary end point
The primary end point was a comparison between the donor and no donor arms of the observed proportion of patients who survived at 2 years after biologic assignment. Regardless of the treatment received, subjects remained in their assigned treatment arm for analysis of survival (intent-to-treat principle) and all other end points. Survival at 2 years in the 2 arms was compared with the goal of establishing if the difference in the proportion of patients surviving at this early time point was no more than 0.15 lower in the donor arm than in the no donor arm. It was assumed that 60 participants would be assigned to the donor arm and 140 to the no donor arm and that, at 2 years, 95% of participants in the no donor arm and 80% of participants in the donor arm would remain alive for the intended binomial comparison. A 0.10, 1-sided significance level set the rejection region at 1.282 standard deviations beyond the null. With this design, there was greater than 80% power to reject an alternative difference of 0.30. There were no interim analyses for efficacy. Stopping guidelines were developed only for the donor arm using a truncated sequential probability ratio test for 100-day and 1-year mortality and day 100 graft failure. End points were monitored separately for HLA-matched sibling and unrelated donor transplantation.
Secondary end points
The occurrence of sickle-related events, including acute chest syndrome, stroke, skin ulcers, and severe vaso-occlusive pain (VOC) crises, was assessed quarterly as the number of events per patient. Health-related quality of life (HRQoL) was assessed annually using scored values from the PROMIS-57 and the stiffness impact short form (Adult Sickle Cell Quality of LIfe Measurement System). Pain intensity was measured annually using both the PROMIS pain intensity score with a 7-day lookback and the mean pain intensity recorded in an electronic pain diary twice daily for 28 consecutive days. Functional assessments, such as the 6-minute walk distance to assess exercise capacity, the tricuspid regurgitant jet velocity, albuminuria, and pulmonary function testing, were also conducted annually. Additional end points for patients in the donor arm who underwent HCT were primary and secondary graft failure, grade 2 to 4 and 3 to 4 acute and chronic GVHD. The occurrence of other HCT-related complications, including infectious complications and viral reactivation, are described. GVHD-free, disease recurrence–free survival (GRFS) was defined as being alive with sustained donor engraftment and without grade 3 to 4 acute GVHD or chronic GVHD that requires systemic immunosuppression.12
Statistical analysis
An exact binomial comparison of the proportion of participants alive at 2 years was planned but was modified with approval from the DSMB. The 2-year survival was calculated for each treatment arm using the method of Kaplan and Meier,13 along with the 95% confidence interval (CI) calculated separately for the donor and the no donor arms using Greenwood’s formula.14 The probability of GRFS was calculated for transplant recipients using the method of Kaplan and Meier13 with the 95% CI calculated using Greenwood’s formula. Comparisons between the donor and no donor arms for the secondary end points were done using the Wilcoxon rank sum test. Binary end points are presented as point estimates with exact CIs, and differences between arms were tested using the Fisher exact test. Modeling was not undertaken. The incidence of grade 2 to 4 acute and chronic GVHD was calculated using the cumulative incidence estimator to accommodate competing risks and tested using the Gray test.15 There were no adjustments for multiplicity of testing.
Data sharing
Deidentified data will be deposited in the National Heart, Lung, and Blood Institute’s Biologic Specimen and Data Repository Information Coordinating Center, a publicly available database. All relevant trial-related documents will also be available via the repository. Data will be accessible 3 years after the end of the trial and 2 years after the primary publication.
Results
Enrollment, biologic assignment, and characteristics of the participants
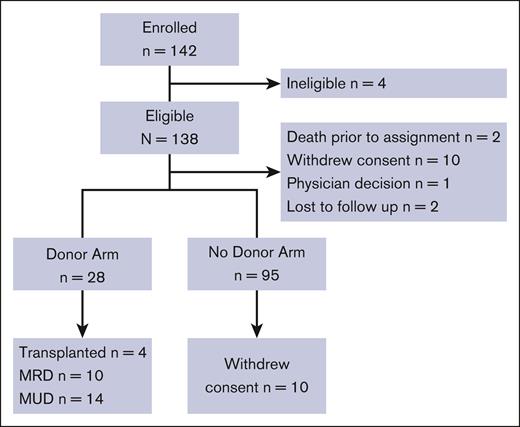
A CONSORT diagram outlines trial participation (Figure 1), and enrollment by site is shown in supplemental Table 2A-B. Ten participants assigned to the no donor arm withdrew all consent, including consent to use data after biologic assignment. Of the remaining 85 participants assigned to the no donor arm, 4 participants received HCT from an HLA-mismatched donor and 1 participant received gene therapy. Four participants assigned to the donor arm did not proceed to HCT as planned (4 of 28; 14%). Of these, 1 failed the assessments after biologic assignment, 1 withdrew consent, 1 delayed HCT until the HLA-matched unrelated donor was no longer available, and the remaining participant was lost to follow-up.
