Key Points
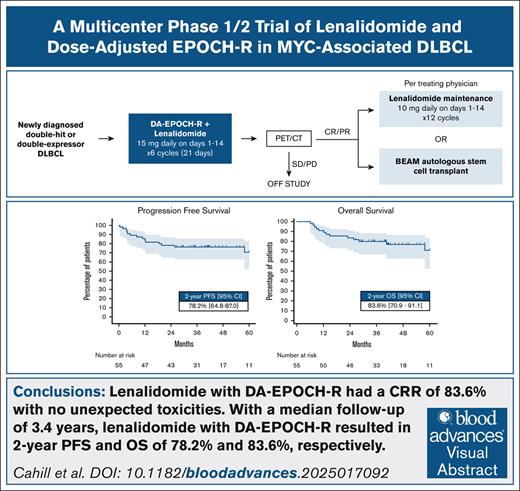
LEN with DA–EPOCH-R had a complete response rate of 83.6% in patients with DHL and DEL with no unexpected toxicities.
With a median follow-up of 3.4 years, LEN with DA–EPOCH-R resulted in 2-year PFS and overall survival rates of 78.2% and 83.6%, respectively.
Visual Abstract
In patients with diffuse large B-cell lymphoma (DLBCL), concurrent deregulation of MYC and BCL2 confers inferior outcomes following R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone). DA–EPOCH-R (dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab) produces favorable results in patients with dual MYC and BCL2 rearrangement (double-hit lymphoma [DHL]), but there are limited prospective data in both DHL and DLBCL with dual protein overexpression of Myc and Bcl2 (double-expressor lymphoma [DEL]). Lenalidomide (LEN) may enhance the response in MYC-driven lymphomas, prompting this investigator-initiated multicenter phase 1/2 study evaluating LEN with DA–EPOCH-R in adults with newly diagnosed DHL and DEL. Fifty-five patients (DHL, 23; DEL, 32) were enrolled and treated. Patients had a median age of 65 years (range, 25-82), International Prognostic Index ≥3 in 69% (38/55), and stage III/IV in 91% (50/55). The overall response rate was 90.9%, with a complete response rate of 83.6%. With a median follow-up of 3.4 years, the primary end point efficacy criterion was met with 1- and 2-year progression-free survival (PFS) rates of 85.5% and 78.2%, respectively. The 2-year overall survival was 83.6%. The most common adverse events (grade ≥3) were neutropenia (67%), anemia (67%), thrombocytopenia (49%), and neutropenic fever (35%). There were no grade 5 events. Second primary malignancy occurred in 6 patients (11%). LEN with DA–EPOCH-R for patients with DEL and DHL has a high response rate, encouraging survival, and met the primary PFS efficacy criterion. A randomized trial of DA–EPOCH-R with and without LEN would be needed to determine the specific benefit of LEN. This trial was registered at www.ClinicalTrials.gov as #NCT02213913.
Introduction
As the most common lymphoma worldwide, diffuse large B-cell lymphoma (DLBCL) is characterized by significant clinical and biologic heterogeneity. Approximately two-thirds of patients are cured with standard R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) chemoimmunotherapy, but improved precision medicine approaches coupled with novel treatments are urgently needed for high-risk disease where a cure remains elusive. DLBCL with dual translocation of MYC and BCL2, also known as double-hit lymphoma (DHL), has inferior outcomes with R-CHOP based on retrospective studies.1,2 DHL represents 5% to 10% of DLBCL cases, and was recently renamed as high-grade B-cell lymphoma with MYC and BCL2 rearrangements in both the 2022 International Consensus Classification and World Health Organization classification systems.3,4 Immunohistochemical overexpression of the Myc and Bcl2 proteins without gene rearrangements, termed double-expressor lymphoma (DEL), occurs in ∼30% of DLBCL-not otherwise specified (NOS) cases, and is an adverse prognostic feature following treatment with R-CHOP.2,5,6 DA–EPOCH-R (dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab) is an intensified regimen with favorable outcomes in DHL based on retrospective analyses, but there are limited prospective data specifically in DHL or DEL.1,7-9
Lenalidomide (LEN) is an immunomodulatory drug with activity in relapsed/refractory DLBCL that has been successfully combined with R-CHOP, and shows particular promise in non-germinal center B-cell (non-GCB) or activated B-cell (ABC) DLBCL, which are subtypes that respond poorly to R-CHOP.10-13 While LEN has multiple direct and indirect antitumor effects, it may enhance the treatment response in MYC-driven lymphomas through inhibition of the interferon regulatory factor 4 (IRF4) and NF-κB pathway with consequent downregulation of MYC signaling.14-18 This investigator-initiated phase 1/2 study was therefore conducted to evaluate the feasibility, safety, and efficacy of combining LEN with DA–EPOCH-R in MYC-associated lymphomas, DHL and DEL. The phase 1 portion has previously been reported, and identified the recommended phase 2 dose of LEN to be 15 mg daily on days 1 to 14 per 21-day cycle with DA–EPOCH-R.19 Here, we report the final analysis with long-term follow-up of the phase 2 portion.
Methods
Patients
Eligible patients had newly diagnosed biopsy-proven DLBCL or high-grade B-cell lymphoma with evidence of DHL or DEL. DHL was defined as the presence of dual MYC and BCL2 and/or BCL6 translocations as determined by fluorescence in situ hybridization (FISH) analysis based on World Health Organization 2016 criteria.20 DEL was defined by ≥40% Myc staining by immunohistochemistry, and ≥50% staining for Bcl2 without respective dual gene rearrangements. Cell of origin was assigned using the Hans algorithm.21 Other eligibility criteria are included in the supplemental Materials. One cycle of anthracycline-based chemotherapy, radiotherapy, or steroids was allowed prior to enrollment in cases where urgent therapy was required. This investigator-initiated study was funded by Celgene, with LEN supplied.
