Key Points
Albuminuria category confirmed on repeat testing predicts kidney disease progression and mortality risk in adults with SCD.
KDIGO heat map integrating albuminuria and estimated glomerular filtration rate further improves prediction of these longitudinal outcomes.
Visual Abstract
Approximately 15% of deaths in adults with sickle cell disease (SCD) are attributed to kidney failure. Although urine albumin-to-creatinine ratio (UACR) is recommended to screen for kidney damage, its utility in predicting long-term complications in SCD remains unclear. We investigated whether “Kidney Disease: Improving Global Outcomes (KDIGO)” algorithms used to assess kidney disease in the general population predicted chronic kidney disease (CKD) progression and mortality in a longitudinal cohort of 379 adults with SCD from 2 academic institutions. KDIGO criteria include UACR detected in 2 consecutive measurements ≥3 months apart and a heat map integrating UACR with estimated glomerular filtration rate. KDIGO-defined CKD was present in 39.8% of individuals in our SCD cohort. Over a median follow-up of 3.3 years, incremental KDIGO-defined UACR category independently predicted a twofold greater risk of CKD progression and 1.8-fold greater risk of mortality (P ≤ .05). KDIGO-defined CKD heat map strengthened the ability to predict CKD progression and mortality risk (P ≤ .0087). Our data provide clinical support for the screening utility of UACR based on repeated abnormal values ≥3 months apart. The KDIGO heat map further refines the risk of long-term outcomes among adults with SCD and should be applied to guide future studies for monitoring and intervention strategies.
Introduction
Chronic organ disease has been increasingly recognized as a major contributor to the morbidity and mortality of sickle cell disease (SCD) and now represents up to half of the known causes of deaths in adults.1,2 Advanced chronic kidney disease (CKD) is consistently associated with greater health care utilization3,4 and early mortality.5-8 Furthermore, approximately one quarter of the patients with SCD who have CKD that progresses to end-stage kidney disease (ESKD) will die within 1 year, representing a threefold greater risk of mortality compared with that of the general population with incident ESKD.9 Tools to risk stratify patients with SCD at increased risk of CKD progression are urgently needed to implement surveillance and treatment strategies in this high-risk population.
The “Kidney Disease: Improving Global Outcomes (KDIGO)” clinical practice guidelines include criteria to guide the care of all people with CKD based on their urine albumin-to-creatinine ratios (UACRs) and estimated glomerular filtration rate (eGFR) values that are abnormal for at least 3 months.10 Although measuring UACR in routine practice for people with SCD is recommended by the National Heart, Lung, and Blood Institute 2014 Guidelines, thresholds to predict longitudinal outcomes, strategies to monitor for abnormal values, and applications to guide clinical practice remain unclear.11 KDIGO developed a heat map integrating UACR and eGFR values to risk stratify for several clinical outcomes, such as progression to kidney failure and all-cause mortality.10 This heat map has been applied to guide the frequency of UACR and eGFR assessments, identify people for the earlier use of disease-modifying therapy, enable personalized discussions of overall goals of care, and prioritize referrals for interdisciplinary care and nephrology services in the general population. The ability of the KDIGO heat map to predict longitudinal outcomes has not been evaluated in the SCD population; however, it could be an important instrument to guide surveillance and treatment strategies and for referral to nephrology care.
