Key Points
A meta-analysis of trials of approved BsAbs and CAR T in B-cell lymphomas was performed to assess infection risk.
Standardizing infection rates per patient-month reveals a significantly greater infection risk for BsAbs, particularly grade 3+ infections.
Visual Abstract
CD3xCD20 bispecific antibody (BsAb) therapy and CD19-directed chimeric antigen receptor T-cell therapy (CAR T) are novel immunotherapies that have shown impressive efficacy in B-cell lymphomas, but also come with significant morbidity and mortality, including infections. This meta-analysis compares rates of infections between commercially approved CAR T and BsAb therapy in patients with B-cell non-Hodgkin lymphoma (B-NHL). We conducted a systematic review for prospective trials assessing commercially approved CAR T and BsAbs in patients with B-NHL. Twenty-five studies comprising 3202 patients were included in the analysis. We used random effects models to evaluate all-grade infections, grade 3+ infections, and infection-related mortality, calculating both pooled rates per patient and per patient-month. While CAR T and BsAbs had similar rates of all-grade infections per patient (0.44 vs 0.54; P = .18), BsAbs had a higher rate of infection per patient-month (0.0397 vs 0.0167; P = .0012). Similarly, CAR T and BsAbs had similar rates of grade 3+ infections per patient (0.16 vs 0.22; P = .08), while BsAbs had a higher rate of grade 3+ infections per patient-month (0.0165 vs 0.0069; P = .0003). CAR T and BsAb products had similar rates of infection-related mortality per patient (0.04 vs 0.03; P = .26) and per patient-month (0.0023 vs 0.0022; P = .96). Our findings point to the potential increased burden of infections over time in patients receiving BsAb therapy, particularly for patients on indefinite therapy.
Introduction
In the past decade, the novel immunotherapies bispecific antibodies (BsAbs) and chimeric antigen receptor T-cell therapies (CAR T) have changed the treatment landscape for many subtypes of B-cell non-Hodgkin lymphoma (B-NHL), particularly in the relapsed/refractory (R/R) setting.1,2 However, there are no direct clinical trial comparisons of CAR T and BsAbs, making treatment selection a clinical challenge.3 A recent meta-analysis sought to compare CAR T with BsAbs in R/R diffuse large B-cell lymphoma (LBCL), finding that CAR T had a higher pooled complete response rate and pooled 1-year progression-free survival compared with BsAbs.4 However, the superior outcomes associated with CAR T also came with significantly higher rates of cytokine release syndrome and neurotoxicity. Though CAR T may be associated with higher initial toxicity rates, patients receiving BsAbs often require prolonged or indefinite treatment until disease progression, which may increase susceptibility to infection.5,6 Infections are a major cause of morbidity and mortality after both CAR T and BsAbs, in part due to prolonged cytopenias and immunosuppression due to hypogammaglobulinemia and B-cell aplasia.7-10 There is a dearth of research assessing comparative infection rates between the two therapies. This meta-analysis aims to compare infection rates of CAR T and BsAbs in patients with B-NHL, both as events per patient and as events per patient-month to account for differences in administration and follow-up times.
Methods
Systematic literature review
This study was performed following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).11 In adherence to this statement, a protocol was registered with PROSPERO international prospective register of systematic reviews (CRD42024517981). A medical librarian performed comprehensive searches to identify studies that addressed rates of infection in patients with B-NHL receiving BsAbs compared with CAR T.
Searches were run in February 2024, with an update in January 2025, in the following databases: Ovid MEDLINE (ALL: 1946 to Present); Ovid Embase (1974 to present); and The Cochrane Library (Wiley). Search strategy included all appropriate controlled vocabulary and keywords for the concepts of “CAR T-cell therapy,” “bispecific antibodies,” and “lymphoma.” The full search strategies for all databases are available in the supplemental Methods. To limit publication bias, there were no language or publication date restrictions on the search strategy.
Study selection
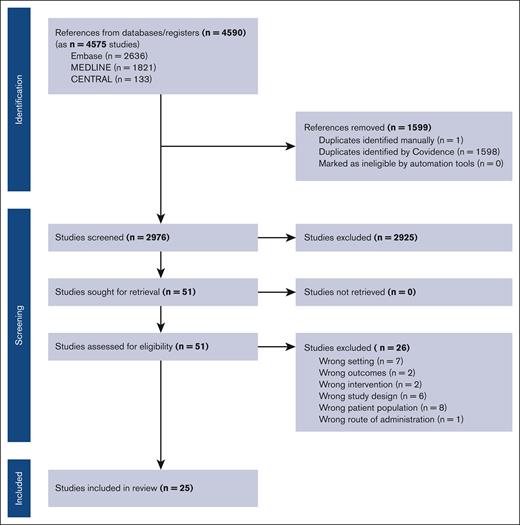
Retrieved studies were screened for inclusion using Covidence systematic review software. Titles and abstracts were reviewed against predefined inclusion/exclusion criteria by 2 independent reviewers. Discrepancies were resolved by consensus. For final inclusion, full text was then retrieved and also screened by 2 independent reviewers. Articles considered for inclusion were prospective clinical trials enrolling adults with either indolent or aggressive lymphoma that were published on or after 2015. We excluded retrospective and registry studies, studies with a sample size <30 patients, patients’ age <18 years, CAR T or BsAb therapy for leukemia (including chronic lymphocytic leukemia) or multiple myeloma, any combination studies using additional agents such as chemotherapy or immunotherapy, any products that have not been approved in the United States or Europe for B-cell lymphoma, or studies that only existed in abstract form. We also excluded studies that were missing necessary details on median follow-up time or mortality data. Figure 1 includes the full PRISMA flow diagram outlining our study selection process.
Data extraction and quality assessment
Data extraction was performed independently in duplicate with predefined, standardized templates. Data points selected for extraction included trial name, trial identifier, publication journal, publication year, first author name, trial phase, regimen, number of patients, median age, B-NHL subtype, proportion of patients with stage III/IV disease (n, %), median number of prior therapies received, and median follow-up in months. For the BsAbs subset, we extracted the number of patients who had received CAR T prior to enrollment. Adverse event outcomes were also collected, including number of patients with infections of any grade, grade 3+ infections, and infection-related mortality including COVID-related deaths. The Joanna Briggs Institute scoring system was used to assess the potential risk of bias of the studies included.12
Statistical analysis
The primary outcome was infection rate, assessed as all-grade infection, grade 3+ infection, and infection-related mortality (grade 5 infections). Total infections were counted regardless of whether or not they were deemed related to study intervention. We included infections that occurred in both the treatment-emergent reporting window, and the reporting window after treatment to minimize ascertainment bias. Infections that occurred upon relapse and initiation of another line of treatment were not counted. Two methods were employed to compare infection rates between CAR T products and BsAb products. The first method compared infection rates as proportions of events per patient, and the second method compared infection rates standardized by patient-month to account for differences in administration as well as highly variable follow-up times across studies.
