Key Points
Dara-KRd–based, MRD-adapted treatment yielded high stringent CR rates and MRD negativity that improved over time.
Results support randomized studies of therapy de-escalation for patients who are MRD(-) and escalation for MRD(+) after initial treatment.
Visual Abstract
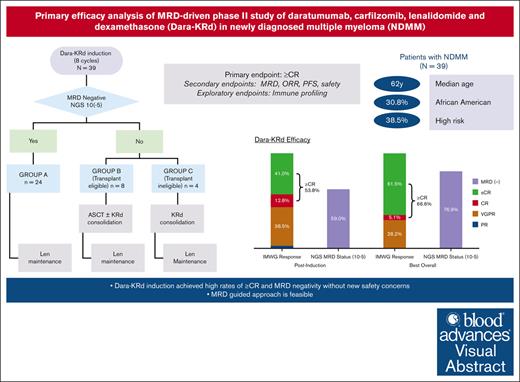
In newly diagnosed multiple myeloma (NDMM), measurable residual disease (MRD) status is prognostically important, but its role in treatment decisions remains unclear. In a phase 2 trial, we assessed daratumumab, carfilzomib, lenalidomide, and dexamethasone (Dara-KRd) induction followed by a next-generation sequencing–based MRD-adapted strategy. The primary outcome was complete response (CR) and stringent CR (≥CR) after induction. Flow cytometry was used to profile T cells. Among 39 patients, 21 (54%) achieved ≥CR after induction (P = .375), with MRD-negative rates of 59% (10−5) and 41% (10−6). Patients who were MRD-negative (n = 24, group A) received lenalidomide maintenance, showing sustained MRD negativity in 14 of 18 (77.8%) for ≥12 cycles. MRD-positive transplant-eligible patients (n = 8, group B) underwent autologous stem cell transplantation, with 62.5% converting to MRD-negative at 10−5 (37.5% at 10−6) posttransplant. MRD-positive, transplant-ineligible patients (n = 4, group C) received KRd consolidation. Best MRD-negative rates improved to 77% (10−5) and 72% (10−6). No new safety concerns were identified for Dara-KRd. With a median follow-up of 30.1 months, 3, 2, and 1 patient(s) in groups A, B, and C, respectively, have progressed or died. We observed that Dara-KRd strongly activated memory T cells, which was associated with an MRD-negative state post induction. Although the primary outcome was not met, Dara-KRd induction in NDMM achieved high ≥CR and MRD-negative rates without new safety concerns. The post induction MRD-adapted strategy deepened responses in MRD-positive patients and maintained durable MRD control in MRD-negative patients. This trial was registered at www.clinicaltrials.gov as #NCT04113018.
Introduction
In recent years, the treatment paradigm of newly diagnosed multiple myeloma (NDMM) has moved toward quadruplet induction therapy. Monoclonal anti-CD38 antibody used in combination with an immunomodulatory drug, proteasome inhibitor, and dexamethasone is becoming the standard of care in the frontline setting based on several studies showing a high proportion of patients achieving negative measurable (or minimal) residual disease (MRD) and improved progression-free survival (PFS).1-6 It is increasingly appreciated that achieving and sustaining undetectable MRD translates to improved outcomes,3,7,8 but additional data are still needed to determine how to best integrate MRD testing to guide treatment decisions.
The combination of daratumumab, carfilzomib, lenalidomide, and dexamethasone (Dara-KRd) has been studied in several trials that reported high rates of MRD negativity.2,9,10 The MANHATTAN trial explored the efficacy of 8 cycles of Dara-KRd induction with postinduction treatment per standard of care.9 The MASTER trial aimed treatment cessation in patients with sustained MRD-negative responses.2,11 Another phase 2 study evaluated the efficacy of 24 cycles of Dara-KRd in patients with NDMM without autologous stem cell transplantation (ASCT).10 This trial, conceptualized contemporaneously with other Dara-KRd trials, features a distinctive adaptive design. We evaluated the quadruplet regimen of Dara-KRd induction, followed by MRD-adapted treatment escalation or deescalation. Additionally, we evaluated the adaptive immune-modulatory effects of Dara-KRd induction in relation to treatment response and MRD status.
Methods
Trial design
The study was an investigator-initiated single-arm, 2-stage, phase 2 trial conducted at the Levine Cancer Institute. The trial followed International Council for Harmonization good clinical practice guidelines and the Declaration of Helsinki. The protocol and amendments were approved by the Advarra institutional review board, and all patients provided written informed consent. All authors confirm that the trial was conducted per the protocol and vouch for the data accuracy. They reviewed, revised, and approved the manuscript for submission.
Patients
Eligible adult patients had NDMM as per the International Myeloma Working Group (IMWG) 2014 criteria12; Eastern Cooperative Oncology Group performance status of 0 to 2; measurable disease; left ventricular ejection fraction of ≥45%; and renal creatinine clearance of ≥30 mL/min. Patients with nonsecretory multiple myeloma (MM), active central nervous system involvement by MM, severe chronic obstructive pulmonary disease, or POEMS (polyneuropathy, organomegaly, endocrinopathy, monoclonal protein, skin changes) syndrome were excluded. No >1 prior cycle of systemic chemotherapy (to accommodate patients who needed emergent therapy at diagnosis) for MM treatment was allowed. Detailed inclusion/exclusion criteria are listed in supplemental Appendix 1. High-risk cytogenetics (HRCs) were defined as ≥1 of the following abnormalities: gain or amplification of 1q, t(4;14), t(14;16), t(14;20), and del (17p). Patients lacking these HRC abnormalities were classified as having standard-risk cytogenetics (SRCs).
Induction treatment
Enrolled patients underwent a total of 8 cycles of Dara-KRd induction (7 for those who received 1 cycle of nonprotocol therapy before enrollment). Dara-KRd induction consisted of subcutaneous daratumumab per standard approved dosing and schedule, IV carfilzomib 56 mg/m2 on days 1, 8, and 15 (except 20 mg/m2, day 1 cycle 1), oral lenalidomide 25 mg on days 1 through 21, and oral or IV dexamethasone 40 mg on days 1, 8, 15, and 22 of each 28-day cycle. Of note, initially, the protocol called for carfilzomib to be administered twice weekly at 27 mg/m2 on days 1, 2, 8, 9, 15, and 16. However, this was changed to weekly dosing at 56 mg/m2 on days 1, 8, and 15 with amendment 2, version 3, dated 5 March 2020. Thromboprophylaxis with either aspirin or direct oral anticoagulants was recommended, based on each patient’s underlying risk factors.