CONSORT diagram. MRD, matched related donor; MUD, matched unrelated donor.
The characteristics (including indications for entry) of the 113 participants who proceeded in the trial are presented in Table 2. The median age was 26 years and 52% were male. Painful VOC events was the most common indication. Clinical features were balanced across the treatment arms except for neurologic deficits that were more common in the donor arm (P = .017). All but 1 participant who proceeded to HCT received regimen A, which was the initial protocol-specified conditioning regimen. One participant received regimen C11 and none received regimen B. The median time from biologic assignment to HCT was 88 days. Six of 24 (25%) in the donor arm were transplanted ≥180 days after biologic assignment with 1 HCT at 466 days.
Characteristics of the 113 participants who were biologically assigned
| . | All N = 113 . | No donor n = 85 . | Donor n = 28 . |
|---|---|---|---|
| Median age, y (range) | 26.2 (15.6-40.8) | 25.9 (15.6-39.3) | 29.1 (15.6-40.8) |
| Sex | |||
| Male | 59 (52%) | 46 (54%) | 13 (46%) |
| Female | 54 (48%) | 39 (46%) | 15 (54%) |
| Ethnicity | |||
| Hispanic or Latino | 12 (11%) | 8 (9.4%) | 4 (14%) |
| Not Hispanic or Latino | 99 (88%) | 75 (88%) | 24 (86%) |
| Not answered | 2 (1.8%) | 2 (2.4%) | 0 (0%) |
| Race (condensed) | |||
| White | 6 (5.3%) | 5 (5.9%) | 1 (3.6%) |
| Black or African American | 99 (88%) | 73 (86%) | 26 (93%) |
| Other | 3 (2.7%) | 2 (2.4%) | 1 (3.6%) |
| Unknown | 3 (2.7%) | 3 (3.5%) | 0 (0%) |
| Not answered | 2 (1.8%) | 2 (2.4%) | 0 (0%) |
| SCD type∗ | |||
| Hb SS | 88 (84%) | 67 (83%) | 21 (88%) |
| Hb SC | 5 (4.8%) | 4 (4.9%) | 1 (4.2%) |
| Hb Sb | 11 (10%) | 9 (11%) | 2 (8.3%) |
| Hb S-OArab | 1 (1%) | 1 (1.2%) | 0 (0%) |
| Unknown | 8 | 4 | 4 |
| Severity of SCD criteria† | |||
| Neurologic | 14 (12%) | 7 (8.2%) | 7 (25%) |
| Acute chest syndrome | 15 (13%) | 14 (16%) | 1 (3.6%) |
| VOC crises | 79 (70%) | 62 (73%) | 17 (61%) |
| Red blood cell transfusion | 20 (18%) | 13 (15%) | 7 (25%) |
| Tricuspid regurgitant jet velocity | 10 (8.8%) | 9 (11%) | 1 (3.6%) |
| High impact chronic pain | 29 (30%) | 19 (27%) | 10 (36%) |
| . | All N = 113 . | No donor n = 85 . | Donor n = 28 . |
|---|---|---|---|
| Median age, y (range) | 26.2 (15.6-40.8) | 25.9 (15.6-39.3) | 29.1 (15.6-40.8) |
| Sex | |||
| Male | 59 (52%) | 46 (54%) | 13 (46%) |
| Female | 54 (48%) | 39 (46%) | 15 (54%) |
| Ethnicity | |||
| Hispanic or Latino | 12 (11%) | 8 (9.4%) | 4 (14%) |
| Not Hispanic or Latino | 99 (88%) | 75 (88%) | 24 (86%) |
| Not answered | 2 (1.8%) | 2 (2.4%) | 0 (0%) |
| Race (condensed) | |||
| White | 6 (5.3%) | 5 (5.9%) | 1 (3.6%) |
| Black or African American | 99 (88%) | 73 (86%) | 26 (93%) |
| Other | 3 (2.7%) | 2 (2.4%) | 1 (3.6%) |
| Unknown | 3 (2.7%) | 3 (3.5%) | 0 (0%) |
| Not answered | 2 (1.8%) | 2 (2.4%) | 0 (0%) |
| SCD type∗ | |||
| Hb SS | 88 (84%) | 67 (83%) | 21 (88%) |
| Hb SC | 5 (4.8%) | 4 (4.9%) | 1 (4.2%) |
| Hb Sb | 11 (10%) | 9 (11%) | 2 (8.3%) |
| Hb S-OArab | 1 (1%) | 1 (1.2%) | 0 (0%) |
| Unknown | 8 | 4 | 4 |
| Severity of SCD criteria† | |||
| Neurologic | 14 (12%) | 7 (8.2%) | 7 (25%) |
| Acute chest syndrome | 15 (13%) | 14 (16%) | 1 (3.6%) |
| VOC crises | 79 (70%) | 62 (73%) | 17 (61%) |
| Red blood cell transfusion | 20 (18%) | 13 (15%) | 7 (25%) |
| Tricuspid regurgitant jet velocity | 10 (8.8%) | 9 (11%) | 1 (3.6%) |
| High impact chronic pain | 29 (30%) | 19 (27%) | 10 (36%) |
Hb SB, hemoglobin SB disease; Hb SC, hemoglobin SC disease; Hb S-O, hemoglobin S-O disease: Hb SS, hemoglobin SS disease.