Infection proportions were pooled using the metaprop function from the R meta package. The Clopper-Pearson binomial interval was used to calculate confidence intervals (CIs) of toxicity rates, rates were pooled using logit transformations, and the inverse variance weighting method with random effects modeling was used to pool the effect sizes. Infection rates per patient-month were calculated using the metarate function. A random-effects model with the inverse variance method was used to pool log-transformed incidence rates, and CIs were computed using normal approximation. In both methods, the restricted maximum-likelihood estimator was used to calculate the heterogeneity variance τ2. Between-study heterogeneity was assessed by τ2 and I2 statistics, and the significance of heterogeneity was tested by Cochran’s Q test. Heterogeneity was assessed as follows: I2 ≤ 25%, low heterogeneity; I2 ≤ 50%, moderate heterogeneity; and I2 ≤ 75%, substantial heterogeneity. Studies with missing values for any particular toxicity rate were removed from the analysis for that particular outcome. Continuity corrections of 0.5 were applied to zero-event studies to ensure they were included in the analyses. Subgroup analyses were conducted to explore differences in infection rates between CAR T and BsAb therapies. Given the potential for differential toxicity between CAR T products, analyses were also performed between BsAbs and CAR T products with CD28 and 4-1BB costimulatory domains. Given the potential impact of the COVID-19 pandemic on infection-related mortality rates during the study period, infection-related mortality analyses were performed both including and excluding COVID-19-related deaths. Given the potential for differential toxicity between BsAbs with different dosing schedules (ie, fixed duration vs continuous), subgroup analyses were also performed comparing infection rates between fixed-duration BsAbs, continuous-therapy BsAbs, and CAR T. The fixed-duration BsAbs included mosunetuzumab (8 or 17 cycles) and glofitamab (maximum 12 cycles), while the continuously dosed BsAbs included epcoritamab and odronextamab (dosed until disease progression or toxicity). Small-study effects and publication bias were assessed using funnel plots and Egger’s test of asymmetry. Leave-one-out analyses were performed to assess robustness of findings. All statistical tests were 2-sided, and P values of <.05 were considered significant. All data analysis was conducted using R Version 4.2.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Literature search
Our initial database search revealed 4590 references. Search terms are included in the supplemental Methods. Of these, 1599 were determined to be duplicates and excluded. After further screening of study titles and abstracts, 51 studies remained. After further exclusion criteria as outlined in the Methods were applied, 25 studies were included in the meta-analysis, with a total of 15 CAR T studies and 10 BsAb studies comprising 3202 total patients (Figure 1).
Baseline study characteristics
Baseline study characteristics are summarized in Table 1. Studies included 4 phase 1/2 trials (results of phase 1 and phase 2 studies published separately), 11 phase 2 trials, 3 phase 3 trials, and 2 seamless trials, with publication years ranging from 2017 to 2024. Of the 15 CAR T trials, 6 studied CD28 costimulatory domain-based CAR T products, including 5 axicabtagene ciloleucel studies and 1 brexucabtagene autoleucel study. The remaining 9 CAR T trials studied 4-1BB costimulatory domain-based CAR T products, including 3 studies of tisagenlecleucel and 6 studies of lisocabtagene maraleucel. Of the 10 BsAb trials, 5 studied BsAbs on a fixed-duration regimen (mosunetuzumab and glofitamab), while the other 5 studied continuous dosing (epcoritamab and odronextamab).
Baseline characteristics of included studies
| Trial name . | Authors (trial identifier) . | Phase . | Regimen . | No. . | Median age, y . | B-NHL type . | Stage, N . | Median no. of prior therapies . | Prior CAR T (no.) . | Median follow-up, mo . |
|---|---|---|---|---|---|---|---|---|---|---|
| CAR T | ||||||||||
| ZUMA-113-15 | Locke et al (NCT02348216) | 2 | Axi-cel | 108 | 58 | R/R LBCL | III-IV 86 | 3 | — | 27.1 |
| ZUMA-216,17 | Wang et al (NCT02601313) | 2 | Brexu-cel | 68 | 65 | R/R MCL | III NR IV 58 | 3 | — | 35.6 |
| ZUMA-518,19 | Neelapu et al (NCT03105336) | 2 | Axi-cel | 152 | 60 | R/R FL | III-IV 139 | 3 | — | 41.7 |
| ZUMA-720,21 | Westin et al (NCT03391466) | 3 | Axi-cel | 170 | 58 | R/R LBCL | III-IV 139 | 1 | — | 47.2 |
