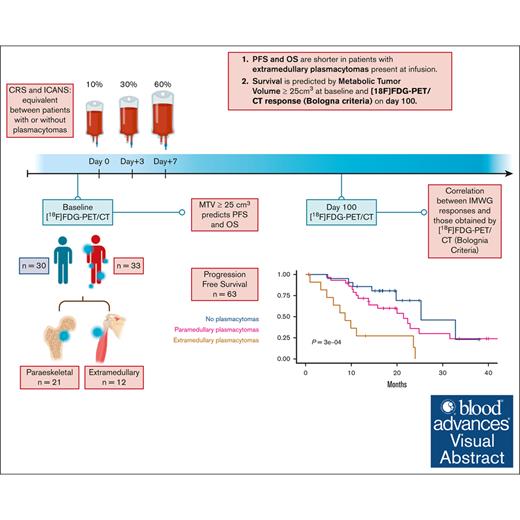
Key Points
PFS and OS are shorter in patients with extramedullary plasmacytomas evaluated by [18F]FDG-PET/CT at infusion time.
Survival is predicted by a metabolic tumor volume of ≥25 cm3 at baseline and [18F]FDG-PET/CT response (Bologna criteria) on day 100.
Visual Abstract
Multiple myeloma (MM) remains incurable, with poor outcomes in heavily pretreated patients. Chimeric antigen receptor (CAR) T-cell therapy has emerged as a promising treatment; however, outcomes after such therapy in patients with soft-tissue plasmacytomas and other bone lesions remain poorly understood. This study included 63 patients with relapsed/refractory MM treated either in the CARTBCMA-HCB-01 clinical trial (ARI0002h; academic B-cell maturation antigen [BCMA]–targeted CAR T-cell therapy) or in compassionate use. The aim was to evaluate the impact of soft-tissue involvement (extramedullary [EMD] and paraskeletal [PS] plasmacytomas) in response, survival and safety. Baseline [18F]fluorodeoxyglucose (FDG)–positron emission tomography (PET)/computed tomography (CT) from 5 participating centers were reviewed centrally. Of 63 patients, 52.4% presented plasmacytomas at the time of inclusion (21 PS, exclusively; and 12 EMD). Per responses, there were no significant differences between patients with and without plasmacytomas. A correlation was present between International Myeloma Working Group responses and those obtained by [18F]FDG-PET/CT at day 100 (Bologna criteria). No differences were observed in progression-free survival (PFS) or overall survival (OS) between patients with or without plasmacytomas. However, both PFS and OS were significantly shorter in patients with EMD. Interestingly, [18F]FDG-PET/CT response assessed on day 100, in accordance with the Bologna criteria, was predictive of survival outcomes. A metabolic tumor volume of ≥25 cm3 at baseline [18F]FDG-PET/CT was associated with earlier disease progression and shorter OS. These results highlight the importance of EMD evaluation by [18F]FDG-PET/CT before and after CAR T-cell infusion. This trial was registered at www.ClinicalTrials.gov as #NCT04309981; and EudraCT, 2019-001472-11.
Introduction
Multiple myeloma (MM) remains an incurable disease. The outcome is extremely poor in triple- or penta-refractory patients, with a reported progression-free survival (PFS) and overall survival (OS) of ∼6 and ∼9 months, respectively.1 In this setting, chemotherapy combinations are limited therapeutic options.2 However, chimeric antigen receptor (CAR) T-cell therapy has proven to be a promising alternative for patients with relapsed/refractory MM (RRMM).3-5 This particular group of patients presents, among other high-risk characteristics, plasmacytomas more frequently (incidence of 0.5%-4.8% at diagnosis vs 3.4%-14% in RRMM setting for the extramedullary [EMD] plasmacytomas, and 15% both at diagnosis and RRMM for paraskeletal [PS] plasmacytomas),6,7 representing one of the factors that may contribute to their adverse prognosis.
To date, evidence is scarce when it comes to evaluating the outcomes of patients with MM and the presence of plasmacytoma and undergoing CAR T-cell therapy; however, such information is critical, because of the underlying biological characteristics of this clinical feature. Ciltacabtagene autoleucel (Cilta-cel)8 and ARI0002h3 have reported to be effective for soft-tissue plasmacytomas. Yet, in terms of survival, there is no detailed data on whether patients with EMD or PS plasmacytomas (at the moment of infusion) reach a lower rate of responses, experience a higher rate of relapses, or behave differently than patients without plasmacytomas.
There is a lack of consensus on the concept of soft-tissue involvement and its subtypes when cases are reported in clinical trials. Therefore, differences in definitions among studies contribute to variability. PS lesions are generated by disruption of the cortical bone from skeletal lesions, whereas true EMD are soft-tissue masses with no contact with bone that are generated through a hematogenous spread of the myeloma clone.9 Imaging techniques may play a key role in assessing PS and EMD plasmacytomas after CAR T-cell therapy. In past decades, cross-sectional techniques such as whole-body low-dose (WBLD) computed tomography (CT), whole-body magnetic resonance imaging (MRI), and [18F]fluorodeoxyglucose (FDG)–positron emission tomography (PET)/CT have progressively replaced conventional skeletal X-ray survey in MM evaluations.10-13 In many trials, following the European Myeloma Network proposals14 and International Myeloma Working Group (IMWG) recommendations,2 [18F]FDG-PET/CT has been selected as the preferred technique. It allows for combined anatomical and functional information, shows a specificity for lesions with high activity and cell density,15,16 and represents a better tool during follow-up because disease activity monitoring is based on the evolution of FDG uptake.17,18 Also, it allows for an earlier evaluation than whole-body MRI19,20 and usually presents higher availability in the clinical setting. Even functional MRI with diffusion weighted imaging can be equally useful. [18F]FDG-PET/CT parameters such as standard uptake value (SUVmax), metabolic tumor volume (MTV), and total lesion glycolysis (TLG), which quantify the metabolic uptake and metabolic tumor burden, have demonstrated prognostic value when stratifying patients with MM.21 However, the potential role of these metabolic [18F]FDG-PET/CT parameters in predicting treatment response and prognosis in patients with MM treated with CAR T-cell therapy has not been fully analyzed.