Percentages are calculated based on the numbers with known disease type (105, 81, 24).
Participants could report multiple criteria. Numbers here represent assessment after eligibility review committee confirmed eligibility.
Overall survival
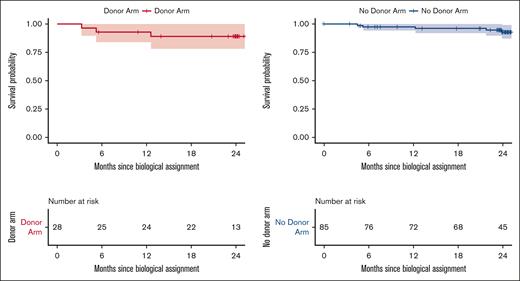
Survival in the 85 participants in the no donor arm and 28 participants in the donor arm is presented in Figure 2. The finding of no difference between the 2 treatment arms was not adequately statistically powered because of incomplete accrual, and a comparative analysis to support a conclusion that survival in the donor arm was not worse than survival in the no donor arm was not possible. Instead, point estimates for this end point in each arm were generated descriptively with 95% CIs. The estimate of overall survival at 2 years after biologic assignment was 89% in the donor arm (95% CI, 78-100) and 93% (95% CI, 87-99) in the no donor arm. There were 8 deaths during the first 2 years after biologic assignment; 5 deaths occurred on the no donor arm and 3 deaths in the donor arm. The primary causes of death in the no donor arm included cardiac arrest (n = 1), congestive heart failure (n = 1), sepsis (n = 1), and SCD-related death (n = 2). The primary causes of death in the donor arm included multiorgan failure (n = 1), graft failure with acute subarachnoid hemorrhage (n = 1), and GVHD (n = 1).
Overall survival. The probabilities of overall survival 2 years after biologic assignment for patients in the no donor and donor arms were 93% (95% CI, 87-99) and 89% (95% CI, 78-100).
Overall survival. The probabilities of overall survival 2 years after biologic assignment for patients in the no donor and donor arms were 93% (95% CI, 87-99) and 89% (95% CI, 78-100).
Sickle cell events of special interest
Events of special interest that occurred between enrollment and biologic assignment are presented in Table 3 by both number of events reported and by number of participants reporting the event. The data obtained before biologic assignment were combined from both arms because the time to assignment was similar in both arms. During this period, the most common event reported was acute pain (severe VOC), defined as requiring either hospitalization or parenteral opioids in the outpatient setting with 52 events reported by 45 participants.
Sickle cell events of special interest
| Event . | . | Before biologic assignment . | ||
|---|---|---|---|---|
| Patients, n (%) . | . | Events, n (%) . | ||
| Number completing event form | 110 | 57 | ||
| Pulmonary hypertension | 1 (1) | 1 (2) | ||
| Significant cerebrovascular event∗ | 0 (0) | 0 (0) | ||
| Renal function compromise | 2 (2) | 2 (4) | ||
| Avascular necrosis | 4 (4) | 4 (7) | ||
| Leg ulceration | 0 (0) | 0 (0) | ||
| Acute chest syndrome with hospitalization | 6 (5) | 6 (11) | ||
| VOC with hospitalization or parenteral opioid drugs in outpatient setting | 43 (39) | 43 (75) | ||
| Any event leading to advanced care setting or intensive care unit admission/transfer | 1 (1) | 1 (2) | ||
| Event | Year 1 after biologic assignment | |||
| Donor arm | No donor arm | |||
| Patients | Events | Patients | Events | |
| Number who completed event form | 27 | 33 | 77 | 207 |
| Pulmonary hypertension | 0 (0) | 0 (0) | 3 (4) | 4 (2) |
| Significant cerebrovascular event∗ | 4 (15) | 7 (21) | 2 (3) | 3 (1) |
| Renal function compromise | 4 (15) | 6 (18) | 7 (9) | 9 (4) |
| Avascular necrosis | 3 (11) | 3 (9) | 8 (10) | 9 (4) |
| Leg ulceration | 0 (0) | 0 (0) | 1 (1) | 2 (1) |