| ZUMA-1222 | Neelapu et al (NCT03761056) | 2 | Axi-cel | 40 | 61 | LBCL | III-IV 38 | 0 | — | 17.4 |
| ALYCANTE23 | Houot et al (NCT04531046) | 2 | Axi-cel | 62 | 70 | R/R LBCL | III-IV 46 | 1 | — | 12 |
| JULIET24,25 | Schuster et al (NCT02445248) | 2 | Tisa-cel | 115 | 56 | R/R LBCL | III-IV 88 | 3 | — | 40.3 |
| BELINDA26 | Bishop et al (NCT03570892) | 3 | Tisa-cel | 155 | 59.5 | R/R LBCL | III-IV 107 | 1 | — | 10.0 |
| ELARA27,28 | Dreyling et al (NCT03568461) | 2 | Tisa-cel | 97 | 57 | R/R FL | III-IV 83 | 4 | — | 29 |
| TRANSCEND29,30 | Abramson et al (NCT03435796) | Seamless | Liso-cel | 270 | 63 | R/R LBCL | NR | 3 | — | 19.9 |
| TRANSCEND FL31 | Morschhauser et al (NCT04245839) | 2 | Liso-cel | 130 | 60 | R/R FL | III-IV 112 | 2 | — | 18.9 |
| TRANSCEND MCL32 | Wang et al (NCT02631044{) | Seamless | Liso-cel | 88 | 68.5 | R/R MCL | NR | 3 | — | 16.1 |
| TRANSFORM33,34 | Abramson et al (NCT03575351) | 3 | Liso-cel | 92 | 60 | R/R LBCL | III-IV 68 | 1 | — | 17.5 |
| OUTREACH35 | Linhares et al (NCT03744676) | 2 | Liso-cel | 82 | 66 | R/R LBCL | III-IV 61 | 2 | — | 10.6 |
| PILOT36 | Sehgal et al (NCT03483103) | 2 | Liso-cel | 61 | 74 | R/R LBCL | NR | 1 | — | 12.3 |
| BsAbs | ||||||||||
| NR37 | Dickinson et al (NCT03075696) | 1/2 | Glofitamab | 154 | 66 | R/R LBCL | III-IV 116 | 3 | 51 | 12.6 |
| NR38 | Phillips et al (NCT03075696) | 1/2 | Glofitamab | 60 | 72.0 | R/R MCL | III-IV 52 | 2 | 2 | 19.6 |
| NR39 | Hutchings et al (NCT03075696) | 1/2 | Glofitamab | 171 | 64 | R/R B-NHL | III-IV 133 | 3 | 3 | 13.5 |
| EPCORE-NHL340 | Izutsu et al (NCT04542824) | 1/2 | Epcoritamab | 36 | 68.5 | R/R LBCL | III-IV 28 | 3 | 2 | 8.4 |
| EPCORE NHL-141,42 | Thieblemont (NCT03625037) | 1/2 | Epcoritamab | 157 | 64.0 | R/R LBCL | III-IV 118 | 3.0 | 61 | 25.1 |
| EPCORE NHL-143 | Linton et al (optimization∗) (NCT03625037) | 1/2 | Epcoritamab | 86 | 64 | R/R FL | III-IV 79 | 2 | 6 | 5.7 |
| EPCORE NHL-143 | Linton et al (pivotal) (NCT03625037)∗ | 1/2 | Epcoritamab | 128 | 65 | R/R FL | III-IV 109 | 3 | 6 | 17.4 |
| NR44-47 | Matasar et al (NCT02500407) | 1/2 | Mosunetuzumab | 218 | 64 | R/R FL, LBCL, Richters, MCL, other∗ | NR | 3 | 30 | 14 |
| NR48,49 | Budde et al (NCT02500407) | 1/2 | Mosunetuzumab | 229 | 60-64† | R/R LBCL, FL, MCL, Richters, other | III-IV 136 | 3 | 19 | 42 |
| ELM-250 | Kim et al (NCT03888105) | 2 | Odronextamab | 128 | 61.0 | R/R FL | III-IV 109 | 3 | 0 | 20.1 |
| ELM-151 | Bannerji et al (NCT02290951) | 1 | Odronextamab | 145 | 67 | R/R LBCL, FL, MCL, MZL | III-IV 123 | 3 | 42 | 4.2 |
| Trial name . | Authors (trial identifier) . | Phase . | Regimen . | No. . | Median age, y . | B-NHL type . | Stage, N . | Median no. of prior therapies . | Prior CAR T (no.) . | Median follow-up, mo . |
|---|---|---|---|---|---|---|---|---|---|---|
| CAR T | ||||||||||
| ZUMA-113-15 | Locke et al (NCT02348216) | 2 | Axi-cel | 108 | 58 | R/R LBCL | III-IV 86 | 3 | — | 27.1 |
| ZUMA-216,17 | Wang et al (NCT02601313) | 2 | Brexu-cel | 68 | 65 | R/R MCL | III NR IV 58 | 3 | — | 35.6 |
| ZUMA-518,19 | Neelapu et al (NCT03105336) | 2 | Axi-cel | 152 | 60 | R/R FL | III-IV 139 | 3 | — | 41.7 |
| ZUMA-720,21 | Westin et al (NCT03391466) | 3 | Axi-cel | 170 | 58 | R/R LBCL | III-IV 139 | 1 | — | 47.2 |
| ZUMA-1222 | Neelapu et al (NCT03761056) | 2 | Axi-cel | 40 | 61 | LBCL | III-IV 38 | 0 | — | 17.4 |
| ALYCANTE23 | Houot et al (NCT04531046) | 2 | Axi-cel | 62 | 70 | R/R LBCL | III-IV 46 | 1 | — | 12 |
| JULIET24,25 | Schuster et al (NCT02445248) | 2 | Tisa-cel | 115 | 56 | R/R LBCL | III-IV 88 | 3 | — | 40.3 |
| BELINDA26 | Bishop et al (NCT03570892) | 3 | Tisa-cel | 155 | 59.5 | R/R LBCL | III-IV 107 | 1 | — | 10.0 |
| ELARA27,28 | Dreyling et al (NCT03568461) | 2 | Tisa-cel | 97 | 57 | R/R FL | III-IV 83 | 4 | — | 29 |
| TRANSCEND29,30 | Abramson et al (NCT03435796) | Seamless | Liso-cel | 270 | 63 | R/R LBCL | NR | 3 | — | 19.9 |
| TRANSCEND FL31 | Morschhauser et al (NCT04245839) | 2 | Liso-cel | 130 | 60 | R/R FL | III-IV 112 | 2 | — | 18.9 |
| TRANSCEND MCL32 | Wang et al (NCT02631044{) | Seamless | Liso-cel | 88 | 68.5 | R/R MCL | NR | 3 | — | 16.1 |
| TRANSFORM33,34 | Abramson et al (NCT03575351) | 3 | Liso-cel | 92 | 60 | R/R LBCL | III-IV 68 | 1 | — | 17.5 |
| OUTREACH35 | Linhares et al (NCT03744676) | 2 | Liso-cel | 82 | 66 | R/R LBCL | III-IV 61 | 2 | — | 10.6 |
| PILOT36 | Sehgal et al (NCT03483103) | 2 | Liso-cel | 61 | 74 | R/R LBCL | NR | 1 | — | 12.3 |
| BsAbs | ||||||||||
| NR37 | Dickinson et al (NCT03075696) | 1/2 | Glofitamab | 154 | 66 | R/R LBCL | III-IV 116 | 3 | 51 | 12.6 |