Postinduction treatment assignment
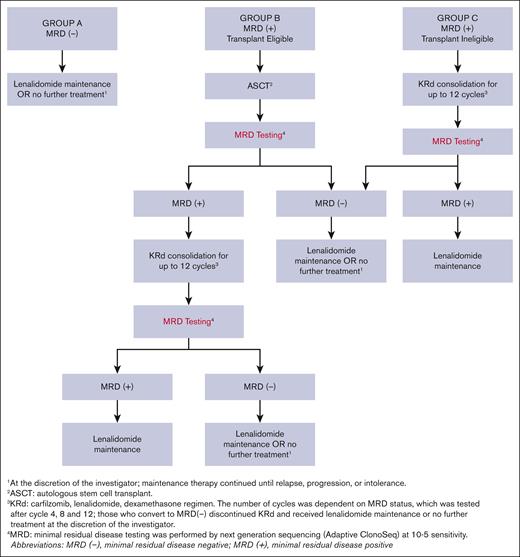
Patients who achieved greater than a very good partial response (VGPR) after induction underwent an MRD assessment and were classified as MRD positive or MRD negative by next-generation sequencing (NGS) at 10−5 sensitivity (clonoSEQ, Adaptive Biotechnologies). Those with less than VGPR after induction or with inadequate baseline bone marrow sample for clonality detection were deemed MRD positive. Further therapy was guided by MRD status and ASCT eligibility (Figure 1): group A (MRD negative) received lenalidomide maintenance or no further treatment at the discretion of the investigator; group B (MRD positive, ASCT eligible) underwent ASCT; and group C (MRD positive, ASCT ineligible) received KRd consolidation for up to 12 cycles. Patients in group B could receive up to 12 cycles of KRd consolidation if they remained MRD positive after ASCT. Conversion to MRD negativity at any protocol specified time point allowed patients to receive lenalidomide maintenance or no further treatment at the investigator’s discretion. The consolidation KRd dose and schedule are provided in supplemental Appendix 2. Maintenance therapy with oral lenalidomide 10 mg on days 1 through 21 of each 28-day cycle continued until disease progression, unacceptable side effects, or patient preference to discontinue.
Post-induction MRD-driven treatment algorithm. Patients were divided into 3 separate groups: group A (MRD negative) received lenalidomide maintenance or no further treatment at the discretion of the investigator; group B (MRD positive, ASCT eligible) underwent ASCT; and group C (MRD positive, ASCT ineligible) received KRd consolidation for up to 12 cycles. Patients in group B could receive up to 12 cycles of KRd consolidation therapy if they remained MRD positive after ASCT. Conversion to MRD-negative status at any protocol specified time point allowed patients to receive lenalidomide maintenance or no further treatment at the investigator’s discretion.
Post-induction MRD-driven treatment algorithm. Patients were divided into 3 separate groups: group A (MRD negative) received lenalidomide maintenance or no further treatment at the discretion of the investigator; group B (MRD positive, ASCT eligible) underwent ASCT; and group C (MRD positive, ASCT ineligible) received KRd consolidation for up to 12 cycles. Patients in group B could receive up to 12 cycles of KRd consolidation therapy if they remained MRD positive after ASCT. Conversion to MRD-negative status at any protocol specified time point allowed patients to receive lenalidomide maintenance or no further treatment at the investigator’s discretion.
Stem cell apheresis
Transplant-eligible patients could undergo stem cell apheresis. Initially, stem cell collection was allowed after completion of Dara-KRd induction, but the protocol was amended to permit it after ≥3 cycles. Patients were mobilized with granulocyte colony-stimulating factor and plerixafor. Mobilization failure was defined as day-4 CD34 count <1/μL or collection of <3 × 106 CD34+ cells per kg in the first attempt.
End points and assessments
The primary end point was rate of responses greater or equal to complete response (≥CR) or stringent CR (sCR) at the end of Dara-KRd induction. Secondary end points included MRD negativity after induction, best response, PFS, overall survival (OS), overall response, and safety. Best response was defined as the best confirmed response that occurred during the trial and overall response was defined as best response ≥ PR. Response and progression were evaluated per IMWG 2016 response criteria.13 PFS was defined as the time from enrollment to progression or death or censored at last disease assessment before subsequent therapy. OS was defined as the time from enrollment to death from any cause.
MRD was assessed using NGS and flow cytometry of bone marrow aspirate (BMA) in accordance with IMWG guidelines. Briefly, NGS-MRD assay was performed on a technical first-pull BMA after needle repositioning, using the ClonoSeq assay (version 2.0; Adaptive Biotechnologies, Seattle, WA). Subsequent second- and third-pull BMA, collected after a 45° bevel rotation, were evaluated for MRD by next-generation flow cytometry (NGF) using a standardized and validated 2-tube–8-color assay developed by the Euroflow consortium.14,15 Both NGS-MRD and NGF-MRD reports included precalculated calls at the MRD sensitivity thresholds of 1 × 10−5, and 1 × 10−6, reporting MRD as positive, negative, or indeterminate. Specimen hemodilution was assessed by flow cytometry using a 0.002% mast cell content threshold for specimen adequacy.14
MRD assessments were performed at the end of 8 induction cycles, after ASCT, every 4 cycles of consolidation (up to 12 cycles), and annually during maintenance. MRD assessment was also done if ≥CR was suspected. MRD negativity at the end of induction was evaluated in the intention-to-treat (ITT) population with sustained MRD negativity defined as lasting for at least 6 or 12 cycles of maintenance.
Adverse events (AE) were monitored continuously and graded according to the National Cancer Institute common terminology criteria for adverse events, version 4.03. AEs were documented until 30 days after last dose of any component of the treatment regimen.