| Acute chest syndrome with hospitalization | 2 (7) | 2 (6) | 10 (13) | 16 (8) |
| VOC with hospitalization or parenteral opioid drugs in outpatient setting | 9 (33) | 10 (30) | 65 (84) | 159 (77) |
| Any event leading to an advanced care setting or intensive care unit admission/transfer | 3 (11) | 5 (15) | 4 (5) | 5 (2) |
| Event | Year 2 after biologic assignment | |||
| Donor arm | No donor arm | |||
| Patients | Events | Patients | Events | |
| Number completing event form | 21 | 8 | 71 | 183 |
| Pulmonary hypertension | 0 (0) | 0 (0) | 3 (4) | 6 (3) |
| Significant cerebrovascular event∗ | 1 (5) | 1 (12) | 4 (6) | 5 (3) |
| Renal function compromise | 2 (10) | 3 (38) | 5 (7) | 7 (4) |
| Avascular necrosis | 1 (5) | 1 (12) | 5 (7) | 7 (4) |
| Leg ulceration | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Acute chest syndrome with hospitalization | 0 (0) | 0 (0) | 7 (10) | 9 (5) |
| VOC with hospitalization or parenteral opioid drugs in outpatient setting | 1 (5) | 2 (25) | 59 (83) | 143 (78) |
| Any event leading to an advanced care setting or intensive care unit admission/transfer | 1 (5) | 1 (12) | 5 (7) | 6 (3) |
| Event . | . | Before biologic assignment . | ||
|---|---|---|---|---|
| Patients, n (%) . | . | Events, n (%) . | ||
| Number completing event form | 110 | 57 | ||
| Pulmonary hypertension | 1 (1) | 1 (2) | ||
| Significant cerebrovascular event∗ | 0 (0) | 0 (0) | ||
| Renal function compromise | 2 (2) | 2 (4) | ||
| Avascular necrosis | 4 (4) | 4 (7) | ||
| Leg ulceration | 0 (0) | 0 (0) | ||
| Acute chest syndrome with hospitalization | 6 (5) | 6 (11) | ||
| VOC with hospitalization or parenteral opioid drugs in outpatient setting | 43 (39) | 43 (75) | ||
| Any event leading to advanced care setting or intensive care unit admission/transfer | 1 (1) | 1 (2) | ||
| Event | Year 1 after biologic assignment | |||
| Donor arm | No donor arm | |||
| Patients | Events | Patients | Events | |
| Number who completed event form | 27 | 33 | 77 | 207 |
| Pulmonary hypertension | 0 (0) | 0 (0) | 3 (4) | 4 (2) |
| Significant cerebrovascular event∗ | 4 (15) | 7 (21) | 2 (3) | 3 (1) |
| Renal function compromise | 4 (15) | 6 (18) | 7 (9) | 9 (4) |
| Avascular necrosis | 3 (11) | 3 (9) | 8 (10) | 9 (4) |
| Leg ulceration | 0 (0) | 0 (0) | 1 (1) | 2 (1) |
| Acute chest syndrome with hospitalization | 2 (7) | 2 (6) | 10 (13) | 16 (8) |
| VOC with hospitalization or parenteral opioid drugs in outpatient setting | 9 (33) | 10 (30) | 65 (84) | 159 (77) |
| Any event leading to an advanced care setting or intensive care unit admission/transfer | 3 (11) | 5 (15) | 4 (5) | 5 (2) |
| Event | Year 2 after biologic assignment | |||
| Donor arm | No donor arm | |||
| Patients | Events | Patients | Events | |
| Number completing event form | 21 | 8 | 71 | 183 |
| Pulmonary hypertension | 0 (0) | 0 (0) | 3 (4) | 6 (3) |
| Significant cerebrovascular event∗ | 1 (5) | 1 (12) | 4 (6) | 5 (3) |
| Renal function compromise | 2 (10) | 3 (38) | 5 (7) | 7 (4) |
| Avascular necrosis | 1 (5) | 1 (12) | 5 (7) | 7 (4) |
| Leg ulceration | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Acute chest syndrome with hospitalization | 0 (0) | 0 (0) | 7 (10) | 9 (5) |
| VOC with hospitalization or parenteral opioid drugs in outpatient setting | 1 (5) | 2 (25) | 59 (83) | 143 (78) |
| Any event leading to an advanced care setting or intensive care unit admission/transfer | 1 (5) | 1 (12) | 5 (7) | 6 (3) |
Includes stroke, transient ischemic attack, and seizure.