| NR38 | Phillips et al (NCT03075696) | 1/2 | Glofitamab | 60 | 72.0 | R/R MCL | III-IV 52 | 2 | 2 | 19.6 |
| NR39 | Hutchings et al (NCT03075696) | 1/2 | Glofitamab | 171 | 64 | R/R B-NHL | III-IV 133 | 3 | 3 | 13.5 |
| EPCORE-NHL340 | Izutsu et al (NCT04542824) | 1/2 | Epcoritamab | 36 | 68.5 | R/R LBCL | III-IV 28 | 3 | 2 | 8.4 |
| EPCORE NHL-141,42 | Thieblemont (NCT03625037) | 1/2 | Epcoritamab | 157 | 64.0 | R/R LBCL | III-IV 118 | 3.0 | 61 | 25.1 |
| EPCORE NHL-143 | Linton et al (optimization∗) (NCT03625037) | 1/2 | Epcoritamab | 86 | 64 | R/R FL | III-IV 79 | 2 | 6 | 5.7 |
| EPCORE NHL-143 | Linton et al (pivotal) (NCT03625037)∗ | 1/2 | Epcoritamab | 128 | 65 | R/R FL | III-IV 109 | 3 | 6 | 17.4 |
| NR44-47 | Matasar et al (NCT02500407) | 1/2 | Mosunetuzumab | 218 | 64 | R/R FL, LBCL, Richters, MCL, other∗ | NR | 3 | 30 | 14 |
| NR48,49 | Budde et al (NCT02500407) | 1/2 | Mosunetuzumab | 229 | 60-64† | R/R LBCL, FL, MCL, Richters, other | III-IV 136 | 3 | 19 | 42 |
| ELM-250 | Kim et al (NCT03888105) | 2 | Odronextamab | 128 | 61.0 | R/R FL | III-IV 109 | 3 | 0 | 20.1 |
| ELM-151 | Bannerji et al (NCT02290951) | 1 | Odronextamab | 145 | 67 | R/R LBCL, FL, MCL, MZL | III-IV 123 | 3 | 42 | 4.2 |
Axi-cel, axicabtagene ciloleucel; Brexu-cel, brexucabtagene autoleucel; FL, follicular lymphoma; LBCL, large B-cell lymphoma; Liso-cel, lisocabtagene maraleucel; MCL, mantle cell lymphoma; MZL, marginal zone lymphoma; NR, Not reported; Tiso-cel, tisagenlecleucel.
‡Median ages were reported separately for 3 different cohorts.
Single publication with 2 cohorts represented and analyzed separately.
One melanoma patient was mistakenly enrolled and treated with mosunetuzumab.
Trials included patients with LBCL, R/R LBCL, R/R mantle cell lymphoma, and R/R follicular lymphoma. The median number of prior therapies was 3 in 14 of the trials.
The incidences of observed infections in each trial are summarized in Table 2.
Infection rates by study
| Study . | Total patients . | All-grade infection . | Grade 3+ infections . | Infection-related mortality . |
|---|---|---|---|---|
| CAR T | ||||
| Locke et al13-15 | 108 | 49 | 30 | 6 |
| Wang et al16,17 | 68 | 45 | 26 | 3 |
| Neelapu et al (2024)18,19 | 152 | 100 | 34 | 14 |
| Westin et al20,21 | 170 | 76 | 28 | 14 |
| Neelapu et al (2022)22 | 40 | 13 | 5 | 1 |
| Houot et al23 | 62 | 33 | 11 | 6 |
| Schuster et al24,25 | 115 | 43 | 22 | 0 |
| Bishop et al26 | 155 | NR | NR | 5 |
| Dreyling et al27,28 | 97 | 16 | 9 | 1 |
| Abramson et al (2024)29,30 | 270 | 135 | 45 | 8 |
| Morschhauser et al31 | 130 | NR | 10 | 3 |
| Wang et al32 | 88 | NR | 13 | 8 |
| Abramson et al (2023)33,34 | 92 | NR | 14 | 4 |
| Linhares et al35 | 82 | 27 | 9 | 3 |
| Sehgal et al36 | 61 | NR | 4 | 3 |
| BsAbs | ||||
| Dickinson et al37 | 154 | 59 | 23 | 7 |
| Phillips et al38 | 60 | 44 | 13 | 9 |
| Hutchings et al39 | 171 | 88 | 30 | 1 |
| Izutsu et al40 | 36 | 16 | 7 | 0 |
| Thieblemont et al41,42 | 157 | 71 | 46 | 6 |
| Linton et al (optimization)43 | 86 | NR | NR | 0 |
| Linton et al (pivotal)43 | 128 | NR | NR | 8 |
| Budde et al48,49 | 229 | NR | NR | 3 |
| Kim et al50 | 128 | 102 | 54 | 14 |
| Bannerji et al51 | 145 | 71 | 33 | 3 |
| Matasar et al44-47 | 218 | 102 | 33 | 3 |
| Study . | Total patients . | All-grade infection . | Grade 3+ infections . | Infection-related mortality . |
|---|---|---|---|---|
| CAR T | ||||
| Locke et al13-15 | 108 | 49 | 30 | 6 |
| Wang et al16,17 | 68 | 45 | 26 | 3 |
| Neelapu et al (2024)18,19 | 152 | 100 | 34 | 14 |
| Westin et al20,21 | 170 | 76 | 28 | 14 |
| Neelapu et al (2022)22 | 40 | 13 | 5 | 1 |
| Houot et al23 | 62 | 33 | 11 | 6 |
| Schuster et al24,25 | 115 | 43 | 22 | 0 |
| Bishop et al26 | 155 | NR | NR | 5 |
| Dreyling et al27,28 | 97 | 16 | 9 | 1 |
| Abramson et al (2024)29,30 | 270 | 135 | 45 | 8 |
| Morschhauser et al31 | 130 | NR | 10 | 3 |
| Wang et al32 | 88 | NR | 13 | 8 |
| Abramson et al (2023)33,34 | 92 | NR | 14 | 4 |
| Linhares et al35 | 82 | 27 | 9 | 3 |
| Sehgal et al36 | 61 | NR | 4 | 3 |
| BsAbs | ||||
| Dickinson et al37 | 154 | 59 | 23 | 7 |
| Phillips et al38 | 60 | 44 | 13 | 9 |
| Hutchings et al39 | 171 | 88 | 30 | 1 |
| Izutsu et al40 | 36 | 16 | 7 | 0 |
| Thieblemont et al41,42 | 157 | 71 | 46 | 6 |
| Linton et al (optimization)43 | 86 | NR | NR | 0 |
| Linton et al (pivotal)43 | 128 | NR | NR | 8 |
| Budde et al48,49 | 229 | NR | NR | 3 |
| Kim et al50 | 128 | 102 | 54 | 14 |
| Bannerji et al51 | 145 | 71 | 33 | 3 |
| Matasar et al44-47 | 218 | 102 | 33 | 3 |
NR, not reported.