Methods
This study was approved by the institutional review boards of the participating institutions and participants provided written informed consent in accordance with the Declaration of Helsinki. The University of Illinois Chicago cohort included 198 adults with SCD recruited into a longitudinal registry between August 2010 and March 2016. Clinical and laboratory data were extracted from the electronic medical charts from the time of enrollment. The Vanderbilt University Medical Center previously developed algorithms for accurate automated SCD cohort creation using International Classification of Diseases (ICD) and laboratory-based algorithms, and 181 adults were included in this analysis.12
Outpatient UACR and eGFR values were used to categorize patients based on the KDIGO thresholds of <30 (A1), 30 to 300 (A2), and >300 mg/g of creatinine (A3) and ≥90 (G1), 60 to 89 (G2), 45 to 59 (G3a), 30 to 44 (G3b), and <30 (G4/G5) mL/min per 1.73 m2, respectively. Patients were categorized by albuminuria status based on the first 2 consecutive measures at a given threshold of UACR for at least 3 months using spot urine samples. The eGFR values, which were calculated using the serum creatinine CKD-Epidemiology 2021 equation (eGFRcr = 142 × min(Scr/κ, 1)α × max(Scr/κ, 1)–1.200 × 0.9938Age × 1.012 (if female), where Scr = standardized serum creatinine in mg/dL; κ = 0.7 (females) or 0.9 (males); α = –0.241 (female) or –0.302 (male); min(Scr/κ, 1) is the minimum of Scr/κ or 1.0; max(Scr/κ, 1) is the maximum of Scr/κ or 1.0; and age (years),13 within 12 months of the UACR values were used for eGFR categorization. If a patient had 1 UACR or eGFR value above and 1 below a threshold, the patient was placed in the less severe category. The patients were also categorized according to the KDIGO heat map definition integrating the UACR and eGFR categories as follows: green zone = A1/G1 or A1/G2; yellow zone = A2/G1, A2/G2, or A1/G3a; orange zone = A3/G1, A3/G2, A2/G3a, or A1/G3b; and red zone = A3/G3a or worse eGFR category, A2/G3b or worse eGFR category, or A1/G4 or G5.10 The KDIGO guidelines recommend continued screening for those in the green zones and treatment for those in yellow, orange, and red zones with diet modification, aggressive blood pressure and glycemic monitoring and control, initiating renoprotective therapies (eg, renin-angiotensin-aldosterone system inhibitors [RAASi]), and referral to a nephrologist to assist in identifying reversible causes of kidney damage and slow progression of CKD. Other clinical data, such as age and treatments (eg, hydroxyurea, RAASi, or chronic red blood cell transfusion therapies), were based on the first of the 2 UACR measures used to define CKD status. CKD progression was defined as a sustained reduction in eGFR by 40% or development of ESKD. Deaths were identified through chart review, including discharge summaries or notification for events that occurred at outside facilities.
Time until CKD progression or death was regressed on predictive categories using Cox proportional hazards model. Covariates included age, sex, severe hemoglobin genotypes (SS or Sβ0), hydroxyurea treatment, RAASi therapy, and chronic red blood transfusion therapy. P values of estimated hazard ratios (HRs) were obtained by Wald test. Cox models with KDIGO heat map as the predictor were compared to those with UACR or eGFR categories by Akaike information criterion (AIC), where a decrease in AIC by 2 units indicates that the model being considered fits the data significantly better relative to the other model. Survival curves were generated by Kaplan-Meier estimator and P values were obtained by the log-rank test. Statistical analyses were conducted using R version 4.2.1.
Results