In this study, we aim to evaluate the efficacy and safety of ARI0002h treatment, an academic B-cell maturation antigen (BCMA)–targeted CAR T-cell therapy, on soft-tissue plasmacytomas and other bone lesions, as assessed by [18F]FDG-PET/CT and CT, in patients with RRMM.
Methods
Study design
This study included 63 patients; 56 were treated in the CARTBCMA-HCB-01 single-arm, multicenter study, which comprises several academic centers in Spain; 7 received treatment on the basis of compassionate use for the same indication.
Patients were diagnosed with RRMM and aged 18 to 75 years. This cohort presented an Eastern Cooperative Oncology Group score of 0 to 2; ≥2 previous lines of therapy, including a proteasome inhibitor, an immunomodulatory agent, and an anti-CD38 antibody; refractoriness to the last line of therapy; and measurable disease according to the IMWG criteria. Patients received a lymphodepletion regimen (cyclophosphamide [300 mg/m2] and fludarabine [30 mg/m2] on days −4, −5, and −6), followed by an initial fractionated infusion of 3 × 106 CAR T cells per kg in 3 aliquots (0.3 × 106, 0.9 × 106, and 1.8 × 106 CAR-positive cells per kg on days 0, 3, and 7) and a nonfractionated booster dose of up to 3 × 106 CAR T cells per kg, at least 100 days after the first infusion.3,22
Study end points were overall response rate at day 100 and month 12 of the first infusion. Responses were evaluated following IMWG criteria.17 The median follow-up was 21.48 months.
[18F]FDG PET/CT assessment
[18F]FDG-PET/CT studies required a 4-hour fasting period before 3.7 MBq/kg [18F]FDG was administered. Before scanning, patients rested for at least 60 minutes. No oral or IV iodine contrast was administered. Scan range included the whole body.
[18F]FDG-PET/CT studies were performed at baseline, at a median of 32 days (range, 5-58) before CAR T-cell infusion. Follow-up included a [18F]FDG PET/CT at day 100 and month 12 of treatment. Fifty-two studies were performed on a Siemens Biograph mCT model PET/CT, 7 on a Philips ingenuity-TF, and 4 on a GE Healthcare Discovery MI PET/TC.
Two nuclear medicine specialists from Hospital Clinic of Barcelona completed a centralized analysis of all [18F]FDG-PET/CT baseline images mentioned previously. The response criteria were established according to the 2021 Bologna criteria (Zamagni et al23). Visual parameters based on the Bologna Italian Myeloma for PET Use were applied at baseline.24 Variables included were bone marrow (BM) diffuse uptake; focal PET-positive lesions with and without osteolytic characteristics, organized by groups (no lesions; 1-3; 4-10; and >10) presence of EMD; and presence of PS disease. Furthermore, SUVmax, MTV, and TLG were measured using MIM Encore software version 7.3.3 (MIM Software Inc, Cleveland, OH), with a fixed 2.5 SUVmax threshold. The lower limit of lesion size was set at 0.5 mm, and only focal lesions with FDG uptake were considered.21 BM uptake in a diffuse pattern is known to be mediated by several external factors (granulocyte colony-stimulating factor, aplasia recovery, and infections). To avoid bias, this parameter was not considered as exclusively associated to disease infiltration; it was, therefore, excluded when assessing MTV and TLG.
Regarding the CT scan, multiplanar reconstructions of the CT component of [18F]FDG-PET/CT were included to assess the focal lesions and evolution of the calcified tissue in a subgroup of 29 patients. CT evaluation was done by 2 specialized musculoskeletal staff radiologists from Hospital Clinic of Barcelona, followed by a third revision from an experienced musculoskeletal radiologist. To avoid heterogeneously patchy lesions associated with osteoporosis, osteolytic lesions were only considered at diameter of >10 mm. A maximum of 3 clear, significant lesions were taken into account for each patient. Marginal ossification and central foci calcifications were also considered.
Statistical analysis
Statistical analyses were conducted using R version 4.3.0 (RStudio, PBC, Boston, MA). PFS was defined as time between ARI0002h administration and either disease progression or death from any cause. OS was defined as time between ARI0002h administration and death from any cause. Responses, survival, and safety data were defined at baseline, before the ARI0002h infusion. A landmark analysis was performed for both PFS and OS, as indicated. The log-rank test (2-sided) and Kaplan-Meier method were used for survival curves. A P value of <.05 was considered statistically significant.
Univariate and multivariate logistic regression using SUVmax, MTV, and TLG values as continuous variables was performed. For the multivariate model, we included SUVmax, MTV, and TLG values. Cox proportional hazards were used to analyze the effects on survival outcomes from these 3 variables. The receiver operating characteristic curve was used as a tool to evaluate the discriminative capacity of MTV when considering the presence of plasmacytomas.
The study protocol was approved by the ethics committee of Hospital Clinic de Barcelona.
Results
Enrolled patient characteristics
The clinical, demographic, and laboratory characteristics of the patients at baseline are presented in Table 1. Of 63 patients, 33 (52.4%) presented plasmacytomas at the time of inclusion (21 were exclusively PS [64%], whereas 12 were EMD [36%]). EMD was localized to a single site. For the remaining cohort (4 patients), multiple EMD localization including hepatic, pancreatic, breast, and subcutaneous tissue was reported (supplemental Table 1). No significant differences were observed between patients with or without plasmacytomas (both EMD and PS) with respect to clinical and laboratory baseline characteristics, and previous lines of therapy. However, patients with plasmacytomas did present a trend toward lower BM plasma cell infiltration (supplemental Table 2).