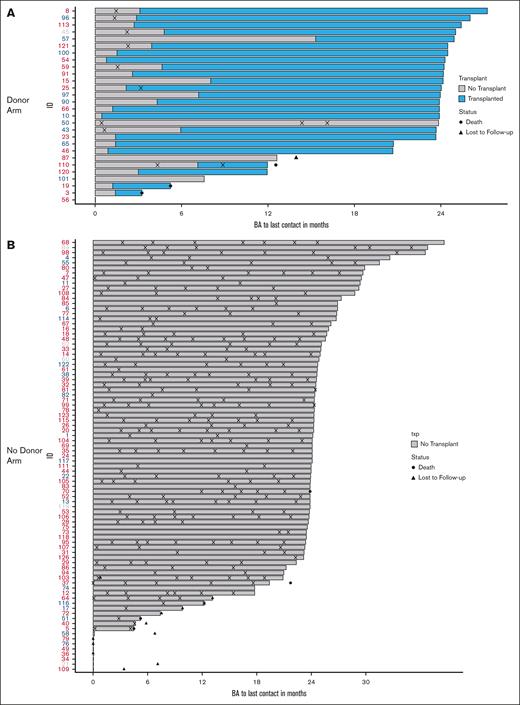
During the first year after biologic assignment, severe VOC was the most common event in both arms and was higher in the no donor arm (donor arm: n = 9 participants, 33%; no donor arm: n = 65 participants, 84%; P = .002). Significant neurologic events were reported by more participants in the donor arm with 4 individuals reporting 7 events (2 events were stroke, the rest were seizures or posterior reversible encephalopathy) in contrast with 2 individuals who reported 3 events in the no donor arm (P = .043) in the first year. Neurologic events included stroke, posterior reversible encephalopathy, and seizures. In the donor arm, in the first year after biologic assignment, there were 10 events of VOC reported by 9 patients as opposed to 159 events reported by 65 patients in the no donor arm (Table 3). In the second year, there were 2 events reported by 1 patient in the donor arm (5%) as opposed to 143 events reported by 59 patients in the no donor arm (83%; P < .001). Thus, a significant difference in VOC in the first year after biologic assignment was followed by a more striking difference in the second year. Other event categories did not differ significantly in the second year after biologic assignment (Table 3). A visual depiction of severe VOC by treatment arms is presented in Figure 3. All but 2 events occurred before HCT in the donor arm (Figure 3A). In contrast, severe VOC occurred in all but 6 participants in the no donor arm during the second year after biologic assignment, suggesting that SOC was not as effective as HCT in eliminating severe VOC (Figure 3B).
Severe vaso-occlusive crisis. (A) Donor arm: severe vaso-occlusive crisis for each patient in the donor arm after biologic assignment, overlaid by the period before and after HCT. (B) No donor arm: severe vaso-occlusive crisis for each patient in the no donor arm after biologic assignment. Patient ID is in red if the severity criteria for pain were met at enrollment, and blue if the severity criteria for pain were not met at enrollment. Patients in gray did not respond about pain at enrollment. VOE, vasocclusive episode.
Severe vaso-occlusive crisis. (A) Donor arm: severe vaso-occlusive crisis for each patient in the donor arm after biologic assignment, overlaid by the period before and after HCT. (B) No donor arm: severe vaso-occlusive crisis for each patient in the no donor arm after biologic assignment. Patient ID is in red if the severity criteria for pain were met at enrollment, and blue if the severity criteria for pain were not met at enrollment. Patients in gray did not respond about pain at enrollment. VOE, vasocclusive episode.
HRQoL
Of the 7 components of the PROMIS-57 surveys, only 2 yielded significant differences in the change from baseline between the donor and the no donor arms (Table 4; supplemental Table 3; supplemental Figure 1). The fatigue component asks about the degree of fatigue, and a higher score indicates more fatigue. There was no change in the no donor arm in contrast with a clinically and statistically significant decrease in fatigue in the donor arm of 13 points (P = .003) (represented as −13, Table 3). Participation in social roles also showed a clinically and statistically significant difference. This component asks about the ability to participate in social roles and activities, and therefore, a higher score is associated with more satisfaction. The median difference showed improvement in both arms, but in the no donor arm, the median change was 2 points as opposed to 15 points improvement in the donor arm (P = .003). None of the other components of the PROMIS-57 surveys showed significant differences between the treatment arms. Pain interference, with higher scores associated with increased interference with daily life attributable to pain, displayed a trend toward a statistically significant difference, although the range of measurements was quite wide. The no donor arm showed a median decrease of 1 point between baseline and 2 years, whereas the donor arm showed a decrease of 12 points (P = .07). Adherence to the pain dairy was poor (data not shown).