The median age of the cohort was 33 years, 55% were female, 75% were hemoglobin SS or Sβ0-thalassemia genotype, 54% were on hydroxyurea, 20% were on RAASi, and 8% were on chronic red blood cell transfusion therapy at baseline (Table 1; supplemental Table 1). The median UACR and eGFR values at enrollment were 27 mg/g of creatinine and 120 mL/min per 1.73 m2, respectively. According to the KDIGO definition of abnormal UACR or eGFR confirmed at least 3 months apart, 38.8% of the cohort had an elevated UACR (≥30 mg/g of creatinine) and 16.4% had a reduced eGFR (<90 mL/min per 1.73 m2).
Baseline characteristics of the combined UIC and VUMC cohorts of adults with SCD
| Variable . | UIC & VUMC cohorts of adults with SCD N = 379 . |
|---|---|
| Age, y | 33.0 (25.8-44.1) |
| Female sex | 209 (55.1%) |
| SCD genotype | |
| Hemoglobin SS or Sβ0-thalassemia | 285 (75.2%) |
| Hemoglobin SC | 66 (17.4%) |
| Hemoglobin Sβ+-thalassemia | 28 (7.4%) |
| Hydroxyurea use | 204 (53.8%) |
| RAASi use | 75 (19.8%) |
| ACE inhibitors | 58 (15.3%) |
| ARBs | 17 (4.5%) |
| Chronic RBC transfusions | 30 (7.9%) |
| Body mass index, kg/m2 | 24.3 (21.5-28.4) |
| Systolic blood pressure, mmHg | 118 (109-129) |
| WBC count (× 103/μL) | 9.4 (7.0-12.0) |
| Hemoglobin, g/dL | 9.3 (8.2-10.5) |
| Platelet count (× 103/μL) | 358 (262-450) |
| Hemoglobin F, % | 7.5 (3.5-13.65) |
| Reticulocyte, % | 8.7 (4.5-12.9) |
| LDH, U/L | 320 (244-460) |
| UACR, mg/g | 27 (10-117) |
| UACR category, mg/g of creatinine | |
| Normal (<30) | 232 (61.2%) |
| Moderately elevated (30-300) | 104 (27.4%) |
| Severely elevated (>300) | 43 (11.3%) |
| eGFR, mL/min per 1.73 m2 | 120 (101-133) |
| eGFR category, mL/min per 1.73 m2 | |
| G1 (>90) | 317 (83.6%) |
| G2 (60-89) | 43 (11.3%) |
| G3a (45-59) | 10 (2.6%) |
| G3b (30-44) | 5 (1.3%) |
| G4 (15-29) | 3 (0.8%) |
| G5 (<15) | 1 (0.3%) |
| Variable . | UIC & VUMC cohorts of adults with SCD N = 379 . |
|---|---|
| Age, y | 33.0 (25.8-44.1) |
| Female sex | 209 (55.1%) |
| SCD genotype | |
| Hemoglobin SS or Sβ0-thalassemia | 285 (75.2%) |
| Hemoglobin SC | 66 (17.4%) |
| Hemoglobin Sβ+-thalassemia | 28 (7.4%) |
| Hydroxyurea use | 204 (53.8%) |
| RAASi use | 75 (19.8%) |
| ACE inhibitors | 58 (15.3%) |
| ARBs | 17 (4.5%) |
| Chronic RBC transfusions | 30 (7.9%) |
| Body mass index, kg/m2 | 24.3 (21.5-28.4) |
| Systolic blood pressure, mmHg | 118 (109-129) |
| WBC count (× 103/μL) | 9.4 (7.0-12.0) |
| Hemoglobin, g/dL | 9.3 (8.2-10.5) |
| Platelet count (× 103/μL) | 358 (262-450) |
| Hemoglobin F, % | 7.5 (3.5-13.65) |
| Reticulocyte, % | 8.7 (4.5-12.9) |
| LDH, U/L | 320 (244-460) |
| UACR, mg/g | 27 (10-117) |
| UACR category, mg/g of creatinine | |
| Normal (<30) | 232 (61.2%) |
| Moderately elevated (30-300) | 104 (27.4%) |
| Severely elevated (>300) | 43 (11.3%) |
| eGFR, mL/min per 1.73 m2 | 120 (101-133) |
| eGFR category, mL/min per 1.73 m2 | |
| G1 (>90) | 317 (83.6%) |
| G2 (60-89) | 43 (11.3%) |
| G3a (45-59) | 10 (2.6%) |
| G3b (30-44) | 5 (1.3%) |
| G4 (15-29) | 3 (0.8%) |
| G5 (<15) | 1 (0.3%) |
Data are presented as median (interquartile values) and frequency (%).
ACE, angiotensin converting enzyme; ARBs, angiotensin receptor blocker; LDH, lactate dehydrogenase; RBC, red blood cell; UIC, University of Illinois Chicago; VUMC, Vanderbilt University Medical Center; WBC, white blood cell.
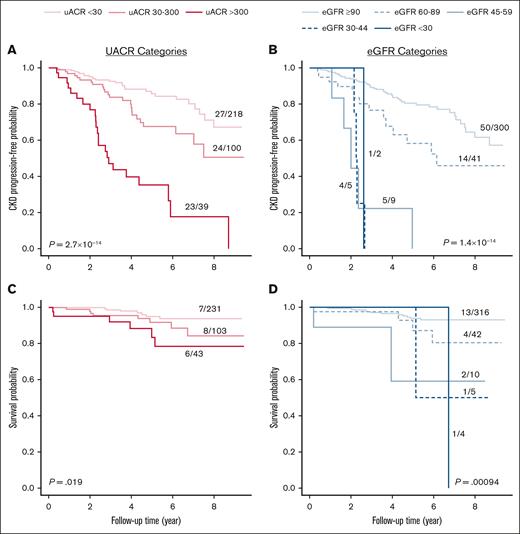
Over a median follow-up of 3.3 (interquartile range, 1.9-5.5) years, we observed CKD progression in 20.7% (74/357) of evaluable patients, of which 72 had an eGFR decline by 40% and 2 progressed to ESKD. Progressively higher UACR (HR, 2.0; 95% confidence interval [CI], 1.4-2.8; P = .0001) and lower eGFR (HR, 1.8; 95% CI, 1.4-2.4; P = 5.6 × 10–5) categories were independently associated with the risk of CKD progression after adjusting for age, sex, SCD genotype, hydroxyurea use, RAASi use, and chronic red blood cell transfusion therapy (Figure 1A-B). We observed 21 deaths over a median follow-up of 4.0 (interquartile range, 2.3-6.3) years. Survival was reduced in patients with higher UACR category (HR, 1.8; 95% CI, 1.0-3.3; P = .05) and in those with lower eGFR category (HR, 1.7; 95% CI, 1.1-2.7; P = .018) after multivariable adjustment (Figure 1C-D).
