In this issue of Blood Advances, 2 pivotal studies led by Kemps et al1 and Ozkaya et al2 present a comprehensive clinicopathologic and genomic analysis of a combined cohort of 40 patients with indeterminate dendritic cell histiocytosis (IDCH). Both studies begin to ask: can we finally “determine” the “indeterminate” of IDCH?
Both studies highlight the clinical, histologic, and genetic diversity of IDCH. Together, the Kemps et al cohort (12 centrally reviewed IDCH cases from the Dutch Nationwide Pathology Databank [Palga] and the University Hospitals Leuven) and the Ozkaya et al cohort (28 centrally reviewed IDHC cases from the US and French databases) highlight the deficiencies in the current IDCH diagnostic criteria, notably in the lack of a clear threshold for the percentage of positive cells with Langerin/CD207 for distinguishing IDCH from Langerhans cell histiocytosis (LCH). Both studies recommended reserving the pathologic diagnosis of IDCH for cases with typical morphology and predominant CD1a expression and <1% Langerin expression. Although S100 and CD68 were generally expressed in most cases, a strong and diffuse expression was not essential for diagnosis. Exclusion of histologic mimics, including CD1a+ reactive dendritic cell hyperplasia and CD1a+ hematopoietic (ie, myeloid or T-cell) malignancies, is also necessary.
The Ozkaya et al series described 15 additional cases with low Langerin expression (1%-20% of cells), whereas the Kemps et al series included 6 additional cases labeled as “potential IDCH” with variable CD1a and cyclin D1 expression. Although pediatric cases were included in each study, the median age ranged from 57.5 to 70 years, which is much higher than that of typical LCH.3,4 The older median age likely reflects referral bias, because pediatric cases with any Langerin expression are typically diagnosed and treated as LCH in most pediatric referral centers. In further support, the pediatric cases described in both cohorts closely resembled LCH, including expressing Langerin in >1% of cells (1%-20% of positive cells, low level), having mixed areas of LCH/IDCH, and also a higher association of BRAF p.V600E. Unlike LCH, bone involvement is rare in IDCH, with cutaneous involvement, especially skin-limited disease, more frequently described (65%-66% of cases), although systemic disease involvement (eg, lungs, breast, lymph nodes, and gastrointestinal tract) was still noted. Both studies report a striking association with hematologic malignancies in adult patients, including 33% (4 of 12 patients) in the Kemps et al cohort, and 46% (13 of 28 patients) in the Ozkaya et al cohort, including patients diagnosed with myeloid (n = 10), lymphoid (n = 2), and lymphoid/myeloid (n = 1) malignancies in the latter.1,2 This rate of “secondary IDCH” was higher than that observed with secondary LCH and Erdheim Chester Disease, although the numbers were overall small.5-7 Most strikingly, secondary IDCH, after or together with another hematopoietic malignancy, was also associated with inferior median and 72-month survival2 as compared with primary IDCH in the Ozkaya et al cohort.
Wood et al8 first described the “indeterminate” histiocytosis nomenclature in 1985 to suggest a questionable cell of origin, given the varied pathologic differences with LCH (ie, lack of Birbeck granules, the defining ultrastructural organelle of LCH, and lack of the epidermotropism, a defining characteristic of cutaneous LCH). Such original cases also had indeterminate clinical features (ie, lack of bone and extracutaneous involvement). The World Health Organization classification of hematolymphoid tumors has long-defined IDCH as a histiocytic/dendritic cell neoplasm9 with histiocytic cells that resemble LCH but lacking Birbeck granules. Langerin/CD207 immunohistochemistry, a surrogate marker for Birbeck granules in the cytoplasm, has largely replaced electron microscopy, although its routine use in clinical practice is variable and nonuniform.
The essential pathologic features (CD1a+ and Langerin negative or <1%) should be considered foremost but are just 1 piece of the indeterminate puzzle, with clinical history and molecular integration also critical for diagnosis. First noted in small case series, the ETV3::NCOA2 fusion in IDCH had been suggested to be pathognomic10; however, both studies1,2 and others have expanded the IDCH molecular landscape beyond ETV3::NCOA2 and now include all other MAPK pathway mutations. Next-generation DNA and RNA sequencing reveal the complex genetic landscape of IDCH. MAPK pathway gene mutations (KRAS, NRAS, BRAF, and MAP2K1) are common drivers, but unlike in LCH, in which BRAF p.V600E is highly prevalent (ie, 50%-60%), IDCH showed far fewer BRAF p.V600E mutations and rather more ETV3::NCOA2, KRAS, and NRAS mutations, all of which are rare in LCH.3,4BRAF p.V600E was more often detected in the mixed (LCH/IDCH) and pediatric cases with low Langerin expression (1%-20%), indicating a close relationship to LCH. The impact of epigenetic regulator mutations (TET2, ASXL1, EZH2, PHF6, KMT2D, and ZRSR2) on these lesions’ biology remains unclear, because these mutations are rare in typical pediatric IDCH/LCH but more frequently found in adult cases.1-3 Additionally, the shared genetic alterations between the secondary IDCH and their associated hematologic malignancy further supports a shared clonal relationship and likely plays a role in their biology (ie, transdifferentiation) and affect the poor outcomes.