Incidence of infections per patient analysis
All-grade infections
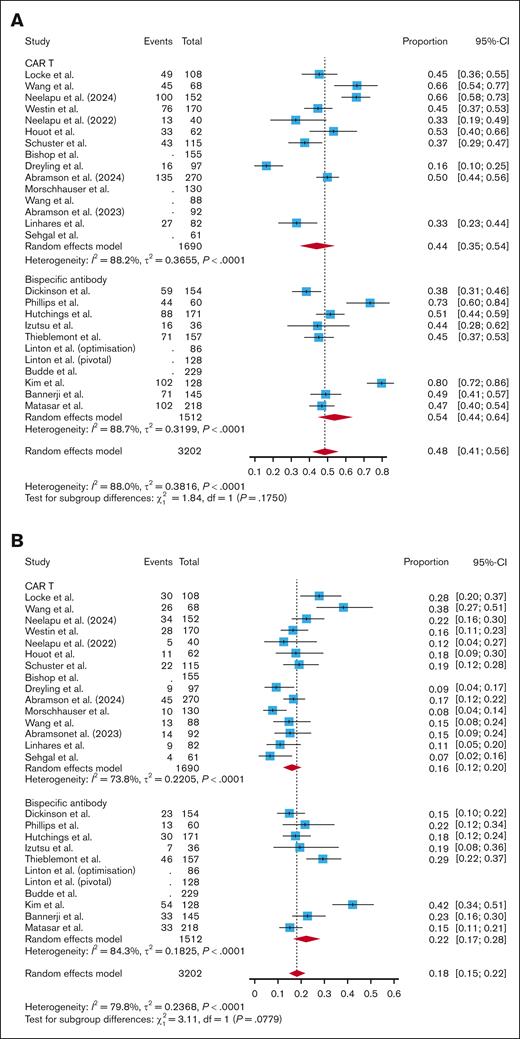
Across 15 CAR T studies, the pooled incidence of all-grade infections per patient was 0.44 (95% CI, 0.35-0.54; I2 = 88.2%). Across 10 BsAb studies, the pooled incidence of all-grade infections was 0.54 (95% CI, 0.44-0.64; I2 = 88.7%). Subgroup analyses showed no significant difference in incidence of all-grade infection between all CAR T studies and BsAb studies (P = .18; Figure 2A). When breaking out CAR T products by costimulatory domain, there was no significant difference in all-grade infections in CD28-based CAR T studies vs BsAb studies (P = .77; supplemental Figure 1). However, BsAb studies had a significantly higher pooled incidence of all-grade infections compared with 4-1BB–based CAR T studies (0.54 vs 0.34; P = .02; supplemental Figure 1). There was no significant difference in incidence of all-grade infections when comparing fixed-duration vs continuous-therapy BsAbs (0.52 vs 0.56; P = .70), CAR T vs fixed-duration BsAbs (0.44 vs 0.46; P = .33), or CAR T vs continuous-therapy BsAbs (0.44 vs 0.47; P = .22; supplemental Figure 2).
Pooled infection rate per patient. A subgroup analysis comparing the incidence of all-grade infections (A), grade 3+ infections (B), and infection-related mortality (C) per patient between CAR T and BsAbs.
Pooled infection rate per patient. A subgroup analysis comparing the incidence of all-grade infections (A), grade 3+ infections (B), and infection-related mortality (C) per patient between CAR T and BsAbs.
Grade 3+ infections
Across 15 CAR T studies, the pooled incidence of grade 3+ infections per patient was 0.16 (95% CI, 0.12-0.20; I2 = 73.8%). Across 10 studies of BsAbs, the pooled incidence of grade 3+ infections was 0.22 (95% CI, 0.17-0.28, I2 = 84.3%). Subgroup analyses showed no significant difference between grade 3+ infection rates with CAR T vs BsAb therapy, though the relationship approached significance with a trend toward higher grade 3+ infections with BsAbs (P = .08; Figure 2B). There was no significant difference in grade 3+ infections between CD28-based CAR T and BsAbs (P = .98; supplemental Figure 3). BsAbs had a significantly higher incidence of grade 3+ infections compared with 4-1BB–based CAR T (0.22 vs 0.13; P = .003; supplemental Figure 3). Continuous-therapy BsAbs had a significantly higher incidence of grade 3+ infections than fixed-duration BsAbs (0.29 vs 0.16; P = .002; supplemental Figure 4). While there was no difference in the incidence of grade 3+ infections when comparing fixed-duration BsAbs with CAR T (0.16 vs 0.16; P = .85; supplemental Figure 4), continuous-therapy BsAbs did have a higher incidence of grade 3+ infections than CAR T (0.29 vs 0.16; P = .003; supplemental Figure 4).
Infection-related mortality (grade 5 infection)
Across 15 CAR T studies, the pooled incidence of infection-related mortality per patient was 0.04 (95% CI, 0.03-0.06; I2 = 36.2%). Across 10 BsAb studies, the pooled incidence of infection-related mortality was 0.03 (95% CI, 0.01-0.05; I2 = 73.9%). Subgroup analyses showed no difference in infection-related mortality between all CAR T products and BsAbs (P = .26; Figure 2C). Even when only assessing infection-related mortality not related to COVID, there was no significant difference between CAR T and BsAbs (0.02 vs 0.02; P = .22; supplemental Figure 5). When breaking out CAR T product by costimulatory domain, CD28-based CAR T had a significantly higher incidence of infection-related mortality compared with BsAbs (0.07 vs 0.03; P = .010; supplemental Figure 6). There was no difference in infection-related mortality per patient when comparing 4-1BB–based CAR T with BsAbs (P = .99; supplemental Figure 6). There was no difference in infection-related mortality per patient when comparing fixed-duration and continuous BsAbs (0.02 vs 0.03; P = .78), CAR T and fixed-duration BsAbs (0.04 vs 0.02; P = .35), and CAR T and continuous-therapy BsAbs (0.04 vs 0.03; P = .52; supplemental Figure 7).