Progressively worse UACR and eGFR categories were associated with CKD progression and survival. CKD progression, defined by a 40% decline in eGFR or development of ESKD, was associated with (A) worsening UACR and (B) eGFR categories based on 2 consecutive measures ≥3 months apart. Overall survival was also associated with (C) worsening UACR and (D) eGFR categories (log-rank P values are provided).
Progressively worse UACR and eGFR categories were associated with CKD progression and survival. CKD progression, defined by a 40% decline in eGFR or development of ESKD, was associated with (A) worsening UACR and (B) eGFR categories based on 2 consecutive measures ≥3 months apart. Overall survival was also associated with (C) worsening UACR and (D) eGFR categories (log-rank P values are provided).
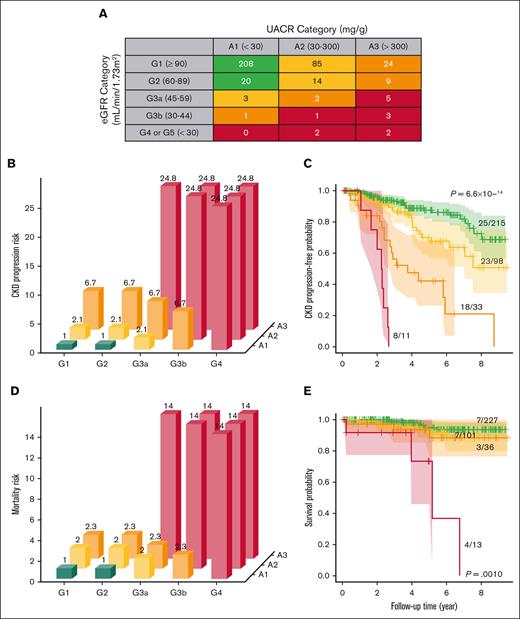
Next, we categorized patients with SCD according to the KDIGO heat map integrating UACR and eGFR values, with green, yellow, orange, and red zones indicating progressively higher risk categories (Figure 2A). In our cohort, 60.2% (228/379) of the individuals were in the green zone, 26.9% (102/379) in the yellow zone, 9.5% (36/379) in the orange zone, and 3.4% (13/379) in the red zone. The KDIGO heat map identified 49 patients with SCD who were in the higher risk orange or red zones, among which 87.8% (43/49) would have been identified as high risk based on severe UACR (≥300 mg/g of creatinine) and 38.8% (19/49) based on severe eGFR (<60 mL/min per 1.73 m2) criteria alone. On follow-up, worsening of KDIGO heat map zone occurred in 34.3% (103/300) and improvement in 6.1% (8/132) of evaluable patients. Progressively higher KDIGO heat map risk was an independent predictor for CKD progression (HR 2.1, 95% CI: 1.6-2.9; P = 6.9 × 10-7) and for mortality (HR 2.0, 95% CI: 1.2-3.3; P = 0.0087) after multivariable adjustment (Figure 2B-E). The KDIGO heat map better predicted CKD progression (UACR: ΔAIC = −15; eGFR: ΔAIC = −26) and mortality risk (UACR: ΔAIC = −3.6; eGFR: ΔAIC = −2.4) compared to the individual kidney function categories, respectively.
KDIGO heat map predicts progressively higher risks of CKD progression and mortality in adults with SCD. (A) We applied the KDIGO heat map according to 2 consecutive UACR and eGFR values ≥3 months apart to categorize adults with SCD. The KDIGO heat map predicted risk of (B-C) CKD progression and (D-E) mortality by HRs and Kaplan-Meier curves, respectively. HRs with the green category as the reference variable and log-rank P values are provided.
KDIGO heat map predicts progressively higher risks of CKD progression and mortality in adults with SCD. (A) We applied the KDIGO heat map according to 2 consecutive UACR and eGFR values ≥3 months apart to categorize adults with SCD. The KDIGO heat map predicted risk of (B-C) CKD progression and (D-E) mortality by HRs and Kaplan-Meier curves, respectively. HRs with the green category as the reference variable and log-rank P values are provided.