Statistical considerations
The primary objective was to evaluate the ≥CR rate after induction and compare with historical controls. Using a minmax 2-stage design, we tested the hypothesis that the ≥CR rate was ≤50%.16-18 The aim was to achieve a ≥CR rate of 70% with Dara-KRd. With a 1-sided α = .10 significance level, 39 total evaluable patients (23 in stage 1, 16 in stage 2) provided 90% power to reject the null hypothesis.
Two Bayesian-based stopping rules (grade ≥3 cardiovascular or pulmonary toxicities related to the Dara-KRd regimen; any grade 5 AE regardless of causality) were used for safety monitoring during induction. Additional stopping rule details are provided in supplemental Appendix 3. Continuous variables were summarized with descriptive statistics, whereas categorical variables were summarized with frequencies and proportions. The ≥CR rate was calculated with a 90% Clopper Pearson confidence interval (CI), and an exact binomial test compared it with historical control. Survival outcomes were analyzed with Kaplan-Meier methods. Concordance between NGS and NGF MRD results were assessed with agreement rates and Cohen κ statistics. A post-hoc Fisher exact test compared stem cell mobilization failure rates. To ensure a fully validated data set, the data cutoff was 30 October 2023.
Immune correlative analyses
Blood specimens were collected every other induction cycle and after induction. T-cell profiling of cryopreserved peripheral blood mononuclear cells (in 90% fetal bovine serum/10% dimethyl sulfoxide) was performed by flow cytometry. A total of 157 blood samples collected from 36 patients were evaluated (supplemental Table 1). Immune profiling and analytic strategies are described in the supplemental Methods, supplemental Tables 2 and 3 and supplemental Figure 1.
Results
Baseline characteristics and patient disposition
Between 12 December 2019 and 18 October 2022, 39 patients were enrolled. Patient characteristics are outlined in Table 1. Median age was 62 years (range, 34-78), 28% were female, and 31% were Black. Revised International Staging System stage 3 disease was present in 4 (10.3%) patients and HRC in 15 (38.5%) patients. Patient disposition is shown in Figure 2.
Baseline patient and disease characteristics
| . | N = 39 . | |
|---|---|---|
| Sex, n (%) | ||
| Male | 28 | 71.8% |
| Female | 11 | 28.2% |
| Race, n (%) | ||
| White | 26 | 66.7% |
| Black | 12 | 30.8% |
| Asian | 1 | 2.6% |
| Ethnicity, n (%) | ||
| Hispanic/Latino | 1 | 2.6% |
| Non-Hispanic | 37 | 94.9% |
| Unknown | 1 | 2.6% |
| Age at on study, median (range), y | 62 | 34-78 |
| M-protein isotype, n (%) | ||
| IgGκ | 20 | 51.3% |
| IgGλ | 10 | 25.6% |
| IgAκ | 2 | 5.1% |
| IgAλ | 2 | 5.1% |
| κ light chain | 4 | 10.3% |
| λ light chain | 1 | 2.6% |
| ISS, n (%) | ||
| I | 23 | 59.0% |
| II | 10 | 25.6% |
| III | 6 | 15.4% |
| R-ISS, n (%) | ||
| I | 21 | 53.8% |
| II | 14 | 35.9% |
| III | 4 | 10.3% |
| HRC∗ | 15 | 38.5% |
| Del17p | 1 | 2.6% |
| t(4;14) | 1 | 2.6% |
| t(14;16) | 2 | 5.1% |
| t(14;20) | 2 | 5.1% |
| Gain or amplification of 1q | 14 | 35.9% |
| Prestudy cycle of therapy, n (%) | 21 | 53.8% |
| Follow-up time, median (range), mo | 30.1 | 1.7-45.0 |
| . | N = 39 . | |
|---|---|---|
| Sex, n (%) | ||
| Male | 28 | 71.8% |
| Female | 11 | 28.2% |
| Race, n (%) | ||
| White | 26 | 66.7% |
| Black | 12 | 30.8% |
| Asian | 1 | 2.6% |
| Ethnicity, n (%) | ||
| Hispanic/Latino | 1 | 2.6% |
| Non-Hispanic | 37 | 94.9% |
| Unknown | 1 | 2.6% |
| Age at on study, median (range), y | 62 | 34-78 |
| M-protein isotype, n (%) | ||
| IgGκ | 20 | 51.3% |
| IgGλ | 10 | 25.6% |
| IgAκ | 2 | 5.1% |
| IgAλ | 2 | 5.1% |
| κ light chain | 4 | 10.3% |
| λ light chain | 1 | 2.6% |
| ISS, n (%) | ||
| I | 23 | 59.0% |
| II | 10 | 25.6% |
| III | 6 | 15.4% |
| R-ISS, n (%) | ||
| I | 21 | 53.8% |
| II | 14 | 35.9% |
| III | 4 | 10.3% |
| HRC∗ | 15 | 38.5% |
| Del17p | 1 | 2.6% |
| t(4;14) | 1 | 2.6% |
| t(14;16) | 2 | 5.1% |
| t(14;20) | 2 | 5.1% |
| Gain or amplification of 1q | 14 | 35.9% |
| Prestudy cycle of therapy, n (%) | 21 | 53.8% |
| Follow-up time, median (range), mo | 30.1 | 1.7-45.0 |
Data for N = 39 patients.
ISS, International Staging System; R-ISS, Revised International Staging System; HRC, High Risk Cytogenetics.
∗High risk cytogenetics is defined as del17p, t(4;14), t(14;16), t(14;20), and/or gain or amplification of 1q.
Thirty-seven patients completed the planned induction therapy. Seven patients received twice-weekly dosing (1 patient through cycle 5, 3 patients through cycle 3, 2 patients through cycle 3, and 1 patient through cycle 2) before the switch to universal weekly dosing schedule was implemented. There was 1 early discontinuation owing to death during cycle 2, and 1 per investigator discretion during cycle 1. Additionally, 1 patient discontinued study treatment after completing induction. This patient had an inadequate baseline bone marrow sample for clonality detection by NGS, and therefore was considered MRD positive and offered ASCT. However, MRD testing by NGF was negative, and the patient elected not to undergo transplant, discontinued treatment on study, and started standard of care lenalidomide maintenance. After induction, 24 patients were assigned to group A, 8 to group B, and 4 to group C. After a median follow-up of 30.1 months (range, 1.7-45.0), 26 remain on treatment, 10 are in follow-up, and 3 are off study (2 deceased, 1 lost to follow-up).