Objectives
The primary objective of the phase 2 portion was to determine the 1- and 2-year progression-free survival (PFS) rates, defined as time from the start of treatment to progression or death (whichever occurred first). Secondary objectives included determining the overall response rate (ORR), defined as complete response (CR) plus partial response (PR) based on positron emission tomography/computed tomography after the end of induction.22 Other secondary end points were overall survival (OS; time from start of treatment to death), duration of response (time from CR or PR to progression or death), and adverse events (AEs) according to Common Terminology Criteria for Adverse Events (version 4.0). All AEs regardless of attribution were included.
Study design and treatment plan
In the phase 2 portion, patients were treated with the recommended phase 2 dose of LEN at 15 mg daily on days 1 to 14 per 21-day cycle with DA–EPOCH-R for up to 6 total cycles of treatment as previously described.23 Patients who received an initial cycle of anthracycline-based chemoimmunotherapy prior to enrolling were treated on protocol with DA–EPOCH-R and LEN for 5 cycles (6 total cycles of induction). Central nervous system (CNS) prophylaxis with intrathecal methotrexate (IT MTX) was given per treating physician discretion, but was not required. Thromboembolic prophylaxis with aspirin 81 mg daily, Pneumocystis pneumonia and herpes simplex virus prophylaxis, and growth factor support were required. Patients who achieved a CR or PR were treated with either high-dose chemotherapy with BEAM (BCNU/carmustine, etoposide, cytarabine, and melphalan) and autologous stem cell transplant, or LEN maintenance at 10 mg daily on days 1 to 14 of every 21-day cycle for 12 total cycles. This decision was made by the treating physician.
Statistics
Safety and efficacy end points were assessed after combining all phase 1 and phase 2 patients on an intention-to-treat basis. Data lock was 29 October 2024. Pre-specified primary evaluation of PFS was done with an optimal 2-stage design, and planned enrollment of at least 46 patients with up to 55 total. The addition of LEN to DA–EPOCH-R was hypothesized to increase the 1-year PFS from 40% (null) to ≥60% (alternative; α = 0.05 and β = 0.20). Rejection of the null hypothesis required 8 or more of the first 16 patients, and 24 or more of the first 46 patients to be alive and progression free at 1 year. Statistical analysis was performed using Stata software (College Station, TX). The Kaplan-Meier method was used for survival analysis, and survival curves were compared between groups with the log-rank test. P < .05 was considered statistically significant.
This study was approved by the institutional review board and registered at ClinicalTrials.gov as #NCT02213913. Participating sites were included through the University of Chicago Personalized Cancer Care Consortium. All participants provided written informed consent.
Results
Patient characteristics
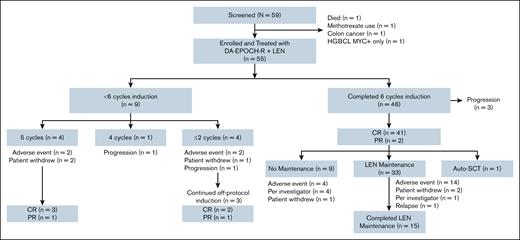
From December 2014 to February 2020, 55 patients were enrolled and treated in this phase 1/2 trial at 8 medical centers in the United States (Figure 1). Overall, patients had a median age of 65 years (range, 25-82). The International Prognostic Index (IPI) was ≥3 in 38 of 55 patients (69%); CNS-IPI ≥4 in 22 of 55 patients (40%); and stage III/IV in 50 of 55 patients (91%; Table 1). These characteristics were similar between patients with DEL and DHL, except for CNS-IPI, where 28% were ≥4 in patients with DEL vs 57% in patients with DHL (supplemental Table 1). Among 4 patients with stage II disease, 1 had bulky disease (12 cm pelvic mass), and the other 3 had extranodal involvement with sites involving breast, adnexa, and tongue. The patient with stage I disease had primary bone DLBCL with a mass in the femur. Thirty-two of 55 patients (58%) had DEL, and 23 of 55 (42%) had DHL. In the patients with DEL, 10 of 32 (31%) had GCB subtype, while 20 of 23 (87%) patients with DHL had GCB subtype. Three patients with DEL had a MYC rearrangement. Five of 32 patients (16%) in the DEL group did not have FISH testing available. Four of these 5 were non-GCB subtype, including 1 patient with Richter transformation, and 1 with Epstein-Barr virus-positive DLBCL. Twenty-two of 23 (96%) of the patients with DHL had MYC and BCL2 rearrangements, while 1 had MYC and BCL6 rearrangements.
Trial profile. Safety and efficacy intent-to-treat population includes the 55 patients enrolled in the study. One patient on screening had HGBCL with MYC rearrangement, but was negative for BCL2 by immunohistochemistry and FISH. HGBCL, high-grade B-cell lymphoma.
Trial profile. Safety and efficacy intent-to-treat population includes the 55 patients enrolled in the study. One patient on screening had HGBCL with MYC rearrangement, but was negative for BCL2 by immunohistochemistry and FISH. HGBCL, high-grade B-cell lymphoma.