Patient characteristics based on the presence or absence of plasmacytomas
| . | No plasmacytomas (n = 30) . | Plasmacytomas (n = 33) . | P value . |
|---|---|---|---|
| Age (y) at inclusion (median, range) | 59 (53-65) | 59 (50-67) | .875 |
| Sex (female, %) | 16 (53.33) | 13 (39.40) | .268 |
| Heavy chain type (n, %) | .827 | ||
| IgG | 14 (46.7) | 17 (54.8) | |
| IgA | 10 (33.3) | 7 (22.6) | |
| IgD | 1 (3.3) | 1 (3.2) | |
| Bence Jones | 5 (16.7) | 6 (19.4) | |
| κ light chain type (n, %) | 18 (60.0) | 18 (54.6) | .662 |
| High-risk cytogenetics∗ (n, %) | 9 (31.0) | 5 (16.7) | .195 |
| PC BM % (median, range) | 20 (13-37) | 2 (1-11) | .093 |
| MC g/L (median, range) | 10.7 (1.2-20.6) | 10.3 (0-19.4) | .465 |
| Triple refractoriness† (n, %) | 23 (76.7) | 21 (63.6) | .260 |
| Penta refractoriness‡ (n, %) | 6 (20.0) | 12 (36.4) | .151 |
| Prior HCT (n, %) | |||
| Autologous | 27 (100) | 27 (93.1) | .165 |
| Allogeneic | 2 (7.4) | 4 (13.8) | .440 |
| . | No plasmacytomas (n = 30) . | Plasmacytomas (n = 33) . | P value . |
|---|---|---|---|
| Age (y) at inclusion (median, range) | 59 (53-65) | 59 (50-67) | .875 |
| Sex (female, %) | 16 (53.33) | 13 (39.40) | .268 |
| Heavy chain type (n, %) | .827 | ||
| IgG | 14 (46.7) | 17 (54.8) | |
| IgA | 10 (33.3) | 7 (22.6) | |
| IgD | 1 (3.3) | 1 (3.2) | |
| Bence Jones | 5 (16.7) | 6 (19.4) | |
| κ light chain type (n, %) | 18 (60.0) | 18 (54.6) | .662 |
| High-risk cytogenetics∗ (n, %) | 9 (31.0) | 5 (16.7) | .195 |
| PC BM % (median, range) | 20 (13-37) | 2 (1-11) | .093 |
| MC g/L (median, range) | 10.7 (1.2-20.6) | 10.3 (0-19.4) | .465 |
| Triple refractoriness† (n, %) | 23 (76.7) | 21 (63.6) | .260 |
| Penta refractoriness‡ (n, %) | 6 (20.0) | 12 (36.4) | .151 |
| Prior HCT (n, %) | |||
| Autologous | 27 (100) | 27 (93.1) | .165 |
| Allogeneic | 2 (7.4) | 4 (13.8) | .440 |
BM, bone marrow; HCT, hematopoietic stem cell transplant; IgH, immunoglobulin heavy chain; MC, monoclonal component; PC, plasma cells.
High-risk cytogenetics were considered for alterations in TP53, t(4,14) and/or t(14,16).
Refractoriness to a proteasome inhibitor, an immunomodulator, and an anti-CD38 drug.
Refractoriness to bortezomib, carfilzomib, lenalidomide, pomalidomide, and an anti-CD38 drug.
[18F]FDG PET/CT baseline characteristics
Baseline [18F]FDG-PET/CT scans of 63 patients were centrally reviewed at Hospital Clinic of Barcelona. All 12 patients with EMD plasmacytomas presented PS involvement as well, except for 1 patient who had a single bile duct plasmacytoma.
With respect to[18F]FDG-PET/CT parameters, diffuse BM uptake, focal lesions, SUVmax, MTV, and TLG were evaluated (Table 2). Diffuse BM uptake was more frequent in patients without plasmacytomas, which is perhaps related to the higher BM infiltration of plasma cells, as shown in Table 1 (median 20% vs 2%, respectively; P = .09).
Visual and volumetric baseline [18F]FDG-PET/CT parameters based on the presence or absence of plasmacytomas
| . | No plasmacytomas (n = 26) . | Plasmacytomas (n = 33) . |
|---|---|---|
| Diffuse BM uptake | 11 (42.3%) | 7 (21.2%) |
| Focal lesions (FL) with [18F]FDG uptake | ||
| No lesions | 11 (42.3%) | 4 (12.1%) |
| 1-3 FL | 6 (23.1%) | 6 (18.2%) |
| 4-10 FL | 5 (19.2%) | 4 (12.1%) |
| >10 FL | 4 (15.4%) | 19 (57.6%) |
| SUVmax (median, range) | 5.1 (3.3-8.2) | 14 (7.0-21.0) |
| MTV (median, range) | 0.85 (0-12.0) | 115.0 (72.0-415.0) |
| TLG (median, range) | 4.8 (0-57.0) | 660.0 (214.0-1360.0) |
| . | No plasmacytomas (n = 26) . | Plasmacytomas (n = 33) . |
|---|---|---|
| Diffuse BM uptake | 11 (42.3%) | 7 (21.2%) |
| Focal lesions (FL) with [18F]FDG uptake | ||
| No lesions | 11 (42.3%) | 4 (12.1%) |
| 1-3 FL | 6 (23.1%) | 6 (18.2%) |
| 4-10 FL | 5 (19.2%) | 4 (12.1%) |
| >10 FL | 4 (15.4%) | 19 (57.6%) |
| SUVmax (median, range) | 5.1 (3.3-8.2) | 14 (7.0-21.0) |
| MTV (median, range) | 0.85 (0-12.0) | 115.0 (72.0-415.0) |
| TLG (median, range) | 4.8 (0-57.0) | 660.0 (214.0-1360.0) |
Additionally, the CT images from baseline [18F]FDG-PET/CT with osteolytic, PS, and EMD lesions were evaluated descriptively in 29 patients. The median diameter of osteolytic lesions of >10 mm at baseline [18F]FDG-PET/CT was 26 mm. With respect to density in Hounsfield units (HU), the median at baseline [18F]FDG-PET/CT was 44 HU. CT images and a representative baseline [18F]FDG-PET are depicted in Figure 1.