Efficacy
IMWG response and MRD negativity
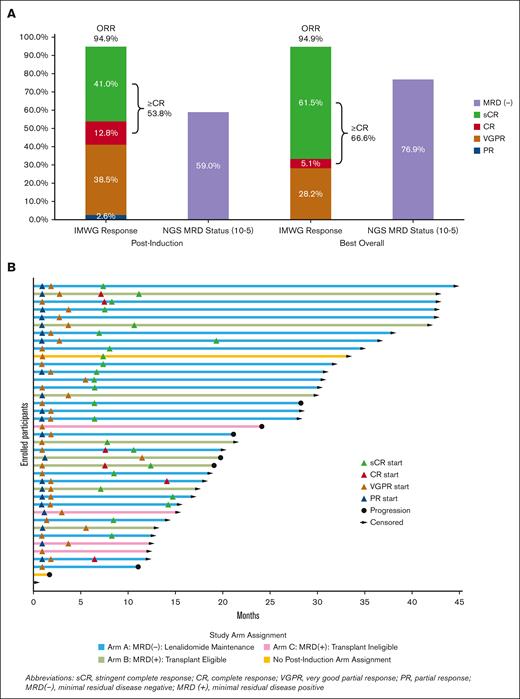
All 39 enrolled patients were considered evaluable for the primary analysis. Thirty-seven patients completed all induction cycles. At the end of induction therapy, ≥CR was achieved in 21 patients (53.8%, 90% CI, 39.6-67.7; P = .375), including sCR in 16 (41%), thereby not meeting the predefined statistical threshold. The overall response rate at the end of induction was 94.9%, including VGPR in 15 (38.5%) and PR in 1 (2.6%, Figure 3A). The daratumumab-specific immunofixation electrophoresis assay (DIRA) was not mandated by the protocol. Among the 20 patients with immunoglobulin Gκ (IgGκ MM, 8 achieved VGPR after induction, and only 3 of those patients underwent DIRA. The ≥CR rate to induction was 53.3% in the HRC group and 54.2% in the SRC group, with both groups achieving a best ≥CR rate of 66.7% over time. Responses over time are shown in Figure 3B.
Response and MRD status. (A) shows the IMWG responses and MRD negativity at the end of induction and best response as of data cutoff (30 October 2023) in N = 39 enrolled patients. (B) shows a swimmer plot over time for the depth and duration of response for each patient. Because 26 patients are still on treatment as of data cutoff, responses could still deepen further.
Response and MRD status. (A) shows the IMWG responses and MRD negativity at the end of induction and best response as of data cutoff (30 October 2023) in N = 39 enrolled patients. (B) shows a swimmer plot over time for the depth and duration of response for each patient. Because 26 patients are still on treatment as of data cutoff, responses could still deepen further.
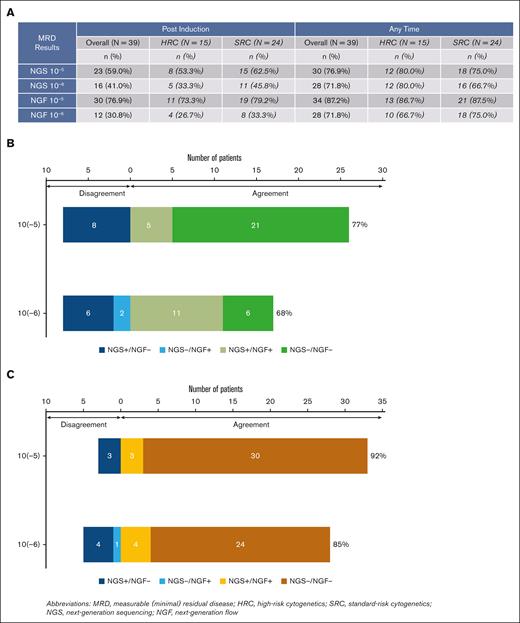
End-of-induction MRD results by NGS were available for 36 of 39 patients; in addition to 2 patients who did not complete induction, 1 patient had no baseline marrow sample. In an ITT analysis, end-of-induction MRD negativity by NGS was 59% at 10−5 and 41% at 10−6. MRD negativity by NGF was 77% at 10−5 and 31% at 10−6 at induction end (N = 39). At any time point, 77% and 72% were MRD negative by NGS at 10−5 and 10−6 sensitivity, respectively. By NGF, 87% and 72% were MRD negative at any time point at 10−5 and 10−6 sensitivity, respectively. There was no notable difference in MRD-negativity rates after induction or at any time between patients with HRC vs SRC (Figure 4A).
MRD-negativity rates during treatment in the intention to treat population (N = 39) and concordance between NGS and NGF methods. (A) This table shows the MRD-negativity rates overall and stratified by cytogenetic risk. HRC is defined as del(17p) and/or t(4;14) and/or t(14;16) and/or t(14;20) and/or gain or amplification of 1q. (B-C) Panel B shows the postinduction MRD concordance between NGS and NGF methods at 10−5 and 10−6 sensitivity, whereas panel C shows the MRD concordance between NGS and NGF methods at 10−5 and 10−6 sensitivity for the best MRD status. Sample size for each of the concordance analyses include patients with results from both NGS and NGF testing at the applicable sensitivity.
MRD-negativity rates during treatment in the intention to treat population (N = 39) and concordance between NGS and NGF methods. (A) This table shows the MRD-negativity rates overall and stratified by cytogenetic risk. HRC is defined as del(17p) and/or t(4;14) and/or t(14;16) and/or t(14;20) and/or gain or amplification of 1q. (B-C) Panel B shows the postinduction MRD concordance between NGS and NGF methods at 10−5 and 10−6 sensitivity, whereas panel C shows the MRD concordance between NGS and NGF methods at 10−5 and 10−6 sensitivity for the best MRD status. Sample size for each of the concordance analyses include patients with results from both NGS and NGF testing at the applicable sensitivity.