Baseline patient characteristics
| Characteristics (N = 55) . | . |
|---|---|
| Median age (range), y | 65 (25-82) |
| Sex | |
| Female | 30/55 (55%) |
| Male | 25/55 (45%) |
| Race | |
| Asian | 2/55 (4%) |
| Black | 6/55 (11%) |
| White | 42/55 (76%) |
| Unknown | 5/55 (9%) |
| Ethnicity | |
| Hispanic | 5/55 (9%) |
| Stage | |
| I | 1/55 (2%) |
| II | 4/55 (7%) |
| III | 13/55 (24%) |
| IV | 37/55 (67%) |
| ECOG performance status | |
| 0-1 | 50/55 (91%) |
| 2 | 5/55 (9%) |
| IPI | |
| 0-1 (low) | 5/55 (9%) |
| 2 (low-intermediate) | 12/55 (22%) |
| 3 (high-intermediate) | 22/55 (40%) |
| 4-5 (high) | 16/55 (29%) |
| CNS-IPI | |
| 2-3 (intermediate) | 28/55 (51%) |
| 4-6 (high) | 22/55 (40%) |
| DEL | 32/55 (58%) |
| GCB | 10/32 (31%) |
| Non-GCB∗ | 22/32 (69%) |
| MYC and BCL2 or BCL6 rearrangements† | 0/27 (0%) |
| MYC rearrangement alone | 3/32 (9%) |
| DHL‡ | 23/55 (42%) |
| GCB | 20/23 (87%) |
| Non-GCB | 3/23 (13%) |
| MYC and BCL2 rearrangements | 22/23 (96%) |
| MYC and BCL6 rearrangements | 1/23 (4%) |
| Characteristics (N = 55) . | . |
|---|---|
| Median age (range), y | 65 (25-82) |
| Sex | |
| Female | 30/55 (55%) |
| Male | 25/55 (45%) |
| Race | |
| Asian | 2/55 (4%) |
| Black | 6/55 (11%) |
| White | 42/55 (76%) |
| Unknown | 5/55 (9%) |
| Ethnicity | |
| Hispanic | 5/55 (9%) |
| Stage | |
| I | 1/55 (2%) |
| II | 4/55 (7%) |
| III | 13/55 (24%) |
| IV | 37/55 (67%) |
| ECOG performance status | |
| 0-1 | 50/55 (91%) |
| 2 | 5/55 (9%) |
| IPI | |
| 0-1 (low) | 5/55 (9%) |
| 2 (low-intermediate) | 12/55 (22%) |
| 3 (high-intermediate) | 22/55 (40%) |
| 4-5 (high) | 16/55 (29%) |
| CNS-IPI | |
| 2-3 (intermediate) | 28/55 (51%) |
| 4-6 (high) | 22/55 (40%) |
| DEL | 32/55 (58%) |
| GCB | 10/32 (31%) |
| Non-GCB∗ | 22/32 (69%) |
| MYC and BCL2 or BCL6 rearrangements† | 0/27 (0%) |
| MYC rearrangement alone | 3/32 (9%) |
| DHL‡ | 23/55 (42%) |
| GCB | 20/23 (87%) |
| Non-GCB | 3/23 (13%) |
| MYC and BCL2 rearrangements | 22/23 (96%) |
| MYC and BCL6 rearrangements | 1/23 (4%) |
ECOG, Eastern Cooperative Oncology Group.
Of the patients with non-GCB DEL, 1 patient had Richter transformation, and another had Epstein-Barr virus-positive DLBCL.
There were 5 patients with DEL who did not have MYC and BCL2 FISH testing available.
Three patients with DHL had transformed follicular lymphoma.
Treatment
Results for the phase 1 portion (n = 15) have previously been reported, and 40 additional patients were treated with DA–EPOCH-R with LEN 15 mg in the phase 2 portion (n = 55).19 For the patients in phase 1, 3 received LEN 10 mg, 7 received LEN 15 mg, and 5 received LEN 20 mg. No patients received LEN 25 mg as there were 2 patients with dose-limiting toxicity (DLT) at LEN 20 mg dose level (DL), thereby establishing 15 mg as the maximum tolerated dose and recommended phase 2 dose. Prior to enrollment, 19 of 55 patients (35%) received 1 cycle of R-CHOP or DA–EPOCH-R, and 2 patients received urgent palliative radiation. The diagnosis-to-treatment interval was median 21 days (range, 2-55). Thirty-four patients (62%) received CNS prophylaxis with IT MTX. Patients received a median of 6 cycles of induction (range, 1-6). Specifically, 46 of 55 patients (84%) received 6 cycles of induction, 4 of 55 patients (7%) received 5 cycles (2 had AEs and 2 withdrew), 1 of 55 patients (2%) received 4 cycles then had CNS progression, and 4 of 55 patients (7%) received ≤2 cycles (2 had an AE, 1 withdrew, and 1 had disease progression). The median DL for DA–EPOCH-R reached per patient was DL 2 (range, 1-4). Thirty-three of the 43 (77%) who completed 6 cycles of induction with a treatment response of CR or PR received LEN maintenance for a median of 10 cycles (range, 1-12); 15 of these 33 (46%) completed full maintenance. For the 10 patients who did not go on to maintenance, the reasons included AEs in 4 (cytopenias in 3 and colitis in 1), investigator decision in 4, 1 patient withdrew, and 1 patient received consolidative high-dose chemotherapy with BEAM followed by autologous stem cell transplant (auto-SCT). The most common reason for stopping maintenance early (n = 18) was AEs in 14 patients (10 with cytopenias, 2 with febrile neutropenia, 1 with sepsis, and 1 with mucositis).
Efficacy
The ORR was 90.9% (50/55), with a CR rate of 83.6% (46/55; Table 2). With a median follow-up of 3.4 years (range, 0.5-7.6), the primary end point was met with 1-year PFS of 85.5% [95% confidence interval (CI), 73-92.5] and 2-year PFS of 78.2% [95% CI, 64.8-87] in the intention-to-treat population (n = 55; Figure 2). Median PFS was not reached. The 2-year OS was 83.6% [95% CI, 70.9-91.1], and median OS was not reached. The 2-year duration of response was 84% [95% CI, 70.5-91.7], with median not yet reached. The 2-year PFS for patients with DEL and DHL was 84.4% [95% CI, 66.5-93.2] and 69.6% [95% CI, 46.6-84.2], respectively (P = .40). The 2-year OS for patients with DEL and DHL was 87.5% [95% CI, 70-95.1] and 78.3% [95% CI, 55.4-90.3], respectively (P = .59). For patients with non-GCB DLBCL, the ORR was 96% (24/25), with CR rate of 92% (23/25). The 2-year PFS and OS for patients with the non-GCB subtype were both 84% [95% CI, 62.8-93.7]. For patients with GCB DLBCL, the ORR was 86.7% (26/30), with a CR rate 23/30 (76.7%). The 2-year PFS and OS for patients with the GCB subtype were 73.3% [95% CI, 53.7-85.7] and 83.3 [95% CI, 64.5-92.7], respectively. There was no significant difference in PFS or OS for the responders who received maintenance (n = 33) compared with no maintenance (n = 10; supplemental Figure 1).