Discussion
Overall, kidney dysfunction, defined by abnormal UACR and/or eGFR values confirmed at least 3 months apart, was present in 39.8% of our cohort and predicted the risk of CKD progression and mortality on longitudinal follow-up. Our findings are clinically important because they provide a simple, improved method to identify patients with SCD who are at greatest risk for CKD-associated morbidity and mortality. Although the National Heart, Lung, and Blood Institute SCD guidelines recommend using UACR, the expert panel noted this recommendation is based on low-quality evidence and provided no recommendations on screening intervals. The potential limitations of this recommendation include the inability of a single UACR measure in the moderately elevated range (30-299 mg/g) to predict progression to stage 2 CKD and the high variability of UACR on repeat annual assessments.14,15 The KDIGO clinical practice guidelines recommend confirming an abnormal UACR on repeat testing at least 3 months apart and integrating the UACR with eGFR to risk stratify the general population for CKD to guide screening, treatment, and referral strategies.10 In our cohort of adults with SCD, the KDIGO heat map integrating UACR and eGFR was an independent predictor for risk of CKD progression and mortality. Based on our findings, we recommend assessing UACR and eGFR annually, rechecking an abnormal UACR in ∼3 months, and applying the KDIGO heat map to stratify adults with SCD for risk of CKD progression and mortality.
CareFirst, the largest health care insurer in the mid-Atlantic region, applied the KDIGO heat map to develop practice guidelines that include the following categories: green zone = annual kidney function testing; yellow zone = increased blood glucose, blood pressure, and kidney function monitoring; orange zone = referral to a nephrologist; red zone = use of local care coordinators to enhance multidisciplinary communication and discuss kidney replacement preparation.16 This tiered approach led to significant reductions in morbidity and improvements in health care costs. To our knowledge, our study is the first to demonstrate that the KDIGO heat map predicts CKD progression and mortality risk in adults with SCD. Therefore, the tiered approach outlined by CareFirst to assess CKD in the general population may benefit individuals with SCD and warrants further investigation in this population.
Our study is limited by the small sample size, relatively short follow-up time, nonstandard assessments of kidney function (eg, timing interval between assessments, technical instructions for random vs first or second urine void) and requires validation in other cohorts. Our cohort represents 2 large academic centers that care for adults with SCD and may be enriched for patients at higher risk for SCD-related adverse outcomes affecting generalizability to the overall US adult population with SCD. We evaluated all-cause mortality, and future studies identifying the causes of death and CKD-specific causes of death would be important. Nonetheless, we provide evidence that (1) UACR should be closely monitored with repeat measurements if ≥30 mg/g of creatinine is detected on a single measurement and that (2) the KDIGO heat map improves prediction of 2 important clinical end points in adults with SCD: CKD progression and mortality. Future studies are warranted to investigate whether the progression of SCD-associated CKD can be reduced by disease-modifying and/or renoprotective therapies in individuals who are deemed to be at risk based on the KDIGO criteria.
Acknowledgments
This work was supported by National Institutes of Health, National Heart, Lung, and Blood Institute grants R01 HL-168179 (A.G.B.), R01 HL-153161 (S.L.S.), and K24 HL-177273 (S.L.S.).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authorship
Contribution: All authors contributed to study conception and design, were involved in data collection and analysis and interpretation of results, participated in draft manuscript preparation, and critically reviewed and approved the final version of this manuscript.
Conflict-of-interest disclosure: M.R.D. reports no relevant conflict-of-interest (COI) related to this manuscript but has served as the chair of a Novartis-sponsored clinical trial. V.R.G. reports no relevant COI related to this manuscript but has served as a consultant for Emmaus Life Sciences, Global Blood Therapeutics/Pfizer, and Modus Therapeutics; and received research funding from Incyte and Novartis. A.G.B. reports no relevant COI related to this manuscript but has served on the scientific advisory board for TenSixteen Bio. M.J.W. reports no relevant COI related to this manuscript but has served as a consultant for Novartis, Vertex Pharmaceuticals, bluebird bio, and Fulcrum Therapeutics. S.L.S. reports no relevant COI related to this manuscript but has served on advisory boards or as a consultant for Agios, Beam Therapeutics, Forma Therapeutics/Novo Nordisk, Novartis, Global Blood Therapeutics/Pfizer, Chiesi Farmaceutici, and Fulcrum Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Santosh L. Saraf, Division of Hematology & Oncology, University of Illinois Chicago, 820 South Wood St, Suite 172, Chicago, IL 60612; email: ssaraf@uic.edu.
References
Author notes
The data that support the findings of this study are available on reasonable request from the corresponding author, Santosh L. Saraf (ssaraf@uic.edu) with appropriate institutional review board approval.
The full-text version of this article contains a data supplement.