Both studies contribute valuable insights into the clinical course of IDCH. Ozkaya et al identified poor clinical prognostic markers including older age at diagnosis, any nodal involvement, (which was highly associated with secondary IDCH), and associated hematologic malignancy, with a 72-month survival rate of 46% (confidence interval, 24.8-64.9). The common theme between outcomes in both studies was that none of the pediatric patients died. Still, among the adults who died, the majority (65%) had an associated hematolymphoid malignancy at some point, highlighting the increased mortality risk in this subpopulation of IDCH and need to separately subclassify this cohort in future studies.
These studies highlight the need for further research to better investigate the underlying biology of IDCH, now with a stricter pathologic classification defined, including typical histiocytes with predominance of CD1a, negative to <1% Langerin expression, variable CD68 and S100 expression (see figure), and a molecular signature that extends beyond the ETV3:NCOA2 fusion to include other known MAPK pathway mutations.
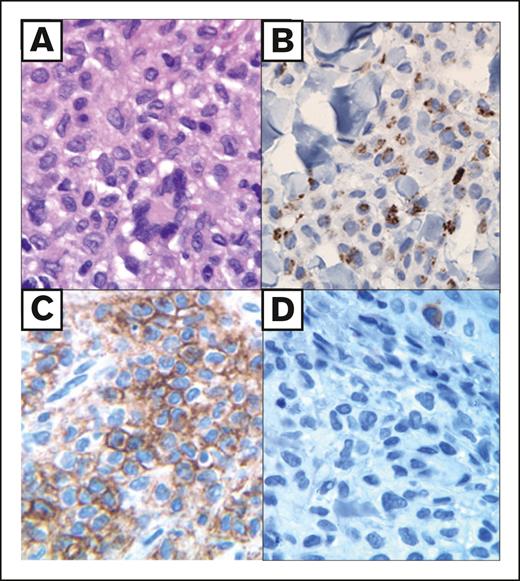
Histologic features of IDCH. Adult skin lesion (multifocal) with a dermal histiocytic infiltrate composed of cytoplasmic rich cells with (A) minimal nuclear folds and occasional Touton-like giant cells (hematoxylin and eosin), (B) an indeterminate cell immunophenotype including paranuclear cytoplasmic dot-like CD68 staining (CD68 PGM1 clone), (C) diffuse membranous CD1a (CD1a), and (D) negative Langerin/CD207 (CD207). The lesional infiltrate was negative for CD163 and Factor XIIIa but diffusely positive for S100 and fascin (not shown).
Histologic features of IDCH. Adult skin lesion (multifocal) with a dermal histiocytic infiltrate composed of cytoplasmic rich cells with (A) minimal nuclear folds and occasional Touton-like giant cells (hematoxylin and eosin), (B) an indeterminate cell immunophenotype including paranuclear cytoplasmic dot-like CD68 staining (CD68 PGM1 clone), (C) diffuse membranous CD1a (CD1a), and (D) negative Langerin/CD207 (CD207). The lesional infiltrate was negative for CD163 and Factor XIIIa but diffusely positive for S100 and fascin (not shown).
Although a bone marrow–derived hematopoietic precursor of the monocytes/dendritic cell spectrum is likely the cell of origin in IDCH, further research is needed to understand the interconnected roles between LCH in children and myeloid/lymphoid malignancies in adults. Further focus on the local tissue microenvironments together with timing of molecular drivers and epigenetic modifiers will likely reveal an interplay into the with indeterminate cell phenotype, biology, and potential transdifferentiation capacity from both myeloid and T-cell origins. Key questions include identifying what mutations/alterations predict disease phenotype and progression, especially in “secondary” IDCH, and what specific drivers predispose adult patients at highest risk of developing secondary IDCH? Addressing these questions will also help expose the complex interrelationship between IDCH, LCH, and myeloid/lymphoid malignancies to develop more effective treatment strategies in the future.
Conflict-of-interest disclosure: The authors declare no competing financial interests.