HRQoL: results of select PROMIS-57 measurements and the Adult Sickle Cell Quality of LIfe Measurement System stiffness scale
| PROMIS-57 . | T-score, median (range) . | P value . | ||
|---|---|---|---|---|
| All . | No donor . | Donor . | ||
| Fatigue | ||||
| Baseline | 57 (33-78) | |||
| Number reporting | 94 | |||
| Change within participant | −4 (−28 to 20) | 0 (−22 to 20) | −13 (−28 to 1) | .003 |
| Number reporting | 47 | 37 | 10 | |
| Participation in social roles | ||||
| Baseline | 45 (26-65) | |||
| Number reporting | 93 | |||
| Change within participant | 3 (−25 to 22) | 2 (−25 to 21) | 15 (−4 to 22) | .003 |
| Number reporting | 46 | 36 | 10 | |
| Pain interference | ||||
| Baseline | 62 (40-77) | |||
| Number reporting | 94 | |||
| Change within participant | −3 (−26 to 22) | −1 (−26 to 22) | −12 (−21 to 16) | .071 |
| Number reporting | 47 | 37 | 10 | |
| PROMIS-57 . | T-score, median (range) . | P value . | ||
|---|---|---|---|---|
| All . | No donor . | Donor . | ||
| Fatigue | ||||
| Baseline | 57 (33-78) | |||
| Number reporting | 94 | |||
| Change within participant | −4 (−28 to 20) | 0 (−22 to 20) | −13 (−28 to 1) | .003 |
| Number reporting | 47 | 37 | 10 | |
| Participation in social roles | ||||
| Baseline | 45 (26-65) | |||
| Number reporting | 93 | |||
| Change within participant | 3 (−25 to 22) | 2 (−25 to 21) | 15 (−4 to 22) | .003 |
| Number reporting | 46 | 36 | 10 | |
| Pain interference | ||||
| Baseline | 62 (40-77) | |||
| Number reporting | 94 | |||
| Change within participant | −3 (−26 to 22) | −1 (−26 to 22) | −12 (−21 to 16) | .071 |
| Number reporting | 47 | 37 | 10 | |
The PROMIS-57 scale items are reported with the median, minimum, and maximum values. The summaries represent individuals who completed the baseline assessment and also the subset that completed the baseline and the 2-year assessments. Full results are shown in supplemental Table 3. Boldface indicates P values that achieved or approached statistical significance.
Functional performance
There were no significant differences observed between study arm participants in the 6-minute walk distance over the 2-year period (supplemental Table 4). Tricuspid regurgitant jet velocity was significantly lower in the donor arm than in the no donor arm at baseline (2.0 vs 2.35 m/s; nominal P = .003) but could not be studied satisfactorily 2 years later because of a lack of adherence to protocol-specified measurements. Similarly, although no significant trends in pulmonary function testing results were observed, fewer than 50% of the participants completed both the baseline and the 2-year time point testing (supplemental Table 5). Albuminuria at 2 years could not be reported because of a lack of adherence to protocol-specified measurements.
Infections
Bacterial, viral, and fungal infections were recorded for participants in both arms and did not differ between the treatment arms (supplemental Table 6). In the donor arm, 12 participants reported a total of 35 infections; viral reactivation and bacterial sepsis were the predominant infections. In the no donor arm, 10 participants reported a total of 12 infections and bacterial sepsis was the predominant infection.
Transplant-specific outcomes for donor arm participants
Graft failure
Of the 24 subjects who underwent transplantation, none documented primary graft failure. There were 3 secondary graft failures, all of which occurred after HLA-matched unrelated transplantation and included 1 fatality. Secondary graft failure occurred on day +43 in 1 participant (day +26 100% donor engraftment in myeloid lineage) who had experienced an acute subarachnoid hemorrhage, and an examination of the bone marrow confirmed aplasia. In another participant, secondary graft failure occurred on day +109 (5% donor cells in the T-cell and myeloid lineages) and was salvaged with a second matched unrelated donor HCT on day +145 after conditioning with fludarabine, cyclophosphamide, TBI at 200 cGy and posttransplantation cyclophosphamide (full donor chimerism). This participant also had resolution of severe VOC after the second HCT but continued to experience chronic pain. The third participant had graft failure on day +131 (absence of donor cells and marrow aplasia) and was salvaged by a second HCT from the same donor on day +196 after conditioning with rabbit antithymocyte globulin, fludarabine, cyclophosphamide, and 200 cGy TBI with post-HCT cyclophosphamide with full donor engraftment and without sickle related events.