Baseline [18F]FDG-PET/CT of a 55-year-old female patient with IgGκ MM showcasing a multifocal pattern with 6 focal osteolytic lesions with FDG uptake, costal plasmacytoma, and extensive EMD involvement. (A) Maximum intensity projection (MIP). (B-C) [18F]FDG-PET/CT and CT axial cuts of a supraclavicular left lymphadenopathy. (D-E) [18F]FDG-PET/CT and CT axial cuts showing multiple focal liver lesions and a retroperitoneal lymphadenopathy. (F) MIP view with overlay of segmented lesions (MTV = 1031 mL; TLG = 3899 g).
Baseline [18F]FDG-PET/CT of a 55-year-old female patient with IgGκ MM showcasing a multifocal pattern with 6 focal osteolytic lesions with FDG uptake, costal plasmacytoma, and extensive EMD involvement. (A) Maximum intensity projection (MIP). (B-C) [18F]FDG-PET/CT and CT axial cuts of a supraclavicular left lymphadenopathy. (D-E) [18F]FDG-PET/CT and CT axial cuts showing multiple focal liver lesions and a retroperitoneal lymphadenopathy. (F) MIP view with overlay of segmented lesions (MTV = 1031 mL; TLG = 3899 g).
Response evaluation
Best responses up to day 100 after ARI0002h treatment were assessed according to IMWG criteria. Of 63 patients, 25 (40%) presented stringent complete response (sCR); 5 (8%) presented CR; 18 (29%) presented very good partial response (PR); 11 (17%) presented partial responses (PR); 1 (2%) presented progression (PD); and 3 (5%) had early deaths before disease evaluation. Responses in 58 patients, per Bologna criteria on day 100 [18F]FDG-PET/CT, were: 62.3% presented complete metabolic response (CMR); 24.5% presented partial metabolic response (PMR); 3.7% presented no metabolic response (NMR); and 9.5% presented PD. Responses in 29 patients at 1-year follow-up ([18F] FDG-PET/CT at month 12) were: 86% presented CMR; 4% presented PMR; 3% presented NMR; and 7% presented PD. There were no significant differences when comparing response rates between patients with plasmacytomas (4% PD, 48% PR, and 48% CR) or without (41% PR and 59% CR) at baseline. No differences were observed when separately considering patients with either PS or EMD plasmacytomas.
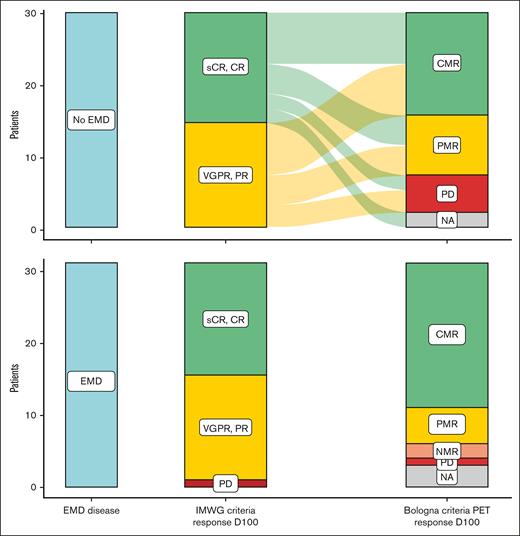
There was a correlation between IMWG responses and those obtained by [18F]FDG-PET/CT at day 100 (Figure 2). For the group of patients without plasmacytomas, the discordant cases were 2 patients with serological CR or sCR (IMWG) who experienced PD in [18F]FDG-PET/CT per Bologna criteria, 5 patients with PR (IMWG) who presented CMR, and 4 patients with CR or sCR (IMWG) who presented PMR (Bologna criteria). For the group of patients with plasmacytomas, the discordant cases between IMWG and [18F]FDG-PET/CT at day 100 were 1 patient with sCR and another patient with very good PR (IMWG) who experienced progression in PET/CT; 7 patients with PR (IMWG) who had CMR; and 2 patients with CR or sCR (IMWG) who showed PMR (Bologna criteria).
Comparison between serological IMWG response and PET/CT response. Description of responses based on IMWG criteria at day 100 (middle column) and PET/CT (right column) at day 100 of ARI0002h infusion based on EMD disease presence (n = 33) or not (n = 30) at screening.
Comparison between serological IMWG response and PET/CT response. Description of responses based on IMWG criteria at day 100 (middle column) and PET/CT (right column) at day 100 of ARI0002h infusion based on EMD disease presence (n = 33) or not (n = 30) at screening.
Additionally, via CT evaluation, median diameter of osteolytic lesions of >10 mm was reduced from 26 mm (baseline) to 16 mm (3 months) and 9 mm (12 months), respectively. Furthermore, when comparing CT and [18F]FDG-PET findings of the same lesions, we observed 3 different patterns of MM (supplemental Figure 1). The first pattern stands for lesions showing uptake at baseline [18F]FDG-PET/CT that have a solid density in CT; after treatment, they cease PET uptake and become more hypodense. The second pattern entails bone lesions that do not show uptake in [18F]FDG-PET and have fatty density in CT; after treatment, they still persist in fatty density. Both former patterns can be seen in the same patient at the same time. The third pattern encompasses osteolytic lesions and plasmacytomas that, after treatment, show calcifications, and even ossification.