Infection rates per patient-month
All-grade infections
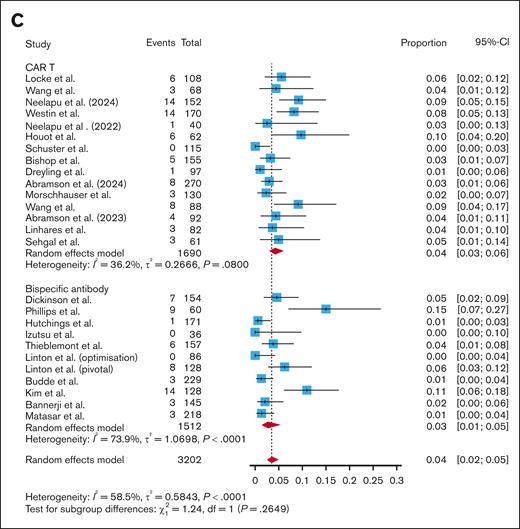
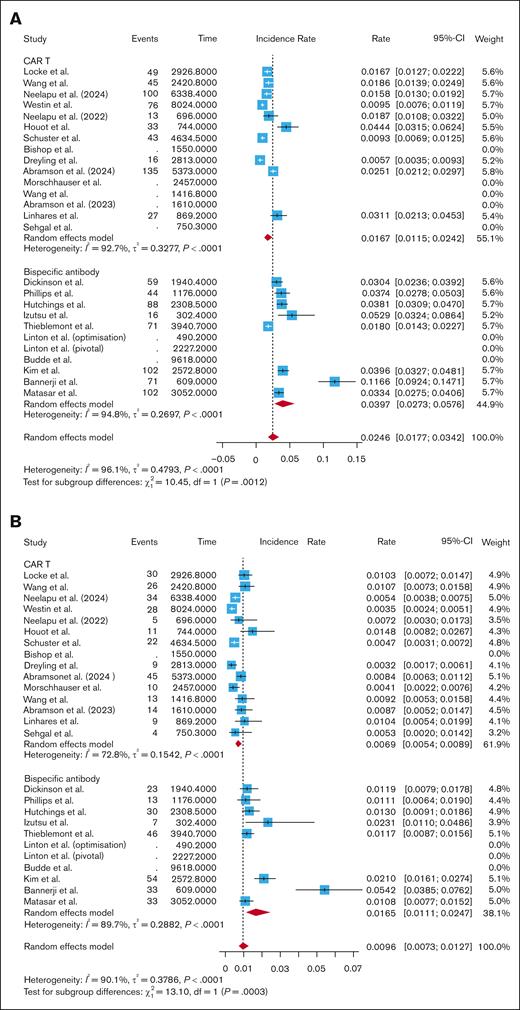
After standardizing by follow-up time, the pooled rate of all-grade infections for CAR T studies was 0.0167 infections per patient-month (95% CI, 0.0115-0.0242; I2 = 92.7%). The pooled rate of all-grade infections for BsAb studies was 0.0397 infections per patient-month (95% CI, 0.0273-0.0576; I2 = 94.8%). Subgroup analysis revealed a significantly higher rate of all-grade infections per patient-month with BsAbs compared with CAR T (P = .0012; Figure 3A). After breaking out CAR T by subtype, BsAbs had a significantly higher rate of all-grade infections per patient-month compared with CD28-based CAR T (0.0397 vs 0.0182; P = .006; supplemental Figure 8), and compared with 4-1BB–based CAR T (0.0397 vs 0.0144; P = .02; supplemental Figure 8). There was no difference in all-grade infections per patient-month when comparing fixed-duration and continuous-therapy BsAbs (0.0347 vs 0.0457; P = .49; supplemental Figure 9). However, the rate of all-grade infections per patient-month was significantly higher in those who received fixed-dose BsAbs compared with CAR T (0.0347 vs 0.0167; P = .0002; supplemental Figure 9). The rate was also higher in those who received continuous-therapy BsAbs vs CAR T (0.0457 vs 0.0167; P = .02; supplemental Figure 9).
Pooled infection rate per patient-month. A subgroup analysis comparing pooled all-grade infections (A), grade 3+ infections (B), and infection-related mortality (C) per patient-month between CAR T studies and BsAbs.
Pooled infection rate per patient-month. A subgroup analysis comparing pooled all-grade infections (A), grade 3+ infections (B), and infection-related mortality (C) per patient-month between CAR T studies and BsAbs.
Grade 3+ infections
The pooled rate of grade 3+ infections per patient-month was 0.0069 infections per patient-month (95% CI, 0.0054-0.0089; I2 = 72.8%) for CAR T studies vs 0.0165 infections per-patient-month (95% CI, 0.0111-0.0247; I2 = 89.7%) for BsAb studies. Subgroup analysis showed that BsAbs had significantly higher grade 3+ infections per patient-month than CAR T (P = .0003; Figure 3B). BsAbs had a higher rate of grade 3+ infections per patient-month compared with CD28-based CAR T (0.0165 vs 0.0076; P = .01; supplemental Figure 9). BsAbs also had a significantly higher rate of grade 3+ infections per patient-month compared with 4-1BB–based CAR T (0.0165 vs 0.0064; P = .0002; supplemental Figure 10). There were more grade 3+ infections per patient-month with continuous-therapy BsAbs than fixed-duration BsAbs (0.0235 vs 0.0117; P < .05; supplemental Figure 11). Fixed-duration BsAbs had a higher rate of grade 3+ infections per patient-month than CAR T (0.0117 vs 0.0069; P = .001; supplemental Figure 11). Continuous-therapy BsAbs also had a higher rate of grade 3+ infections per patient-month than CAR T (0.0235 vs 0.0069; P = .0006; supplemental Figure 11).