Induction treatment response
| Response . | All patients . | DEL . | DHL . |
|---|---|---|---|
| ORR | 90.9% (50/55) | 93.8% (30/32) | 87% (20/23) |
| CR | 83.6% (46/55) | 84.4% (27/32) | 82.6% (19/23) |
| PR | 7.3% (4/55) | 9.4% (3/32) | 4.3% (1/23) |
| PD | 9.1% (5/55) | 6.3% (2/32) | 13% (3/23) |
| Response . | All patients . | DEL . | DHL . |
|---|---|---|---|
| ORR | 90.9% (50/55) | 93.8% (30/32) | 87% (20/23) |
| CR | 83.6% (46/55) | 84.4% (27/32) | 82.6% (19/23) |
| PR | 7.3% (4/55) | 9.4% (3/32) | 4.3% (1/23) |
| PD | 9.1% (5/55) | 6.3% (2/32) | 13% (3/23) |
PD, progressive disease.
PFS and OS in the intent-to-treat population. (A) PFS for all patients. (B) OS for all patients. (C) PFS for patients with DEL vs patients with DHL. (D) OS for patients with DEL vs patients with DHL.
PFS and OS in the intent-to-treat population. (A) PFS for all patients. (B) OS for all patients. (C) PFS for patients with DEL vs patients with DHL. (D) OS for patients with DEL vs patients with DHL.
There were 13 total deaths: 11 due to relapsed/refractory lymphoma, 1 due to pancreatic cancer, and 1 due to acute myeloid leukemia. Of the lymphoma-related deaths, there were 5 patients with primary refractory disease (3 with DHL and 2 with DEL), and 6 patients with relapse (3 with DHL and 3 with DEL), and their median OS was 12.2 months (range, 6.1-40.6). The relapses for these patients occurred at a median of 11.5 months (range, 5.2-19.6) from the start of treatment. Two patients with primary refractory disease developed CNS involvement, and both received prophylactic IT MTX. There were no late CNS relapses. Only 2 patients with relapsed/refractory disease later received anti-CD19 chimeric antigen receptor T-cell therapy. A 68-year-old patient with primary refractory DHL received third-line tisagenlecleucel, and had persistent disease resulting in death at 12 months. The other was a 73-year-old patient with relapsed DHL who received third-line tisagenlecleucel resulting in a CR, but relapsed at day 180. This patient later received an allo-SCT, and died of relapsed disease at 41 months.
Of the 4 patients with a response of PR, 1 received LEN maintenance and converted to CR and remained alive at 41 months. Another did not receive maintenance and converted to CR during follow-up. One patient had a PR by computed tomography imaging only (no positron emission tomography done) at the end of induction, did not receive maintenance, and remained alive at 70 months. One patient did not receive LEN maintenance due to neutropenic fever, and then had disease relapse and died at 7.4 months.
Safety
There were no grade 5 AEs. The most common grade 3 to 4 AEs (Table 3) were neutropenia (67%), anemia (67%), thrombocytopenia (49%), and neutropenic fever (35%). Peripheral sensory neuropathy was the most common nonhematologic toxicity (67% all grades), which was mostly grade 1 to 2. Other common nonhematologic toxicities were fatigue (65%), constipation (62%), nausea (55%), and diarrhea (53%), which were all mostly grade 1 to 2. Eleven of 55 patients (20%) experienced a thromboembolic event. Twenty-nine of 55 patients (53%) experienced an infection, with grade 3 to 4 in 13 of 55 (24%). The most common infection was upper respiratory infection in 7 of 55 patients (13%), and sepsis was the most common infectious grade 3 to 4 AE in 4 patients (7%; supplemental Table 2).
Summary of adverse events
| AE . | All grades . | % . | Grades 3-4 . | % . |
|---|---|---|---|---|
| Anemia | 43 | 78 | 37 | 67 |
| Neutropenia | 37 | 67 | 37 | 67 |
| Peripheral sensory neuropathy | 37 | 67 | 3 | 5 |
| Fatigue | 36 | 65 | 3 | 5 |
| Constipation | 34 | 62 | 1 | 2 |
| Thrombocytopenia | 33 | 60 | 27 | 49 |
| Nausea | 30 | 55 | 2 | 4 |
| Diarrhea | 29 | 53 | 4 | 7 |
| Infection | 29 | 53 | 13 | 24 |
| Hypokalemia | 27 | 49 | 10 | 18 |
| Mucositis oral | 25 | 45 | 1 | 2 |
| Alopecia | 22 | 40 | 0 | 0 |
| Edema | 20 | 36 | 0 | 0 |
| Insomnia | 20 | 36 | 0 | 0 |
| Anorexia | 19 | 35 | 1 | 2 |
| Neutropenic fever | 19 | 35 | 19 | 35 |
| Vomiting | 19 | 35 | 2 | 4 |
| Dyspnea | 18 | 33 | 0 | 0 |
| White blood cell decreased | 17 | 31 | 11 | 20 |
| Abdominal pain | 16 | 29 | 2 | 4 |
| Headache | 16 | 29 | 1 | 2 |
| Dizziness | 16 | 29 | 0 | 0 |
| Fever | 14 | 25 | 2 | 4 |
| Hypomagnesemia | 14 | 25 | 1 | 2 |
| Myalgia | 14 | 25 | 0 | 0 |
| Pain | 14 | 25 | 0 | 0 |
| ALT increased | 13 | 24 | 3 | 5 |
| Hypertension | 13 | 24 | 6 | 11 |
| Hypotension | 13 | 24 | 3 | 5 |
| Hypoalbuminemia | 12 | 22 | 1 | 2 |
| Bone pain | 11 | 20 | 2 | 4 |
| Cough | 11 | 20 | 0 | 0 |