Of 23 patients with MRD negativity after induction by NGS (10−5), 20 had enough follow-up to assess sustained MRD negativity after 6 maintenance cycles, and 18 after 12 cycles. MRD negativity was sustained in 16 of 20 (80%) patients after 6 cycles and in 14 of 18 (77.8%) patients after 12 cycles. One patient progressed (without interval conversion to MRD positive) after cycle 4 of maintenance, 1 converted to MRD positive after cycle 4, and 2 after cycle 5. Three patients in Group A have experienced disease progression, all of whom had achieved MRD negativity at 10−6 by NGS after induction. In group B, 5 (62.5%) patients converted to MRD negative (10−5, NGS) after ASCT, with 3 (37.5%) converting to MRD negative at 10−6. In group C, none converted to MRD negative (10−5, NGS) within 1-year of starting consolidation. Two patients in group B and 1 patient in group C experienced disease progression or death. Of 7 who did not achieve MRD negativity in groups B and C with consolidation treatment, 4 remain alive and free of disease progression. Specimen hemodilution limited the sensitivity of NGF-MRD testing, with 13 of 36 (34%) assays after induction reporting mast cell content of <0.002%. Postinduction concordance of MRD status by NGS/NGF at 10−5 was 26 of 34 (77% agreement, Cohen κ: 0.436; 95% CI, 0.153-0.718), and at 10−6 was 17 of 25 (68% agreement, Cohen κ: 0.351; 95% CI, 0.001-0.700; Figure 4B). Concordance of best MRD status by NGS/NGF at 10−5 was 33 of 36 (92% agreement, Cohen κ: 0.25, 95% CI, 0.248-1.000), and at 10−6 was 28 of 33 (85% agreement, Cohen κ: 0.527; 95% CI, 0.173-0.881; Figure 4C).
Time to event outcomes
The 2-year PFS was 82.5% (95% CI, 62.2-92.5), with the median PFS not reached (supplemental Figure 2A). The 2-year OS was 97.4% (95% CI, 83.2-99.6), with the median OS not reached (supplemental Figure 2B). The 2-year PFS for patients with HRC was 70.7% (95% CI, 32.5-89.9; 3 [20%] events), for those with SRC, it was 89.4% (95% CI, 62.9-97.4; 4 (17%) events). The 2-year OS for patients with HRC was 93.3% [95% CI, 61.3-99.0; 1 (7%) event) and for those with SRC, it was 100.0% (95% CI, not estimable to 1 (4%) event.
Safety
All patients experienced at least 1 treatment-emergent AE. The most common (≥25%) AEs were diarrhea (64.1%), constipation (38.5%), fatigue (35.9%), other infections and infestations (33.3%), upper respiratory infection (33.3%), cough (30.8%), nausea (30.8%), neutropenia (30.8%), hypophosphatemia (28.2%), hypokalemia (25.6%) and vomiting (25.6%). Grade ≥3 AEs were seen in 32 (82.1%) patients. The most common grade ≥3 AEs (≥10% of patients) included neutropenia (23.1%) and hypophosphatemia (23.1%, Table 2). COVID-19 infection occurred in 11 (28.2%); only 1 patient needed hospitalization. Non–COVID-19 infections of any grade were seen in 23 (59.0%) patients. Fifteen (38.5%) patients experienced at least 1 serious AE, of which 9 (23.1 %) patients had a treatment-related serious serious adverse event (SAE). During induction, 2 patients (5.1%) experienced grade ≥3 treatment-related cardiovascular or pulmonary-related toxicities, and 1 patient (2.6%) died from treatment-related respiratory failure. Thromboembolic events were observed in 3 (7.7%) patients. There was no halt in enrollment due to stopping rule events during induction.
Most common grade 3 or higher treatment emergent AEs, select AEs, and other safety data for enrolled patients
| Safety outcome summaries . | N = 39 . | |
|---|---|---|
| n . | % . | |
| Grade ≥3 treatment emergent AEs in >10% patients | ||
| Hypophosphatemia | 9 | 23.1 |
| Neutrophil count decreased | 9 | 23.1 |
| Hypertension | 5 | 12.8 |
| Syncope | 5 | 12.8 |
| Hyperglycemia | 4 | 10.3 |
| Platelet count decreased | 4 | 10.3 |
| Other specific AEs | ||
| COVID-19 infection | 11 | 28.2 |
| Other infections (any grade) | 23 | 59.0 |
| Thromboembolic events | 3 | 7.7 |
| Dose reductions during induction | ||
| ≥1 dose reduction of carfilzomib | 10 | 25.6 |
| ≥1 dose reduction of lenalidomide | 9 | 23.1 |
| ≥1 dose reduction of dexamethasone | 8 | 20.5 |
| Safety outcome summaries . | N = 39 . | |
|---|---|---|
| n . | % . | |
| Grade ≥3 treatment emergent AEs in >10% patients | ||
| Hypophosphatemia | 9 | 23.1 |
| Neutrophil count decreased | 9 | 23.1 |
| Hypertension | 5 | 12.8 |
| Syncope | 5 | 12.8 |
| Hyperglycemia | 4 | 10.3 |
| Platelet count decreased | 4 | 10.3 |
| Other specific AEs | ||
| COVID-19 infection | 11 | 28.2 |
| Other infections (any grade) | 23 | 59.0 |
| Thromboembolic events | 3 | 7.7 |
| Dose reductions during induction | ||
| ≥1 dose reduction of carfilzomib | 10 | 25.6 |
| ≥1 dose reduction of lenalidomide | 9 | 23.1 |
| ≥1 dose reduction of dexamethasone | 8 | 20.5 |
Data refer to AE that occurred in >10% of N = 39 participants.
Stem cell mobilization
In total, 34 patients pursued stem cell collection. Of 23 patients who collected stem cells upon completion of induction (7-8 cycles of Dara-KRd), 7 (30.4%) failed to collect 3 million CD34+ cells per kg on first attempt. Comparatively, after the amendment allowed for earlier stem cell collection, 0 of 11 patients who collected after 3-5 cycles experienced stem cell collection failure (P = .069).