Infection-related mortality
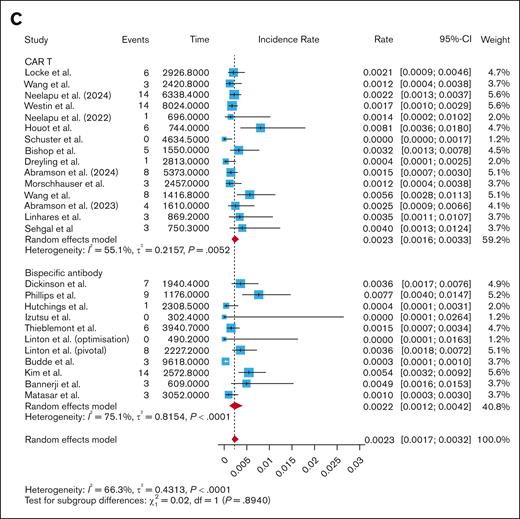
The CAR T studies had a pooled infection-related mortality rate of 0.0023 infections per patient-month (95% CI, 0.0016-0.0033; I2 = 55.1%), while the BsAb studies had a pooled infection-related mortality rate of 0.0022 infections per patient-month (95% CI, 0.0012-0.0042; I2 = 75.1%). Subgroup analysis showed no difference in infection-related mortality per patient-month when comparing CAR T and BsAbs (P = .89; Figure 3C), CD28-based CAR T and BsAbs (P = .89; supplemental Figure 11), and 4-1BB–based CAR T and BsAbs (P = .96; supplemental Figure 12). Even when removing COVID-related deaths from the analysis, there was no difference in infection-related mortality per patient-month between CAR T and BsAbs (0.0015 vs 0.0011; P = .31; supplemental Figure 5). There was no difference in infection-related mortality per patient-month when comparing fixed-duration with continuous-therapy BsAbs (0.0015 vs 0.0033; P = .24; supplemental Figure 13), nor when comparing fixed-duration BsAbs with CAR T (0.0015 vs 0.0023; P = .48; supplemental Figure 13) or continuous-therapy BsAbs with CAR T (0.0033 vs 0.0023; P = .29; supplemental Figure 13).
Table 3 summarizes the results of the infection rate per patient and infection rate per patient-month analyses for convenient comparison.
The results of the infection per patient analysis and the infection rate per patient-month analysis
| Infection type . | Product . | Pooled infection rate per patient . | P value . | Pooled infection rate per patient-month . | P value . |
|---|---|---|---|---|---|
| All-grade infections | CAR T | 0.44 | .18 | 0.0167 | .0012∗ |
| CD28 CAR T | 0.52 | .77 | 0.0182 | .006∗ | |
| 4-1BB CAR T | 0.34 | .02∗ | 0.0144 | .02∗ | |
| BsAbs | 0.54 | — | 0.0397 | — | |
| Grade 3+ infections | CAR T | 0.16 | .08 | 0.0069 | .0003∗ |
| CD28 CAR T | 0.22 | .98 | 0.0076 | .01∗ | |
| 4-1BB CAR T | 0.13 | .003∗ | 0.0064 | .0002∗ | |
| BsAbs | 0.22 | — | 0.0165 | — | |
| Infection-related mortality | CAR T | 0.04 | .26 | 0.0023 | .89 |
| CD28 CAR T | 0.07 | .01∗ | 0.0024 | .89 | |
| 4-1BB CAR T | 0.03 | .76 | 0.0023 | .96 | |
| BsAbs | 0.03 | — | 0.0022 | — |
| Infection type . | Product . | Pooled infection rate per patient . | P value . | Pooled infection rate per patient-month . | P value . |
|---|---|---|---|---|---|
| All-grade infections | CAR T | 0.44 | .18 | 0.0167 | .0012∗ |
| CD28 CAR T | 0.52 | .77 | 0.0182 | .006∗ | |
| 4-1BB CAR T | 0.34 | .02∗ | 0.0144 | .02∗ | |
| BsAbs | 0.54 | — | 0.0397 | — | |
| Grade 3+ infections | CAR T | 0.16 | .08 | 0.0069 | .0003∗ |
| CD28 CAR T | 0.22 | .98 | 0.0076 | .01∗ | |
| 4-1BB CAR T | 0.13 | .003∗ | 0.0064 | .0002∗ | |
| BsAbs | 0.22 | — | 0.0165 | — | |
| Infection-related mortality | CAR T | 0.04 | .26 | 0.0023 | .89 |
| CD28 CAR T | 0.07 | .01∗ | 0.0024 | .89 | |
| 4-1BB CAR T | 0.03 | .76 | 0.0023 | .96 | |
| BsAbs | 0.03 | — | 0.0022 | — |
The P values are taken from the subgroup analyses described in the text comparing all CAR T, CD28-based CAR T, and 4-1BB–based CAR T with BsAbs.
Boldface values are statistically significant.
Sensitivity and bias analyses
In a sensitivity analysis using the “leave-one-out” method, no single study appeared to influence the overall pooled infection rates per patient and per patient-month (supplemental Tables 1-6). Funnel plots can be found in supplemental Figures 13-21. Our risk of bias assessment showed only 2 studies (Bishop et al26; Linton et al43 [optimization]) had a bias score of 2; all other studies had a bias score of <2.
Discussion
To our knowledge, this is the first systematic review and meta-analysis comparing infection rates between CAR T and BsAbs in patients with B-NHL. Using 15 studies with 1690 patients receiving CAR T and 10 studies with 1512 patients receiving BsAbs, we were able to determine that pooled rates of all-grade and grade 3+ infections were similar. However, when accounting for time and the longer duration of follow-up of CAR T studies by calculating rates per patient-month, we discovered a higher rate of all-grade and grade 3+ infections per patient-month in BsAb trials. In general, BsAbs appeared to carry a higher risk of infection when comparing directly with 4-1BB costimulatory domain CAR T (tisagenlecleucel/lisocabtagene maraleucel) compared with the CD28-based CAR T products (axicabtagene ciloleucel/brexucabtagene autoleucel). There was no difference between BsAbs and CAR T in infection-related mortality in either the per patient or per patient-month analysis. This may be because infection-related mortality after these therapies is rare, and there may not have been enough power to detect a difference even in this large sample size.