| Dysgeusia | 11 | 20 | 0 | 0 |
| Hypocalcemia | 11 | 20 | 0 | 0 |
| Hyponatremia | 11 | 20 | 1 | 2 |
| Pain in extremity | 11 | 20 | 1 | 2 |
| Thromboembolic event | 11 | 20 | 4 | 7 |
| Lymphocyte count decreased | 10 | 18 | 10 | 18 |
| Weight loss | 10 | 18 | 0 | 0 |
| Allergic rhinitis | 9 | 16 | 0 | 0 |
| Anxiety | 9 | 16 | 0 | 0 |
| Arthralgia | 9 | 16 | 0 | 0 |
| Back pain | 10 | 18 | 0 | 0 |
| Dehydration | 9 | 16 | 1 | 2 |
| Hyperglycemia | 9 | 16 | 3 | 5 |
| Sinus tachycardia | 9 | 16 | 0 | 0 |
| AST increased | 8 | 15 | 1 | 2 |
| Gastroesophageal reflux disease | 8 | 15 | 0 | 0 |
| Sore throat | 8 | 15 | 0 | 0 |
| Urinary frequency | 8 | 15 | 0 | 0 |
| Peripheral motor neuropathy | 7 | 13 | 1 | 2 |
| Pruritus | 7 | 13 | 0 | 0 |
| Creatinine increased | 7 | 13 | 0 | 0 |
| Dysphagia | 6 | 11 | 0 | 0 |
| Hemorrhoids | 6 | 11 | 0 | 0 |
| Hoarseness | 6 | 11 | 1 | 2 |
| Hypophosphatemia | 6 | 11 | 1 | 2 |
| Neck pain | 6 | 11 | 0 | 0 |
| Oral pain | 6 | 11 | 1 | 2 |
| Weight gain | 6 | 11 | 0 | 0 |
| Neoplasms benign, malignant, and unspecified | 5 | 9 | 2 | 4 |
| Respiratory, thoracic, and mediastinal disorders | 3 | 5 | 1 | 2 |
| Blood bilirubin increased | 2 | 4 | 1 | 2 |
| Colitis | 2 | 4 | 2 | 4 |
| Flu-like symptoms | 2 | 4 | 1 | 2 |
| Leukocytosis | 2 | 4 | 2 | 4 |
| Myelodysplastic syndrome | 2 | 4 | 2 | 4 |
| Respiratory failure | 2 | 4 | 2 | 4 |
| Syncope | 2 | 4 | 2 | 4 |
| Chest pain (cardiac) | 1 | 2 | 1 | 2 |
| Hemolytic uremic syndrome | 1 | 2 | 1 | 2 |
| Hip fracture | 1 | 2 | 1 | 2 |
| Small intestinal obstruction | 1 | 2 | 1 | 2 |
| Urostomy stenosis | 1 | 2 | 1 | 2 |
| AE . | All grades . | % . | Grades 3-4 . | % . |
|---|---|---|---|---|
| Anemia | 43 | 78 | 37 | 67 |
| Neutropenia | 37 | 67 | 37 | 67 |
| Peripheral sensory neuropathy | 37 | 67 | 3 | 5 |
| Fatigue | 36 | 65 | 3 | 5 |
| Constipation | 34 | 62 | 1 | 2 |
| Thrombocytopenia | 33 | 60 | 27 | 49 |
| Nausea | 30 | 55 | 2 | 4 |
| Diarrhea | 29 | 53 | 4 | 7 |
| Infection | 29 | 53 | 13 | 24 |
| Hypokalemia | 27 | 49 | 10 | 18 |
| Mucositis oral | 25 | 45 | 1 | 2 |
| Alopecia | 22 | 40 | 0 | 0 |
| Edema | 20 | 36 | 0 | 0 |
| Insomnia | 20 | 36 | 0 | 0 |
| Anorexia | 19 | 35 | 1 | 2 |
| Neutropenic fever | 19 | 35 | 19 | 35 |
| Vomiting | 19 | 35 | 2 | 4 |
| Dyspnea | 18 | 33 | 0 | 0 |
| White blood cell decreased | 17 | 31 | 11 | 20 |
| Abdominal pain | 16 | 29 | 2 | 4 |
| Headache | 16 | 29 | 1 | 2 |
| Dizziness | 16 | 29 | 0 | 0 |
| Fever | 14 | 25 | 2 | 4 |
| Hypomagnesemia | 14 | 25 | 1 | 2 |
| Myalgia | 14 | 25 | 0 | 0 |
| Pain | 14 | 25 | 0 | 0 |
| ALT increased | 13 | 24 | 3 | 5 |
| Hypertension | 13 | 24 | 6 | 11 |
| Hypotension | 13 | 24 | 3 | 5 |
| Hypoalbuminemia | 12 | 22 | 1 | 2 |
| Bone pain | 11 | 20 | 2 | 4 |
| Cough | 11 | 20 | 0 | 0 |
| Dysgeusia | 11 | 20 | 0 | 0 |
| Hypocalcemia | 11 | 20 | 0 | 0 |
| Hyponatremia | 11 | 20 | 1 | 2 |
| Pain in extremity | 11 | 20 | 1 | 2 |
| Thromboembolic event | 11 | 20 | 4 | 7 |
| Lymphocyte count decreased | 10 | 18 | 10 | 18 |
| Weight loss | 10 | 18 | 0 | 0 |
| Allergic rhinitis | 9 | 16 | 0 | 0 |
| Anxiety | 9 | 16 | 0 | 0 |
| Arthralgia | 9 | 16 | 0 | 0 |
| Back pain | 10 | 18 | 0 | 0 |
| Dehydration | 9 | 16 | 1 | 2 |
| Hyperglycemia | 9 | 16 | 3 | 5 |
| Sinus tachycardia | 9 | 16 | 0 | 0 |
| AST increased | 8 | 15 | 1 | 2 |
| Gastroesophageal reflux disease | 8 | 15 | 0 | 0 |
| Sore throat | 8 | 15 | 0 | 0 |
| Urinary frequency | 8 | 15 | 0 | 0 |
| Peripheral motor neuropathy | 7 | 13 | 1 | 2 |
| Pruritus | 7 | 13 | 0 | 0 |
| Creatinine increased | 7 | 13 | 0 | 0 |
| Dysphagia | 6 | 11 | 0 | 0 |
| Hemorrhoids | 6 | 11 | 0 | 0 |
| Hoarseness | 6 | 11 | 1 | 2 |
| Hypophosphatemia | 6 | 11 | 1 | 2 |
| Neck pain | 6 | 11 | 0 | 0 |
| Oral pain | 6 | 11 | 1 | 2 |
| Weight gain | 6 | 11 | 0 | 0 |
| Neoplasms benign, malignant, and unspecified | 5 | 9 | 2 | 4 |
| Respiratory, thoracic, and mediastinal disorders | 3 | 5 | 1 | 2 |
| Blood bilirubin increased | 2 | 4 | 1 | 2 |
| Colitis | 2 | 4 | 2 | 4 |
| Flu-like symptoms | 2 | 4 | 1 | 2 |
| Leukocytosis | 2 | 4 | 2 | 4 |
| Myelodysplastic syndrome | 2 | 4 | 2 | 4 |
| Respiratory failure | 2 | 4 | 2 | 4 |
| Syncope | 2 | 4 | 2 | 4 |
| Chest pain (cardiac) | 1 | 2 | 1 | 2 |
| Hemolytic uremic syndrome | 1 | 2 | 1 | 2 |
| Hip fracture | 1 | 2 | 1 | 2 |
| Small intestinal obstruction | 1 | 2 | 1 | 2 |
| Urostomy stenosis | 1 | 2 | 1 | 2 |
All AEs regardless of attribution were included. AEs reported for all grades were those that occurred in ≥10% of patients. All grade 3 to 4 AEs were included.
ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Second primary malignancy (SPM) occurred in 6 patients (11%). Two patients (4%) had therapy-related myeloid neoplasms (t-MN). One patient received palliative spinal radiation prior to induction on study, and then received 3 cycles of LEN maintenance before later developing a t-MN with a chromosome 7 deletion and ETV6 pathogenic mutation at 18.3 months after treatment. Another patient who did not receive LEN maintenance developed a t-MN at 6 months after treatment, and was found to have DNMT3A, HIST1H3B, and RUNX1 pathogenic mutations, and a 7q deletion. Germ line mutational testing in this patient, including RUNX1 assessment, was negative. Other SPMs included pancreatic cancer (stage I) at 28 months, colon cancer (stage I) at 5.8 months, squamous cell carcinoma of the skin at 9.9 months, and well-differentiated neuroendocrine tumor of the ampulla at 6.1 months. None of these patients received LEN maintenance, except for the patient with squamous cell carcinoma of the skin (2 cycles).
Discussion
DLBCL is a heterogeneous entity, with multiple established and emerging high-risk features predicting a poor outcome following R-CHOP chemoimmunotherapy. The intensified regimen, DA–EPOCH-R, has become a standard option for some DLBCL subsets, but relapse continues to be problematic. LEN was combined with DA–EPOCH-R based on the hypothesis that LEN targets MYC signaling to promote a synergistic antilymphoma effect with DA–EPOCH-R in MYC-driven DLBCL.14-18 This trial sought to establish preliminary efficacy and toxicity data when LEN is added to DA–EPOCH-R in patients with high-risk lymphomas characterized by dual MYC and BCL2 translocations, or with dual Myc/Bcl2 protein overexpression.
With a median follow-up of 3.4 years, the combination of LEN with DA–EPOCH-R in DEL and DHL was feasible, and resulted in a high ORR of 90.9%, and met its primary end point with 1-year PFS of 85.5% and 2-year PFS of 78.2%. Considering that most treatment failures occur within 2 years of initiating treatment, we find these results to be highly encouraging. Standard dose escalation of EPOCH-R was also feasible with this combination, although recent data suggest dose escalation and level may be less important for survival benefit.24 In the phase 3 study of DA–EPOCH-R vs R-CHOP in patients with DLBCL-NOS, the median DL was 3 (range 1-5) compared with a DL of 2 (range 1-4) in this study with the addition of LEN.9 A second goal of the study was to evaluate the feasibility of delivering maintenance LEN. Thirty-three of the 43 patients (77%) who completed 6 cycles of induction with CR or PR went on to receive LEN maintenance, and only 46% completed the full 12 cycles. LEN was most commonly discontinued due to cytopenias. At the time this study was designed, the role of maintenance therapy and consolidation in DLBCL was still evolving. Only 1 patient received a consolidative auto-SCT, which likely reflects a movement away from this strategy given data suggesting no known PFS or OS benefit coupled with the emergence of more effective second- and third-line strategies.7
There are limited prospective data on the optimal management of patients with DHL or DLBCL-NOS DEL. One of the few prospective data sets regarding DA–EPOCH-R in MYC-rearranged DLBCL included only 24 patients with DHL, and that study preceded the designation of DLBCL-NOS with DEL.8 The CR rate was 74% for all patients with MYC rearrangement. Patients with DHL had 4-year PFS and OS of 73% and 82%, respectively. In a preliminary report of the prospective phase 2 trial HOVON-152, DA–EPOCH-R in 97 patients with DHL resulted in a lower CR rate of 66%, and follow-up for survival is ongoing.25 More recent retrospective studies of patients with DHL with MYC and BCL2 rearrangements treated with DA–EPOCH-R reported 2-year PFS of 63% to 74%, and 2-year OS of 66% to 78%.24,26-28 In other studies of patients with DHL treated with R-CHOP, the 2-year PFS was 52% to 62%, and 2-year OS of 43% to 66%.2,29,30 Acknowledging the challenges with cross-study comparisons, the current trial found a 2-year PFS and OS of 69.6% and 78.3%, respectively, for DHL. While there appears to be an efficacy signal of LEN with DA–EPOCH-R, a randomized trial would be needed to confirm the benefit of the addition of LEN.