Survival outcomes
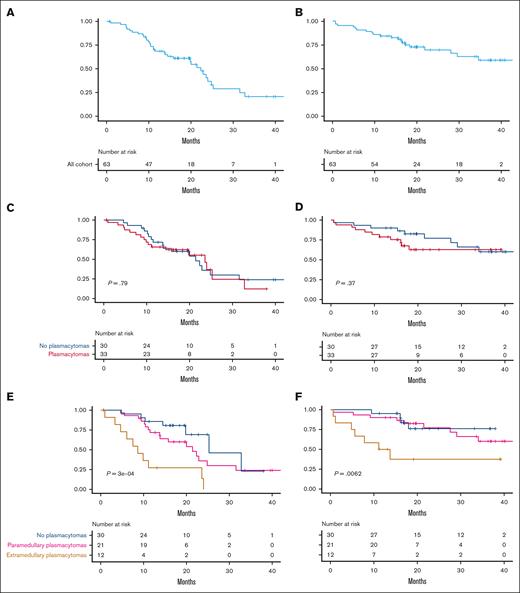
Median PFS and OS of the full cohort were 22.3 months (95% confidence interval [CI], 15.7-31.6) and 34 months (95% CI, 29 to not reached [NR]), respectively (Figure 3A-B). There were no significant differences in PFS or OS between patients with and without plasmacytomas treated with ARI0002h (Figure 3C-D). However, both PFS and OS were significantly shorter for patients with true EMD plasmacytomas (Figure 3E-F). Median PFS for patients with plasmacytomas (both PS and EMD) was 21.42 months (95% CI, 13.83 to NR); for those with EMD, it was 8.63 months (95% CI, 6.07 to NR). Median OS was not reached for patients without plasmacytomas or PS; however, it was 12.43 months (95% CI, 5.61 to NR) for those with EMD plasmacytomas (Figure 3F; P = .0062).
Survival curves after ARI0002h infusion. (A-B) PFS and OS of the cohort. (C-D) PFS and OS based on the absence (blue, n = 30) or presence (red, n = 33) of EMD disease. (E-F) PFS and OS based on the absence (blue, n = 30) or presence of PS (pink, n = 21) or EMD (brown, n = 12) plasmacytomas at inclusion.
Survival curves after ARI0002h infusion. (A-B) PFS and OS of the cohort. (C-D) PFS and OS based on the absence (blue, n = 30) or presence (red, n = 33) of EMD disease. (E-F) PFS and OS based on the absence (blue, n = 30) or presence of PS (pink, n = 21) or EMD (brown, n = 12) plasmacytomas at inclusion.
When patients with plasmacytomas (EMD or PS) progressed after ARI0002h infusion, there was a trend toward a shorter OS than those patients without plasmacytomas at baseline: median OS of 5.1 months (95% CI, 3.7-9.4) vs 11.3 months (95% CI, 8.1-14.8; P = .06). When considering the 3 groups separately at the moment of progression (patients without plasmacytomas vs patients with PS vs patients with EMD involvement), those with EMD had a shorter OS. However, no statistical differences were observed (P = .13).
Outcomes based on [18F]FDG-PET/CT
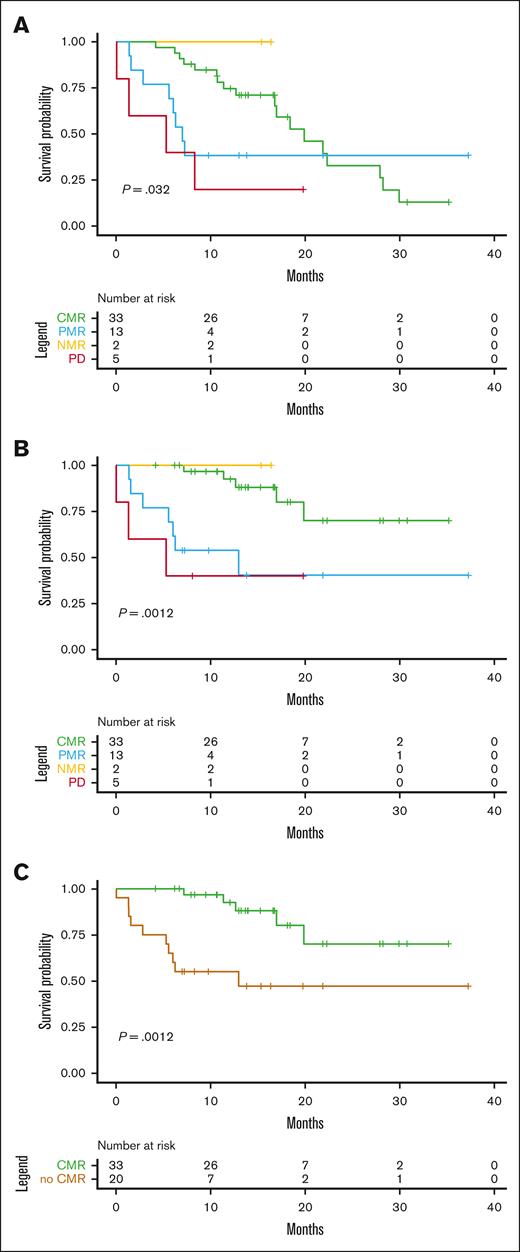
[18F]FDG-PET/CT response assessment at day 100, per the Bologna criteria, was predictive of survival outcomes (Figure 4). Interestingly, when considering responses in [18F]FDG-PET/CT performed on day 100 after infusion (n = 53), patients who reached CMR had significantly better OS (1 year estimated median OS, 92%; 95% CI, 83-100) than those with PMR, NMR, or PD (1 year estimated median OS 55%; 95% CI, 37-81; P = .012). An analysis separating CMR and PMR vs NRM and PD did not show any significant differences.