Immune profiling analyses
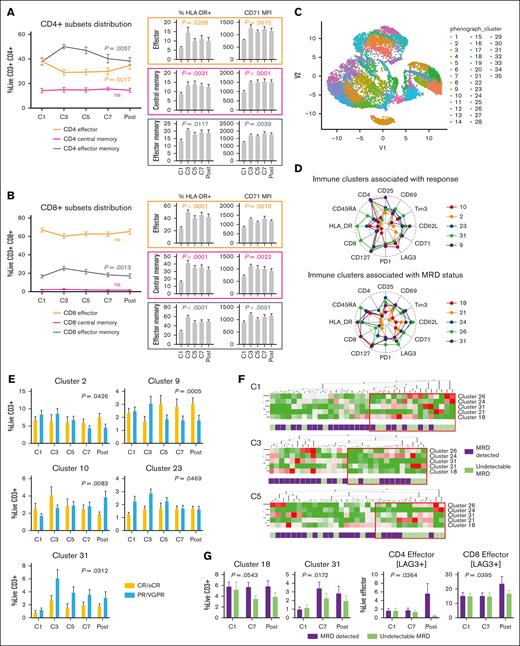
We assessed the immune modulatory activity of Dara-KRd on conventional T cells (CD4+, CD8+). Effector memory T cells showed rapidly expanded during early induction (cycle 3 to 5), whereas effector T cells decreased (Figure 5A-B). Dara-KRd induction sustained T-cell activation (HLA-DR+) and proliferation (CD71 mean fluorescence intensity) and promoted the expansion of inducible regulatory T cells by the end of induction (P = .0058). Central memory T-cell distribution remained unchanged. Immune modulatory effects were further analyzed in relation to MRD status through dimensional reduction analyses (Figure 5C-E; supplemental Figures 3 and 4; supplemental Table 3). Patients with undetectable MRD had higher baseline levels of circulating γδ T cells (clusters 24 or 26), which became activated during Dara-KRd induction, as well as higher baseline levels of activated CD8+ effector memory cells (cluster 18) and terminally differentiated effector memory (TEMRA) CD8+ cells, which were rapidly eliminated by Dara-KRd during cycles 3-5 (Figure 5D,F; supplemental Table 3). After induction, patients with undetectable MRD exhibited lower circulating CD8+ TEMRA titers (P < .05) while maintaining higher overall effector T-cell fitness, as evidenced by no gain in immune checkpoint LAG3 expression (Figure 5G).
Immune modulatory activity of Dara-KRd on both conventional and unconventional T cells during induction therapy. Peripheral blood mononuclear cells (PBMCs), collected from 36 patients before every other cycle of induction therapy (cycle 1-7) and after induction, were evaluated by flow cytometry. (A-B) CD4 and CD8 T-cell subsets (manually gated from live CD3+ cells) distribution, activation (% HLA-DR expression), and proliferation (CD71 MFI) assessment. (C) Conventional (CD4+, CD8+) and unconventional (CD4−/CD8−) T-cell identification through dimensional reduction analysis (Uniform Manifold Approximation and Projection; R export from live CD3+ cells). (D) Radar plots representing immunological clusters associated with treatment outcomes. (E) Mixed model analysis of immune clusters associated with depth of clinical response after Dara-KRd induction (CR/sCR vs PR/VGPR). (F) Hierarchical clustering analysis of immune clusters associated with MRD positivity. For the immune analysis MRD positivity is defined as detectable MRD at the highest level of sensitivity by either NGS or NGF (MRD positive vs MRD negative). The red box indicates immunotypes enriched for patients with MRD negativity. (G) Mixed model analysis of immune clusters and T-cell fitness markers (LAG3) associated MRD status after induction; P values indicate time × MRD status interaction. Error bars represent standard error of the mean. MFI, mean fluorescence intensity.
Immune modulatory activity of Dara-KRd on both conventional and unconventional T cells during induction therapy. Peripheral blood mononuclear cells (PBMCs), collected from 36 patients before every other cycle of induction therapy (cycle 1-7) and after induction, were evaluated by flow cytometry. (A-B) CD4 and CD8 T-cell subsets (manually gated from live CD3+ cells) distribution, activation (% HLA-DR expression), and proliferation (CD71 MFI) assessment. (C) Conventional (CD4+, CD8+) and unconventional (CD4−/CD8−) T-cell identification through dimensional reduction analysis (Uniform Manifold Approximation and Projection; R export from live CD3+ cells). (D) Radar plots representing immunological clusters associated with treatment outcomes. (E) Mixed model analysis of immune clusters associated with depth of clinical response after Dara-KRd induction (CR/sCR vs PR/VGPR). (F) Hierarchical clustering analysis of immune clusters associated with MRD positivity. For the immune analysis MRD positivity is defined as detectable MRD at the highest level of sensitivity by either NGS or NGF (MRD positive vs MRD negative). The red box indicates immunotypes enriched for patients with MRD negativity. (G) Mixed model analysis of immune clusters and T-cell fitness markers (LAG3) associated MRD status after induction; P values indicate time × MRD status interaction. Error bars represent standard error of the mean. MFI, mean fluorescence intensity.
Discussion
This phase 2 study investigated the efficacy of Dara-KRd using an MRD-adapted design in NDMM. Induction treatment with Dara-KRd led to ≥CR in 54% of patients. Furthermore, end-of-induction MRD negativity by NGS was achieved in 59% at 10−5 and 41% at 10−6 in the ITT population. The responses deepened over time, with the ≥CR rate increasing to 66.7% and MRD negativity increasing to 77% (10−5) and 72% (10−6) by data cutoff. There was a high level of agreement between MRD detected by NGS and NGF. Importantly, there was no difference in ≥CR rate or MRD negativity in patients with HRC and SRC.
Based on the observed ≥CR rate, we could not reject the null hypothesis for the primary objective. We recognize that our assumption of achieving a ≥CR rate of 70% with the addition of daratumumab to KRd, compared with the historically observed 50% with KRd alone, was ambitious. In the MASTER study, the ≥CR rate was 36% after 4 cycles of Dara-KRd induction.2 The MANHATTAN trial reported a combined VGPR and CR rate, making ≥CR unclear.18 A recent phase 2 study with 40 evaluable patients reported a ≥CR rate of 70%.10 We included all patients who initiated treatment in the primary end point analysis and did not exclude any from response evaluation. Additionally, daratumumab can interfere with accurate response assessments using gel-based techniques, especially in patients with IgGκ MM. Although we considered DIRA for more precise response evaluation, it was not mandatory, and only 3 of the 8 patients with IgGκ in VGPR underwent DIRA, potentially underestimating the true CR rate to induction.