GVHD
Nine patients developed grade 2 to 4 acute GVHD, and the day 180 incidence was 39% (95% CI, 19-59; supplemental Figure 2; grade 2 [n = 5]; grade 3 [n = 2]; grade 4 [n = 2]). By donor type, the corresponding incidences were 30% (95% CI, 6.2-59) and 46% (95% CI, 18-71) after HLA-matched sibling and unrelated donor transplantation, respectively (P = .313). Eleven patients developed chronic GVHD, 6 of whom also reported acute GVHD (supplemental Figure 3). Chronic GVHD severity was mild (n = 5), moderate (n = 5), and severe (n = 1). The day 600 incidence of chronic GVHD was 46% (95% CI, 25-65). By donor type, the incidence was 30% (95% CI, 6.2-59) and 57% (95% CI, 26-79) after HLA-matched sibling and unrelated donor transplantation, respectively (P = .233). The 1- and 2-year probabilities of GRFS for the 24 subjects who underwent transplantation were 42% (95% CI) and 38% (95% CI), respectively.
Discussion
The STRIDE2 trial was conducted to determine if HCT from an HLA-matched sibling or an unrelated donor might extend a survival advantage in severe SCD when compared with SOC. Failure to accrue as planned and limited donor availability prompted the DSMB, after consultation with the sponsor, to close the trial. These barriers prevented an objective comparison of survival. Nevertheless, the results showed that the mortality in adolescents and young adults with severe SCD and who received SOC was 7% (1%-13%) in a 2-year window. This was balanced by a 2-year mortality of 11% (0%-22%). Among those assigned to the donor arm, the transplant-related mortality was not excessive after an HLA-matched sibling or an unrelated donor HCT. Furthermore, those assigned to the donor arm experienced a clinically important and statistically significant reduction in severe VOC, the hallmark of this disease, and from a patient’s perspective, significant improvements in fatigue and social function. Thus, the results of this trial following a descriptive analysis supports the notion that HCT with an HLA-matched sibling or an unrelated donor is a suitable treatment option in adolescents and young adults with severe SCD. Published reports support a plateau in deaths beyond the first year after transplantation and an increase in deaths in adults treated with disease-modifying treatments.3,16,17 Taken together, we hypothesize that a longer follow-up of the trial cohort is likely to show a survival advantage in the donor arm.
Allogeneic HCT has a risk for severe acute GVHD, chronic GVHD, disease recurrence (graft failure), and death from a transplant-related complication. The myeloablative regimen used in STRIDE2 generated comparable survival and supports the hypothesis that conditioning regimen intensity is not a critical determinant of survival among adults with severe SCD.18 However, higher GVHD risks led to a less than desirable 2-year GRFS. We hypothesize that the higher GVHD risks in STRIDE2 could be attributed, in part, to the patient age, unrelated donor HCT, and a myeloablative regimen. In addition, the GVHD risk did not seem to cause excessive mortality after HCT and might indicate improvement in GVHD therapy in the current era. In contrast, in an earlier trial of unrelated donor transplantation in children and adolescents with SCD using a reduced intensity condition regimen, chronic GVHD developed exclusively in patients aged 14 to 19 years and was the sole cause for death.11 There is an unmet and urgent need to incorporate novel GVHD prophylaxis regimens for allogeneic HCT for SCD in adolescents and adults. Although the risks of GVHD were low with minimal intensity regimens,19 the risk for myeloid malignancy after transplantation was high.19
VOC impairs the HRQoL and is the most common reason adults with SCD are willing to consider HCT despite its risks. Our observations confirmed that successful HCT largely eliminated VOC events. Similarly, the results of autologous gene therapy trials have shown a significant benefit in terms of pain resolution in roughly 90% of recipients.20-22 We attempted to characterize differences between the donor and no donor arms, but limited participation throughout the 2-year period after biologic assignment limited an opportunity for efficient comparison between the treatment arms. Nonetheless, significant improvements among patients in the donor-arm were observed in fatigue and participation in social roles and activities. Other reports on HRQoL have shown improvements in other domains, and variability between reports is best explained by the variability of self-reported outcomes, coupled with modest sample sizes that may have prevented consistent observation across the domains.8,23 It is also likely that short-term differences in HRQoL were impacted by ongoing HCT-related toxicities within the 2-year period after biologic assignment. A quarter of patients in the donor arm underwent HCT ≥6 months after biologic assignment, and recovery from HCT-related toxicities likely influenced self-reporting of HRQoL. We noted a heterogeneity in the pain interference scores in the donor arm. Given that only 10 patients completed the PROMIS after transplantation, we are unable to draw definitive conclusions about what may have contributed to this heterogeneity. We do not have information on the prevalence of chronic pain among the study subjects. Although acute pain is the hallmark of SCD, more than half of the adults with SCD also suffer from chronic pain and may, in part, have contributed to the observed heterogeneity in the pain interference scores after transplantation.24 Screening for chronic pain and chronic pain–related disability at baseline may have been informative about its contribution to pain interference after transplant.