Other studies have evaluated adding LEN to an anthracycline-based regimen in first-line treatment of DLBCL, but included only a minority of patients with DEL and DHL. LEN was added to R-CHOP in patients with MYC-rearranged DLBCL, including single MYC rearrangements and DHL.31 In 53 patients with DHL treated with LEN and R-CHOP, the CR rate was 66%, 2-year PFS was 60%, and 2-year OS was 66%. Apart from DHL, LEN in combination with cytotoxic therapy has also been explored mostly in non-GCB or ABC subtype DLBCL, which constitutes most of DEL. Patients with ABC DLBCL have an inferior prognosis independent of DEL status when treated with R-CHOP, and improved treatment strategies are needed for this DLBCL subtype.32 Given LEN activity in ABC DLBCL, LEN with R-CHOP was compared with R-CHOP in the phase 3 ROBUST study of patients with ABC DLBCL, although the specific number of patients with DEL was not reported.33 Overall, there was no difference in survival with the addition of LEN with 2-year PFS and OS rates of ∼66% and 80%, respectively. In patients with non-GCB DLBCL treated with LEN and DA–EPOCH-R in our trial, the 2-year PFS and OS rates were 84%. Recent phase 1/2 studies using LEN-based combinations such as rituximab, LEN, and ibrutinib with chemotherapy as well as LEN with obinutuzumab and CHOP have also reported encouraging efficacy in the first-line treatment of non-GCB DLBCL, including some patients with DEL.34,35
In DLBCL-NOS with DEL, the optimal treatment is unknown, and intensive chemotherapy is controversial. In the only prospective trial comparing R-CHOP with DA–EPOCH-R in patients with DLBCL, there was no difference in PFS and OS, although only 16% were patients with DEL.9 In the largest multicenter retrospective study of DEL comparing R-CHOP with DA–EPOCH-R, the 3-year PFS was 33.2% vs 57.2% (P = .063), and 3-year OS was 72.2% vs 71.6% (P = .43), respectively.36 Of note, more patients treated with R-CHOP received a consolidative auto-SCT. Patients aged <65 years had improved PFS with DA–EPOCH-R, but not OS. In a recent retrospective study including 81 patients with DEL treated with DA–EPOCH-R, the 2-year PFS and OS were 75% and 86%, respectively.26 The 2-year PFS and 2-year OS rates of 84.4% and 87.5% in our study of patients with DEL treated with LEN and DA–EPOCH-R compare favorably with these historical data. While the optimal backbone for LEN-based front-line therapy remains unclear, more recent data support the use of R-CHOP. Prospective studies with LEN and R-CHOP in ABC DLBCL did not report DEL status, but this regimen may be a less toxic approach with similar efficacy for this group.31,33
Beyond LEN, other novel agents have been combined with cytotoxic therapy in both DHL and DEL. The oral proteasome inhibitor, ixazomib, was successfully combined with DA–EPOCH-R in a phase 1/2 trial demonstrating an ORR of 97% and CR rate of 69%.37 For DHL, the 1-year PFS and OS rates were 53% and 65%, respectively. For DEL, the 1-year PFS and OS rates were 95% and 100%, respectively. Similar to our study, the combination was feasible with a high efficacy signal for DEL over DHL, and cytopenias and neuropathy were common. Other studies have evaluated combinations to target Bcl2 in DHL and DEL. In the randomized phase 2/3 trial Alliance A051701, the addition of venetoclax to DA–EPOCH-R in DHL resulted in excess treatment-related mortality compared with DA–EPOCH-R alone, prompting early discontinuation of the study.38 In the DEL cohort, the addition of venetoclax to R-CHOP did not improve PFS, and also resulted in increased toxicity.39 In an interim analysis of a phase 3 trial of patients with DEL, the histone deacetylase inhibitor, tucidinostat, plus R-CHOP had a higher 2-year event-free survival of 59% vs placebo plus R-CHOP at 46%.40 There was increased hematologic toxicity, but most patients completed treatment. To our knowledge, this is the first randomized study focusing primarily on DEL, and the results from the final analysis with longer follow-up may support this epigenetic targeting strategy.
There were no unexpected toxicities observed with LEN plus DA–EPOCH-R; the incidence of AEs such as cytopenias, neutropenic fever, and infection were similar to previous experience with DA–EPOCH-R alone, and there were no treatment-related deaths.9,41 Neutropenic fever occurred in 35% of patients, which is comparable to other studies with DA–EPOCH-R, but higher than R-CHOP at 8% to 18%.9,42 The incidence of 20% thromboembolic events observed in our study, which included deep vein thrombosis, pulmonary embolism, and catheter-associated thrombosis, was higher than the thromboembolic events with LEN with R-CHOP of ∼2% in E1412 and LEN with O-CHOP of ∼8%.35,43 In contrast, the incidence of thromboembolic AEs in studies of DA–EPOCH-R varies, but has been as high as 35%.8,38,41,44 We report 7% grade 3 to 4 thromboembolic events, which was similar to 10% with the DA–EPOCH-R control arm in A051701, though we recognize that any thromboembolic event of any grade is serious.38 The frequency of thromboembolic events despite aspirin prophylaxis is a disadvantage with this approach, and an alternative prophylaxis strategy such as high-dose aspirin (325 mg) or low-molecular weight heparin may be needed in future studies. In E1412, patients received aspirin 325 mg and those unable to receive aspirin received therapeutic anticoagulation.