Outcomes according to PET/CT response. PET/CT response at day 100 was predictive of PFS (A) and OS (B). Reaching CMR at PET/CT D100 vs the rest of responses (PMR, NRM, and PD) was associated with a longer survival (C).
Outcomes according to PET/CT response. PET/CT response at day 100 was predictive of PFS (A) and OS (B). Reaching CMR at PET/CT D100 vs the rest of responses (PMR, NRM, and PD) was associated with a longer survival (C).
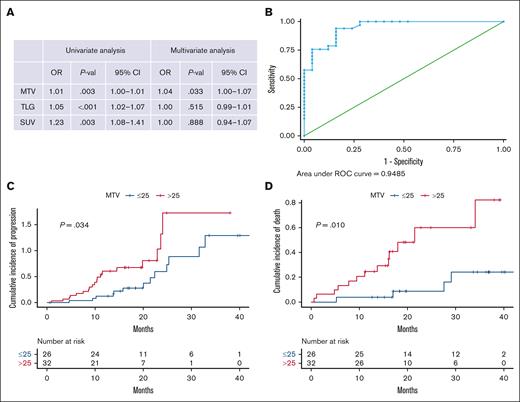
To assess the performance of the [18F]FDG-PET/CT values as potential biomarkers of disease progression, we first analyzed its association with the presence of plasmacytomas, fitting a multivariate logistic regression model using the SUVmax, MTV, and TLG values as continuous variables. As described in Figure 5A, only the MTV values were independently associated with the presence of plasmacytomas at baseline, with an area under the curve of 0.95. An MTV of 25 cm3 was the 1 variable with the highest sensitivity (90.9%) and specificity (84%) for the presence of plasmacytoma (Figure 5B). This cutoff value at baseline [18F]FDG-PET/CT was also associated with a higher risk of disease progression (hazard ratio, 2.16; 95% CI, 1.04-4.49; P = .034) and worst OS (hazard ratio, 4.0; 95% CI, 1.29-12.43; P = .010; Figure 5C-D).
Biomarkers of disease progressions based on PET/CT values. (A) Univariate and multivariate analysis of TLG, MTV, and SUVmax with the presence of plasmacytomas. All values were considered as continuous, being analyzed for an association with the presence of plasmacytomas. For the multivariate logistic regression model, we included SUVmax, MTV, and TLG values. All 3 values were significant on the univariate model, but only MTV remained significant (P = .033) in the multivariate model. (B) ROC curve showing that MTV of 25 cm3 shows highest sensitivity (90.9%) and specificity (84%) for predicting plasmacytomas presence. All values were considered as continuous, being analyzed for an association with the presence of plasmacytomas. For the multivariate logistic regression model, we included SUVmax, MTV, and TLG values. All 3 values were significant in the univariate model, but only MTV remained significant (P = .033) in the multivariate model. (C) MTV of ≥25 cm3 at baseline PET/CT was associated with higher disease progression (D) and worst OS.
Biomarkers of disease progressions based on PET/CT values. (A) Univariate and multivariate analysis of TLG, MTV, and SUVmax with the presence of plasmacytomas. All values were considered as continuous, being analyzed for an association with the presence of plasmacytomas. For the multivariate logistic regression model, we included SUVmax, MTV, and TLG values. All 3 values were significant on the univariate model, but only MTV remained significant (P = .033) in the multivariate model. (B) ROC curve showing that MTV of 25 cm3 shows highest sensitivity (90.9%) and specificity (84%) for predicting plasmacytomas presence. All values were considered as continuous, being analyzed for an association with the presence of plasmacytomas. For the multivariate logistic regression model, we included SUVmax, MTV, and TLG values. All 3 values were significant in the univariate model, but only MTV remained significant (P = .033) in the multivariate model. (C) MTV of ≥25 cm3 at baseline PET/CT was associated with higher disease progression (D) and worst OS.
Safety
Overall, 86% of all patients developed cytokine release syndrome (CRS) and only 1% developed immune effector cell–associated neurotoxicity syndrome (ICANS). The emergence of CRS and ICANS was equivalent between patients with or without plasmacytomas (P = .84 and P = .95, respectively) as grade 2; superior CRS was present in 30% (no plasmacytomas) and 24% (PS and EMD plasmacytomas) of cases. ICANS grade 1 was reported in 1% of both groups. No differences were observed when considering grade ≥2 CRS.
Discussion
Some preliminary reports25-27 and a recent larger retrospective study28 have evaluated the impact of CAR T-cell therapy in soft-tissue involvement.25-27 However, to the best of our knowledge, this is the first study to address this topic specifically, employing a systematic review of the [18F]FDG-PET/CT findings in a group of patients treated with the same CAR T-cell product.
Regarding overall responses, our data show that patients with both PS and EMD plasmacytomas do have a similar response rate by metabolic ([18F]FDG-PET/CT Bologna criteria) and conventional (IMWG) criteria when compared with patients without plasmacytomas before infusion. Response rate in RRMM with PS or EMD involvement treated with conventional regimens has been reported to be ∼50%.9 In our cohort, although, only 1 patient was primary refractory and 2 patients with EMD plasmacytomas were not evaluable because of an early death. The overall response rate was 91% in patients with plasmacytomas vs 97% in those without plasmacytomas. Therefore, CAR T-cell therapy with ARI0002h appears promising in terms of efficacy in this specific subset of patients.