An earlier publication described the barriers to recruitment and follow-up in STRIDE2.25 In brief, major barriers included a lower than expected frequency of HLA-matched related and unrelated donors and a lack of collaboration between SCD and HCT physicians that severely impaired achieving the secondary end points of the trial. The follow-up of trial participants was further compromised by the COVID-19 pandemic during which a substantial number of subjects reached their 2-year assessment mark. Because hospitals temporarily suspended all patient-related research, these participants could have been brought to hospitals for trial-specified assessments. Although we extended our window for the end of trial assessments, we believe that there was a reluctance on the part of the trial participant to visit hospitals. For those assigned to the no donor arm, routine care was provided by their SCD physician. By using an HCT network, the BMT CTN, that rely solely on HCT centers, coupled with a less than desirable collaboration between HCT and referring SCD physicians, impacted our ability to complete the trial as planned.
It is fair to question whether a comparative study design with survival as the primary end point is feasible in curative therapies for SCD because a survival advantage is challenging to determine in the short term.25 Specific to the current trial, a definitive comparison of treatment options, HCT or SOC, relied on ensuring that all participants met the inclusion criteria for our trial, and enrollment was not biased by knowledge of the availability of a suitable donor.16,17 The trial also hinged on enrolling 200 participants with at least 60 assigned to the donor arm based on the assumption that approximately a third of participants would have a suitable HLA-matched donor.26,27 However, only 20% of participants identified a suitable HLA-matched donor. Maintaining contemporaneous comparison groups in a real-world database is very challenging and unlikely to overcome the challenges faced during the conduct of this trial. Our trial illustrates the relative benefits and shortcomings of a traditional trial design, yet generated several clinically relevant outcome comparisons. A major limitation of our trial was lack of adherence to assessments for secondary outcomes among all participants, including those who received HCT, despite the reliance on the BMT CTN. Thus, conclusions were limited to clinical events and patient-reported outcomes with very little to add about the impact of HCT on renal, pulmonary, and cardiac function when compared with SOC, which we agree is critical to understand.
Nevertheless, several important lessons were learned. Our findings support that HCT with an HLA-matched sibling or unrelated donor is a suitable treatment option in adolescents and young adults with severe SCD. Furthermore, we demonstrated a clinically important and statistically significant reduction in severe VOC, the hallmark of this disease, and from a patient’s perspective, significant improvements in fatigue and social function. The choice to pursue a curative treatment is the patient’s decision and the findings of this trial extend generalizable knowledge for informed decision-making.
Acknowledgments
This trial was funded by National Heart, Lung, and Blood Institute grants U01 HL128568 (M.E.) and U01 HL128566 (L.K.) and National Heart, Lung, and Blood Institute and National Cancer Institute grant U24 HL138660 to the Blood and Marrow Transplant Clinical Trials Network. N.D. and N.G. are US Government employees (National Heart, Lung, and Blood Institute).
The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health.
Authorship
Contribution: M.C.W., M.E., A.M., K.S., D.N., and L.K. designed the trial; Y.L. and D.N. assembled and analyzed the data, prepared the data reports, and interpreted the results; M.C.W. wrote the first draft of the manuscript; M.E., D.N., and L.K. critically reviewed and edited the draft and finalized the manuscript; Y.L., F.E.-R., E.K.W., J.E.L., J.J.S., J.H.A., S.H.P., N.B., C.D., J.J.J., S.B., T.W., V.K., B.P., L.J.L., P.L., R.S.N., K.A.K, U.P., W.S., L.Y., N.D., N.G., N.K., E.S.K., K.H., A.M., and K.S. critically reviewed later drafts of the manuscript; and all authors reviewed the data reports, interpreted the results, and approved the final version for submission.
Conflict-of-interest disclosure: M.C.W. reports serving as a consultant for Vertex Pharmaceuticals Inc, Encoma Inc, Sanofi Inc, and BioChip Labs Inc. P.L. reports serving as a consultant for Janssen Therapeutics and receiving research funding from Bristol Myers Squibb and Marker Therapeutics. J.J.S. reports serving as a consultant for Editas Medicine. The remaining authors declare no competing financial interests.
Correspondence: Lakshmanan Krishnamurti, Pediatric Hematology/Oncology/BMT, Yale School of Medicine, 2073 A Laboratory of Medicine and Pediatrics Building, 333 Cedar St, New Haven, CT 06510; email: lakshmanan.krishnamurti@yale.edu.
References
Author notes
Deidentified individual participant data that underlie the reported results will be made available 3 months after publication at the National Institutes of Health Biologic Specimen and Data Repository Information Coordinating Center (BioLINCC). Proposals for access should be sent to BioLINCC. The study protocol will also be included in BioLINCC.
The full-text version of this article contains a data supplement.