Studies consistently show that patients who achieve MRD negativity after frontline therapy for NDMM have better PFS and OS compared to those with detectable MRD.7,8 Although using MRD status to guide postinduction treatment is promising, few MM studies have explored this approach. In this study, treatment decisions were influenced by MRD at end of induction, with ASCT offered to eligible patients, and KRd consolidation for ASCT-ineligible patients. Patients with undetectable MRD proceeded to maintenance therapy.
Although physicians had the option to discontinue treatment in group A, all patients in this group pursued maintenance with lenalidomide. MRD negativity was sustained in 16 of 20 (80%) patients after 6 cycles and in 14 of 18 (77.8%) patients after 12 cycles of maintenance in this group. Importantly, none of the patients with sustained MRD negativity in this study progressed, supporting its role as a key prognostic indicator. Other trials using Dara-KRd explored an ASCT-free approach. In the MANHATTAN trial, 8 cycles of the Dara-KRd yielded a 71% rate of MRD negativity at a sensitivity of 10−5.9 In another study, extended Dara-KRd for up to 13 cycles resulted in MRD-negativity rate of 75% at sensitivity of 10−5 for 8 patients who achieved ≥CR.19 These findings, along with ours, support a randomized trial comparing upfront ASCT vs no ASCT for patients achieving MRD negativity after induction. The ongoing MIDAS trial (ClinicalTrials.gov identifier: NCT04934475) is testing an MRD-driven approach in which, after isatuximab-KRd induction, patients with MRD-positivity are randomized to either ASCT + consolidation or tandem ASCT, whereas those who are MRD negative receive 6 additional cycles of isatuximab-KRd or ASCT.
Consolidation with ASCT beyond 8 cycles proved beneficial for the subgroup remaining MRD-positive at the end of induction, as evidenced by the progressive deepening of responses observed in our study. In group B 5 of 8 (62.5%) patients who received ASCT transitioned to MRD negativity after the transplant, with 3 (37.5%) achieving MRD- negativity at 10−6 sensitivity. This observation aligns with another study where the rate of MRD negativity at the 10−6 threshold was 35% after 8 cycles of therapy but increased to 53% as a best response over the course of extended consolidation.10 The phase 2 MASTER trial asked whether an MRD-adaptive design for deescalation of therapy can generate sustained responses while offering a treatment free interval to patients with NDMM undergoing ASCT.2 Among 123 patients, 38% achieved MRD negativity (10−5) after 4 cycles of Dara-KRd induction.2 MRD kinetics were evaluated after induction, after ASCT, and every 4 cycles (up to 8) of Dara-KRd consolidation with protocolized treatment-free MRD surveillance after 2 consecutive MRD-negativity (10−5) results. Overall, MRD-negativity rates were 80% (10−5) and 66% (10−6), and 71% entered MRD surveillance. Other trials are exploring MRD adapted posttransplant strategies for treatment escalation and deescalation, such as MIDAS (ClinicalTrials.gov identifier: NCT04934475), PERSEUS (ClinicalTrials.gov identifier: NCT03710603), RADAR (ISCRTN46841867), and ADVANCE (ClinicalTrials.gov identifier: NCT04268498). The results of these studies will inform the role of MRD-dependent tailoring of treatment, either to intensify or modify therapy to prevent relapse, or to deescalate therapy to reduce treatment-related morbidity and mortality.
Most patients received a more convenient Dara-KRd regimen using once-weekly carfilzomib (56 mg/m2) with favorable toxicity profile. The safety of this regimen was consistent with what is known about this combination. No major cardiac events were noted. One patient died from suspected (but not confirmed) pulmonary edema attributed to therapy. COVID infection occurred in 11 (28%); only 1 patient needed hospitalization. Early in the study when stem cell collection was allowed after 8 cycles of Dara-KRd, 30% of patients did not collect adequate numbers of stem cells despite granulocyte colony-stimulating factor and plerixafor mobilization. After the study was amended to allow earlier collection, the stem cell mobilization failure dropped to 0%. The number of cycles affected successful stem cell collection in the MANHATTAN trial, as well as the Intergroupe Francophone du Myelome phase 2 study in high-risk MM.9,20 Therefore, these study protocols were amended to allow stem cell collection earlier in induction therapy. Although there was a signal in the MASTER trial (in which cells were routinely collected after 4 cycles), mobilization appeared to be less problematic.2 Based on our results and others, we recommend stem cell collection after 3 to 4 cycles of Dara-KRd.
Previous reports suggested that Daratumumab, with or without lenalidomide, depleted CD38-expressing effector T cells and regulatory T cells.21-23 However, in our study, activated effector T-cell counts initially decreased but rapidly rebounded during Dara-KRd induction. We also noted activation of both effector and memory T-cell subsets, particularly an expansion of effector memory T cells, whereas central memory T cells did not increase. This contrasts with effector-to–central memory T-cell conversion with elotuzumab-KRd,24 suggesting that daratumumab might disrupt T-cell terminal differentiation, possibly through its influence on the CD 8–NAD+ axis.25
In our immune profiling of the peripheral blood, we found distinct differences in immune cell composition related to MRD status. Higher baseline levels of γδ T cells and CD8+ TEMRA were associated with MRD negativity. Among the MRD-negative group, we observed activation of γδ T cells, known for anti-MM activity,26 and a rapid reduction in CD8+ TEMRA, which is linked to immune senescence and poor clinical outcomes in MM.27,28 Dara-KRd could potentially ameliorate TEMRA-associated T-cell dysfunction in MM, either by directly targeting CD38-expressing TEMRA29 or by indirectly inhibiting terminal differentiation processes. Further mechanistic studies are needed to confirm these findings, establish causality, and explore therapeutic implications.