Secondly, an important concern in RRMM with PS or EMD involvement is the limited duration of responses, reported as lasting ∼4 months.9 In our cohort, although overall responses were observed in patients with either PS or EMD plasmacytomas, median PFS for patients with PS plasmacytomas was longer than for those with exclusive EMD. Interestingly, the efficacy of ARI0002h is similar between patients with PS plasmacytomas and those without plasmacytomas. It has already been proven that patients with EMD plasmacytomas, arising because of hematogenous spread, experience worse outcomes compared with those with PS involvement. This is in line with the concept that EMD hematogenous spreading leads to a more immature morphology of plasmatic cells with particular genetic and histological features.9,29
Once patients with plasmacytomas (PS and EMD) progress, a nonstatistically significant trend toward a shorter OS is observed. More follow-up is needed; however, patients with plasmacytomas who experience progression after CAR T-cell therapy may pose a highly challenging therapeutic scenario, in which outcomes are poorer.
In terms of safety, the presence of EMD plasmacytomas at infusion has been proposed as possibly resulting in a higher rate of CRS and ICANS. However, in our study, grade ≥2 CRS occurred in 30% (no plasmacytomas) and 24% (PS and EMD plasmacytomas), whereas ICANS emerged in 1% of both groups. Therefore, plasmacytomas do not appear to compromise safety. Many factors such as the specific structure of ARI0002h or the fractionated infusion can contribute to this fact, so further studies with different constructs are needed to confirm these findings.
Imaging techniques play a key role for plasmacytoma evaluation. [18F]FDG-PET/CT has been known to show high sensitivity and specificity at MM diagnosis, and can help determine prognosis in patients with newly diagnosed and RRMM.30-33 It is worth considering whether [18F]FDG-PET/CT could also prove useful in predicting survival in patients treated with CAR T cells.
The use of imaging techniques such as [18F]FDG-PET/CT may be critical, not only during MM evaluations at diagnosis and relapse, but also within the context of CAR T-cell therapy. Prospective data showed that the presence of >3 focal lesions at baseline had an independent effect on survival.31 This was also observed in smaller retrospective cohorts.34,35 Some data consider 1 or 10 focal lesions30,36 as a significant threshold. Several reports regarding the role of SUVmax and lower vs higher MTV16 have been reported, but results are inconclusive. For this reason, the IMWG consensus statement on [18F]FDG-PET/CT in the diagnosis and management of MM37 concludes that the presence of EMD plasmacytomas and the detection of >3 focal lesions at diagnosis have a negative, independent prognostic impact, whereas the prognostic role of SUVmax remains to be explored. IMWG consensus criteria for response and minimal residual disease assessment in MM also consider [18F]FDG-PET/CT as a useful tool for prognostic purposes, acknowledging that more evidence is needed, nonetheless.17
There are, to our knowledge, no data regarding these criteria in the specific context of CAR T-cell therapy. However, our work evaluates new [18F]FDG-PET/CT parameters not studied previously in this setting, including TLG and MTV at baseline, alongside SUVmax. In our cohort, the univariate model reported that TLG, MTV, and SUVmax were all associated with the presence of plasmacytomas; by definition, SUVmax is expected to have higher values in this population. Yet, only the MTV value remained significant in the multivariate model. Moreover, an MTV of ≥25 cm3 was associated with a higher rate of disease progression and shorter OS. We recognize several limitations, including the use of only 1 CAR T-cell product and the relatively small sample size. That stated, validation of these results in larger cohorts is warranted.
A correlation was found, as expected, between IMWG and metabolic responses per Bologna criteria, with [18F]FDG-PET/CT response at day 100 predictive of survival outcomes reported in previous studies.17,37 Moreover, in our cohort, reaching CR per Bologna criteria on day 100 after infusion was associated with a longer OS in comparison with other response categories, such as PMR, NMR, or PD. This also highlights the importance of adding [18F]FDG-PET/CT during response evaluation after CAR T-cell therapy, complementing serological IMWG criteria.
Because of WBLDCT capacity for evaluating tissular density, MM lesions in the BM has been reported previously as being generally hyperdense (mean, 55 HU) in adults, in contrast with healthy marrow tissue. In this context, 2 different patterns of lesions have been reported in literature: trabecular bone replaced by fat (HU lesion of <0) and cell infiltration (HU of >0).38,39 Our results are in line with this data because we have found an evolution from hyperdense, active lesions in baseline [18F]FDG-PET/CT to hypodense, fat-rich “inactive” lesions in posttherapy follow-up [18F]FDG-PET/CT. However, this part of the study that focused on WBLDCT presents limitations, because of the limited sample size that only allows for descriptive work to be done.
Clinical trials combining other immunotherapy strategies are currently ongoing for RRMM with plasmacytomas, such as the combination of teclistamab and talquetamab simultaneously targeting BCMA and G protein–coupled receptor class C group 5 member D (RedirecTT-1 study40). Results are promising.
In summary, ARI0002h may improve the negative impact of PS plasmacytomas, albeit the efficacy is more limited in patients with EMD. Furthermore, responses and survival appear to be better when compared with historical conventional treatments in patients with RRMM. The concordance between serological IMWG criteria and [18F]FDG-PET/CT evaluation after CAR T-cell infusion, and the potential incorporation of CT patterns, suggest that centralized PET/CT evaluations could prove crucial when reporting efficacy results in CAR T-cell recipients. MTV was identified as an easily available prognostic marker in patients treated with this academic CAR T-cell product; this variable from baseline [18F]FDG-PET/CT could be incorporated into the selection of CAR T-cell candidates and taken into consideration for further decision-making processes, such as that of bridging therapy.
Overall, these results highlight that plasmacytomas, particularly true EMD, is a key player when treating patients with RRMM. This population remains a challenge and constitutes a particular therapeutic need.