In our study, SPM occurred in 11% of patients, which is higher than 7% with LEN plus R-CHOP, and 2% to 4% with R-CHOP alone.45,46 Other studies report SPM incidence of 4% to 13% for patients with DLBCL.47-50 Notably, there were 2 patients (4%) with t-MN compared with 2% with LEN plus R-CHOP, and <1% with R-CHOP alone.43,46 While LEN exposure is associated with TP53-mutated t-MN in other settings, neither of the patients with t-MN in this study had TP53 mutations, latency was quite short, and 1 patient had spinal radiation exposure,51 thus we are unsure if they were directly related to LEN exposure. LEN use in lymphoma does not appear to increase the risk of SPMs in other larger studies, but the signal observed here supports the need for SPM monitoring and reporting in trials that use LEN-based therapy.52
Since our trial was launched, the landscape of treatment options for first-line high-risk DLBCL has continued to expand, with exciting advances in cellular therapy, bispecific antibodies, antibody-drug conjugates, and other novel agents. For non-GCB/ABC DLBCL and DEL, there was a signal for PFS benefit with polatuzumab vedotin plus R-CHP (pola-R-CHP) over R-CHOP, and pola-R-CHP was approved for new DLBCL, with IPI ≥2.42,53,54 In a phase 1 trial of patients with aggressive large B-cell lymphoma including DHL (33%) and DEL (39%), pola with DA–EPCH-R had an acceptable safety profile with an ORR of 100%, and is being explored further.44 The novel oral cereblon E3 ligase modulator, golcadomide, has dual immunomodulatory and direct cell-killing antitumor activity with a high response rate in combination with R-CHOP in high-risk DLBCL.55 Golcadomide with R-CHOP is being evaluated in the phase 3 GOLSEEK-1 trial (ClinicalTrials.gov identifier: NCT06356129). In the phase 2 trial ZUMA-12 of patients with untreated high-risk DLBCL including DHL, axicabtagene ciloleucel demonstrated a high rate of durable responses and manageable safety profile, and the phase 3 trial ZUMA-23 of axicabtagene ciloleucel vs standard chemoimmunotherapy is ongoing.56,57 Bispecific antibodies have also been successfully combined with R-CHOP or pola-R-CHP in the first-line treatment of DLBCL, and the pivotal phase 3 trials using epcoritamab (ClinicalTrials.gov identifier: NCT05578976), glofitamab (ClinicalTrials.gov identifier: NCT06047080), and odronextamab (ClinicalTrials.gov identifier: NCT06091865) also seek to improve the frontline treatment of DLBCL.58,59
There are several limitations to this trial, primarily due to modest sample size and a single-arm design. However, as a multicenter study, we were able to show feasibility of the induction regimen with promising efficacy and reasonable toxicity. The median diagnosis-to-treatment interval of 21 days and the lab-based eligibility criteria also result in inadvertent selection bias and more favorable results.60,61 Our study focused on DHL and DLBCL-NOS with DEL, but these entities are being refined further with more unified biomarker strategies. For example, gene expression profiling has identified a “double-hit gene signature” and “molecular high grade” signature that identify MYC-driven DLBCL that only partially overlaps with DHL, but still exhibit inferior outcomes with R-CHOP.62,63 “Double-hit gene signature” has been renamed the “dark zone signature,” and identifies a poor-prognosis subgroup of DLBCL that extends beyond DHL. It includes patients missed by routine FISH testing who should be considered for treatment intensification or novel therapies in prospective trials.29
In conclusion, this phase 1/2 trial of patients with MYC-associated DLBCL including DEL and DHL, LEN with DA–EPOCH-R resulted in a high response rate, and met the primary end point criteria for improved PFS. In this high-risk group of patients, the immunomodulatory agent, LEN, was successfully added to the intensive backbone of EPOCH-R, leading to prolonged PFS compared with historical data sets of DA–EPOCH-R alone. There were no unexpected toxicities with the combination, and the safety signals for thromboembolism and SPM need to be closely followed in future trials. A randomized trial of DA–EPOCH-R with and without LEN in DHL and DEL is needed to determine the specific benefit of LEN. Regardless of the cytotoxic backbone, approaches incorporating novel immunomodulatory agents may be beneficial for MYC-associated DLBCL.
Acknowledgments
This work was supported by the University of Chicago National Institutes of Health grant T32 CA 9566-35 and Hoogland Family Foundation (K.C.). This investigator-initiated study received funding from Celgene.
Authorship
Contribution: C.N. and S.M.S. designed the study; J.G., K.C., J.R., and S.M.S. collected and analyzed the results; K.C. and S.M.S. wrote the manuscript; T.G.K. performed statistical analysis and reviewed the manuscript; and J.K., P.A.R., K.S.C., S.N., R.K., P.V., S.-H.K., A.P.R., S.T.L., J.L., P.A.S.F., I.I., M.V., L.K., and S.M.S. provided patient care on the trial and critically reviewed the manuscript.
Conflict-of-interest disclosure: K.C. is part of the advisory board for Eli Lilly. C.N. is employed at Ryght, Inc. J.K. is part of the advisory boards for Seagen, Genmab, AbbVie, Bristol Myers Squibb (BMS), ADC Therapeutics, and BeiGene; declares consulting for AbbVie and ADC Therapeutics; and has received research support from Merck and Verastem. P.A.R. has served as a consultant or advisor to AbbVie, ADC Therapeutics, BeiGene, BMS/Celgene, CVS, Genentech/Roche, Genmab, Kite, Novartis, and Pharmacyclics/Janssen; has received travel support from Adaptive Biotechnologies; and has received institutional research funding from BMS/Celgene, Calibr, Cellectis, CRISPR Therapeutics, Fate Therapeutics, Genentech/Roche, Kite, Novartis, Tessa Therapeutics, CARGO Therapeutics, and Xencor. R.K. has received honoraria from Genmab, Genentech/Roche, AbbVie, and BMS; and has been part of the speakers bureau for Ipsen, BeiGene, AstraZeneca, and Incyte. P.A.S.F. declares being a current equity holder in a publicly traded company, Novartis. I.I. declares consulting fees for an advisory board with Celgene in March 2024. S.M.S. declares consultancy for Regeneron, Genmab, Ono Pharmaceutical, and Foresight Diagnostics; and their spouse is employed by Caris Life Sciences. The remaining authors declare no competing financial interests.
Correspondence: Sonali M. Smith, Section of Hematology/Oncology, Department of Medicine, University of Chicago Medicine, MC 2115, 5841 S. Maryland Ave, Chicago, IL 60637; email: smsmith@bsd.uchicago.edu.
References
Author notes
Deidentified data will be made available on reasonable request after the publication.
The full-text version of this article contains a data supplement.