The limitations of this study include the small sample size and nonrandomized design. The study was not designed to separately answer the benefit of escalating treatment by risk groups, although we did not observe any differences in the primary end point or MRD-negativity rate between patients with HRC or SRC. Although the trial aimed to be inclusive, it had a skewed representation, with a predominance of younger and male patients. The limited number of patients in the MRD-positive groups (B and C) makes it challenging to assess the benefit of consolidation. Additionally, the study was not designed to evaluate the role of daratumumab in the maintenance setting, so the effectiveness of a more intensive maintenance approach incorporating daratumumab with lenalidomide or carfilzomib remains undetermined.
In conclusion, although we failed to reject the null hypothesis for our primary end point, our data confirm high rates of sCR and MRD negativity with the Dara-KRd quadruplet regimen in NDMM. No new safety signals were observed with this combination. Longitudinal immune profiling during induction revealed activation of both conventional and unconventional T cells and changes in immune subsets correlating with MRD. The results support a randomized trial comparing upfront ASCT vs no upfront ASCT for patients achieving MRD negativity after induction, and escalated treatment for MRD positivity.
Acknowledgments
The authors thank the patients and their families for their participation in this research. Additionally, the authors thank the Sponsored Research Coordinating Center and the phase 2/3 clinical trial team at Atrium Health for their support in this study. Special thanks are also extended to the Hematology Oncology Research Laboratory at Atrium Health Levine Cancer Institute and to Tahj Jones from the Immune Monitoring Core Laboratory for their valuable technical assistance throughout the study.
This work was funded by grants from Bristol Myers Squibb (RV-CL-MM-PI-12836), Amgen (20167250), and Janssen (54767414MMY2021), and supported, in part, by the Gayle J. and Charles C. Tallardy III Foundation (D.F.) and Leukemia Lymphoma Society CDP Award.
The funding companies had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Authorship
Contribution: M.B. drafted, reviewed, and edited the manuscript, contributed to study design, data collection, analysis, and interpretation, provided medical care, and is the principal investigator of the study; M.R. performed statistical analysis, contributed to study design, data analysis and interpretation, and reviewed and edited the manuscript; D.F., F.G., J.M.G., and A.I.-F. designed and performed study assays, contributed to data analysis and interpretation, and reviewed and edited the manuscript; S.A., B.P., M.P.-R., C.V., R.F., and C.J.F. contributed to the data collection, data analysis, and interpretation, provided medical care, and reviewed and edited the manuscript; X.B., S.N., T.D., and M.B.A. provided trial management, contributed to the data collection, data analysis, and interpretation, and reviewed the manuscript; J.T.S. provided oversight, contributed to the study design, data analysis, and interpretation, and reviewed the manuscript; P.M.V. contributed to study design, data collection, data analysis, and interpretation, provided resources and medical care, and reviewed and edited the manuscript; S.Z.U. conceived and designed the study, contributed to data collection, data analysis, and interpretation, provided resources and medical care, reviewed and edited the manuscript, and was the principal investigator of the study; and all authors have reviewed and/or revised the article for important intellectual content and approve the version to be submitted.
Conflict-of-interest disclosure: M.B. reports research funding (to institute) from Janssen, Amgen, Bristol Myers Squibb/Celgene, Takeda, and Caribou Biosciences. S.A. reports honoraria and research funding from GlaxoSmithKline, Amgen, and Karyopharm; and honoraria from Janssen. B.P. reports membership on an entity's board of directors or advisory committees with AbbVie and Janssen; and research funding (to institute) from Bristol Myers Squibb. J.T.S. reports consultancy with Astellas Pharma, Camurus, Eli Lilly and Co, CARsgen, Fusion Pharmaceuticals, Jazz Pharmaceuticals, and Immatics. P.M.V. reports consultancy with, and honoraria from, AbbVie, Amgen, Bristol Myers Squibb, GlaxoSmithKline, Karyopharm, Novartis, Oncopeptides, Pfizer, Sanofi, and SecuraBio. S.Z.U. reports research funding from AbbVie, Amgen, Bristol Myers Squibb, Celgene, EdoPharma, Genentech, Gilead, GlaxoSmithKline, Janssen Pharmaceuticals, Oncopeptides, Sanofi, SeaGen Inc (formerly Seattle Genetics, Inc), Secura Bio Inc, SkylineDx, Takeda, TeneoBio, Amgen, Array Biopharma, Bristol Myers Squibb, Celgene, GlaxoSmithKline, and Janssen; consultancy with Amgen, Array Biopharma, Bristol Myers Squibb, Celgene, GlaxoSmithKline, Janssen Pharmaceuticals, Merck, Pharmacyclics, Sanofi, SeaGen Inc (formerly Seattle Genetics, Inc), SkylineDx, and Takeda; and serving on the speakers bureau of Amgen, Bristol Myers Squibb, Janssen Pharmaceuticals, and Sanofi. The remaining authors declare no competing financial interests.
Correspondence: Manisha Bhutani, Department of Hematologic Oncology and Blood Disorders, Atrium Health Levine Cancer Institute, 1021 Morehead Medical Dr, Building 2, Charlotte, NC 28204; email: manisha.bhutani@atriumhealth.org; Peter M. Voorhees, Department of Hematologic Oncology and Blood Disorders, Atrium Health Levine Cancer Institute, 1021 Morehead Medical Dr, Building 2, Charlotte, NC 28204; email: peter.voorhees@atriumhealth.org; and Saad Z. Usmani, Myeloma Service, Memorial Sloan Kettering Cancer Center, 530 East 74th St, Room 20-228, New York, NY 10021; email: usmanis@mskcc.org.
References
Author notes
Presented at the 62nd American Society of Hematology annual meeting (virtual), 5 to 8 December 2020; the 64th American Society of Hematology annual meeting, New Orleans, LA, 10 to 13 December 2022; and the 65th American Society of Hematology annual meeting, San Diego, CA, 9 to 12 December 2023.
The deidentified data supporting this study’s findings are available on reasonable request from the corresponding authors, Manisha Bhutani (manisha.bhutani@atriumhealth.org); Peter M. Voorhees (peter.voorhees@atriumhealth.org); and Saad Z. Usmani (usmanis@mskcc.org).
The full-text version of this article contains a data supplement.