Acknowledgments
The authors thank the patients and their families, as well as to the committed team from various departments including Immunology, Hematology, and others at Hospital Clinic of Barcelona; Hospital Clínico Universitario de Salamanca; Hospital Clínico Universitario Virgen de la Arrixaca, Murcia; Hospital Doce de Octubre, Madrid; and Hospital Universitario Virgen del Rocío, Sevilla, who were involved in patient care. The authors also acknowledge the efforts of researchers involved in the preclinical advancement of this academic CAR T-cell construct.
This study was supported by grants from the Instituto de Salud Carlos III (cofounded by the European Union) and the Spanish Ministry of Health (ICI19/00025 and PI22/00647), the RICORS-TERAV network (RD21/0017/0009 and RD21/0017/0019, RD21/0017/0001, RD21/0017/0006 and RD21/0017/0030), Red de Terapia Celular TERCEL (RD16/0011/0005), Fondo Europeo de Desarrollo Regional, 2021-SGR-01292 (AGAUR; Generalitat de Catalunya), Centro de Investigación Biomédica en Red de Cáncer (CB16/12/00369 and CB16/12/00489), La Caixa Foundation (CP042702/LCF/PR/GN18/50310007), Asociación Española Contra el Cancer LABAE21971FERN, and Fundació Bosch I Aymerich support. A.O.-C. from the resident grant Ajut Clínic-La Pedrera 2019, granted by Hospital Clínic de Barcelona. L.G.R.-L. has received funding from the CRIS Cancer Foundation (project number: PR_TMT_2023-6).
Authorship
Contribution: I.Z., M.T.-R., A.O.-C., D.M., X.S., and C.F.d.L. participated in conceptualization and design of the study, and participated in the writing of the manuscript draft; M.T.-R. and X.S. reviewed the PET/CT images from the 5 participating centers; J.C.S-P., A.B.-S., and X.T. reviewed the CT images; R.M.A.P., P.T., L.F., A.G.G., M.T., and X.S. performed and analyzed the PET/CT images collection; I.Z., M.T., A.O.-C., D.M., J.C.S-P., and A.B.-S. contributed to data collection; I.Z., M.T., A.O.-C., D.M., and C.F.d.L. performed data analysis and interpretation; A.O.-C., D.M., X.T., X.S., and C.F.d.L. have verified the data; and all authors revised the article and gave approval of the final version to be published.
Conflict-of-interest disclosure: A.O.-C. declares nonfinancial support from Janssen, outside from the submitted work. V.C. received honoraria for lectures, consulting, and advisory boards from Johnson&Johnson, Bristol Myers Squibb, Sanofi, Amgen, Glaxo, Pfizer, BioGene, and Menarini; and received travel grants from Johnson&Johnson, Bristol Myers Squibb, Amgen, and BioGene; served on a speakers bureau for Bristol Myers Squibb; and reports financing of scientific research from Janssen. L.G.R.-L. declares honoraria and travel grants from Janssen, Amgen, Bristol Myers Squibb, GlaxoSmithKline, Menarini, and Sanofi. C.F.L. declares receiving grants through his institution from Bristol Myers Squibb, Janssen, and Amgen; reports honoraria from Amgen, Janssen, Bristol Myers Squibb, GlaxoSmithKline, and Sanofi; reports support for attending meetings or travel from Janssen, Bristol Myers Squibb, GlaxoSmithKline, and Amgen; and reports participation on advisory boards with Janssen, Bristol Myers Squibb, Amgen, Pfizer, and Sanofi. The remaining authors declare no competing financial interests.
Correspondence: Carlos Fernández de Larrea, Department of Hematology, Amyloidosis and Myeloma Unit, Hospital Clínic de Barcelona, Institut d'Investigacions Biomèdiques August Pi i Sunyer, Universitat de Barcelona, Villarroel 170, 08036 Barcelona, Spain; email: cfernan1@clinic.cat.
References
Author notes
I.Z. and M.T.-R. contributed equally to this study.
X.S. and C.F.d.L. contributed equally to this study and are joint last authors.
The data sets generated and/or analyzed during the current study are available from the corresponding author, Carlos Fernández de Larrea (cfernan1@clinic.cat) on reasonable request.
The full-text version of this article contains a data supplement.

![Baseline [18F]FDG-PET/CT of a 55-year-old female patient with IgGκ MM showcasing a multifocal pattern with 6 focal osteolytic lesions with FDG uptake, costal plasmacytoma, and extensive EMD involvement. (A) Maximum intensity projection (MIP). (B-C) [18F]FDG-PET/CT and CT axial cuts of a supraclavicular left lymphadenopathy. (D-E) [18F]FDG-PET/CT and CT axial cuts showing multiple focal liver lesions and a retroperitoneal lymphadenopathy. (F) MIP view with overlay of segmented lesions (MTV = 1031 mL; TLG = 3899 g).](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/9/3/10.1182_bloodadvances.2024014360/4/m_blooda_adv-2024-014360-gr1.jpeg?Expires=1765010392&Signature=ztoobSvuEhdmXb7bNSnSpPmbkTq37yBgSeAsgxicmyKRACvg9R4tA3XotpZw5MyKh7icPCcVR32nwxxclqjH6GQBn8WiA0A7cVxNhsXJNB0yJxQ-Fd9DWtCMRym7IpPqA2b-5p8MA31cVlmRi~gKhFuXCj1n0qdgrsKmVhy~ZANp571mn~pcREsaoU6dPy34EdDcwgvV~kLCSF91h3gGsgPmIpH0c9Cg~qTG2hWmjcBnOT3hZP45C~hzL5Ry4LjvUojFerNNE4Dns2ZgcS28tDNrlsnzjxPlIk8EHS7atoBvOmmRaDT1tj1VKHLhF3TsuZk97xjSF2gQxh9vsgW6cg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)