Infection is the leading cause of nonrelapse mortality for CAR T52 and for BsAbs.53 As these 2 modalities have become critical in the treatment of B-cell lymphomas, comparisons are inevitable. While CAR T is classically seen as a more effective but potentially more toxic treatment, the cumulative burden of prolonged treatment with BsAbs cannot be ignored, and may be a driver of increased infection rates when accounting for follow-up times. Indeed, our analysis demonstrated that continuously dosed BsAbs had significantly higher grade 3+ infection rates per patient and per patient-month compared with fixed-duration BsAbs and compared with CAR T, as well as higher all-grade infections per patient-month compared with CAR T. Thus, our findings showed that although BsAbs may be seen as a more tolerable treatment option with less toxicities upfront, prolonged dosing schedules may be associated with higher infection rates over time.
Furthermore, there is some evidence that infection risk from BsAbs is higher in the real world than what has been reported in the literature from clinical trials.6,53 Longer-term follow-up is needed to understand the true burden of infections from BsAbs, and to help determine what preventive strategies can be implemented to reduce this burden. Limited-duration regimens of BsAbs could be beneficial in mitigating the prolonged infection risk. Alternatively, more aggressive infection prophylaxis incorporating growth factors or immune globulin treatment may be warranted.
Our study comes with some limitations. In all studies, reporting on infectious etiologies is incomplete outside of severe and/or fatal infections. Furthermore, most studies do not report on changing infection incidence over time. One notable exception is the ELM-2 study, which seems to show a plateau of infection rate for both all-grade and grade 3+ infections by month 24, even for a continuous-dosing treatment strategy.50 Although our study was not able to capture these data, other studies have shown that for CAR T, bacterial infections pose the highest risk in the first month of treatment, and are followed by a low level of persistent risk of bacterial, viral, and fungal infections.54,55 For BsAb therapy, 1 study suggests that COVID-19 represents most fatal infections, followed by a relatively equal distribution of other viral, bacterial, or fungal causes.6
Many studies, particularly phase 1 dose-escalation studies, do not report the median duration of follow-up, and in some cases did not report infections outside of grade 5 events. This is in keeping with the evidence that clinical trials often report infections in an incomplete manner.56 The differences in study protocol may have some impact on infection reporting, particularly in the designation of infections as treatment-related or unrelated to intervention. In general, CAR T studies have a period of hospitalizations but relatively infrequent follow-up a few months after therapy, whereas BsAb trials have less inpatient time upfront but more regular follow-up, which may impact the accurate collection of infection rates. Furthermore, patients were censored upon disease progression or death, although there is likely no significant imbalance across studies. In many CAR T trials, all-grade infections are only counted during a prespecified reporting window, although there are more stringent criteria for reporting ≥grade 3 adverse events. As an example, the ZUMA studies incorporate an adverse event reporting window for the first 3 months after infusion, but report targeted serious adverse events, including infection, up to 24 months after infusion or until disease progression.13,16,18,20,22 Similar protocols or follow-up safety analyses are incorporated for the other studies to ensure that ≥grade 3 toxicities are reported accurately and for a long time.
The impact of the COVID-19 pandemic on infection rates and infection mortality cannot be understated, as many COVID infections and COVID deaths were reported in both CAR T and BsAb studies. Out of 76 total infection-related deaths in the CAR T studies, 31 (40.8%) were due to or related to COVID infection. Out of 54 total infection-related deaths in the BsAb studies, 31 (57.4%) were due to or related to COVID-19. Our analysis showed that removing these deaths from our infection-related mortality data did not have a significant impact on our findings. The earliest data cut-off date for all of these studies was February 2020,24 and the latest accrual start date was 19 March 2021,23 so all studies were analyzing COVID infection rate to some degree.
The effect of multiple prior lines of treatment cannot be elucidated due to small size of varying subgroups. Some patients in the BsAb trials had received CAR T as a prior line of therapy, which is described in Table 1. The potential compound infectious risk of the 2 therapies in sequence warrants further investigation. Subgroup analysis of subtype and stage of lymphoma cannot be done without individual patient data from the clinical trials. Our focus on prospective trials excluded the analysis of real-world and registry studies, which may also have led to some degree of underreporting. Bridging therapies and lymphodepletion prior to CAR T, and various premedications for both CAR T and BsAbs (ie, steroids, anti-CD20 monoclonal antibodies) are potential infectious confounders. Outside of these standard additions to regimens, we excluded studies that combined other agents with our target drugs to avoid these confounders as much as possible.
Many of the pooled CAR T and BsAbs analyses had substantial heterogeneity with I2 > 75%, and Egger’s test for some funnel plots was consistent with small-study or publication bias. This may be due to differences in patient population and disease types, variability in products and regimens, and differences in reporting. Despite this, the leave-one-out analyses showed that no single study significantly influenced our results.
In conclusion, patients with B-NHL treated with either CAR T or BsAb therapy face a substantial risk of infection related to impaired humoral immunity from targeting CD19 or CD20 on B cells. The continuous dosing of BsAbs may lead to a longer duration of increased risk of infection.
Authorship
Contribution: H.v.B. and S.Y. designed the research; H.v.B., S.Y., and M.D. contributed data; H.v.B., N.E., and S.Y. collected and interpreted the data; N.E. analyzed the data; N.E., H.v.B., M.D., M.P., T.S., J.L., J.B., P.M., and S.Y. wrote the manuscript; and all authors approved the final version of the manuscript.
Conflict-of-interest disclosure: J.L. declares consulting fees from AstraZeneca, BeiGene, Bristol Myers Squibb, Caribou, Eisai, Foresight, Genentech, Grail, Kyowa Kirin, Novartis, Ono, Pfizer, Regeneron, Sail Biomedicines, Teva, and Treeline; and research funding from the National Institutes of Health, Genentech Foundation, and Leukemia & Lymphoma Society. P.M. declares consulting fees from AbbVie, AstraZeneca, BeiGene, Genentech, Janssen, Merck, and PeproMene. S.Y. declares membership on the advisory board for and consulting fees and travel fees from Bristol Myers Squibb; membership on the advisory board for and consultancy fees from Kite Pharma; and membership on the advisory board for AbbVie and Genmab. The remaining authors declare no competing financial interests.
Correspondence: Samuel Yamshon, Weill Cornell Medicine, 520 E 70th St, Starr 3, New York, NY 10021; email: sjy9001@med.cornell.edu.
References
Author notes
H.v.B. and N.E. contributed equally to this work and are joint first authors.
The data used in this study were extracted from the primary sources cited in this article. Original data are available on request from the corresponding author, Samuel Yamshon (sjy9001@med.cornell.edu).
The full-text version of this article contains a data supplement.