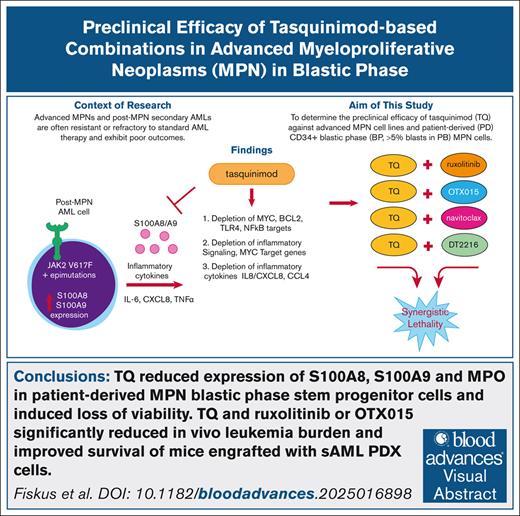
Key Points
TQ reduced expression of S100A8, S100A9, and myeloperoxidase in PD MPN-BP stem progenitor cells and induced loss of viability.
TQ and ruxolitinib or OTX015 significantly reduced leukemia burden and improved survival of mice engrafted with secondary AML PDX cells.
Visual Abstract
The alarmins, S100A8 (A8) and S100A9 (A9), are low molecular weight proteins belonging to the S100 protein family. A8 and A9 are secreted into the extracellular space and plasma, in which they interact with Toll-like receptor 4, receptor for advanced glycation end products, and CD33. In these studies, we determined the preclinical efficacy of tasquinimod (TQ) against advanced myeloproliferative neoplasm (MPN) cell lines and patient-derived (PD) CD34+ blastic phase (BP; >5% blasts in the peripheral blood) MPN cells. TQ induced loss of viability in cell lines and PD MPN-BP cells, but not in normal CD34+ progenitor cells. In TQ-treated PD MPN–acute myeloid leukemia (AML) cells, RNA-sequencing analysis showed negative enrichment of the gene sets of MYC and E2F targets, interleukin-6–JAK-STAT3 signaling, and of inflammatory response. In phenotypically defined, PD CD34+ MPN-BP stem progenitor cells, cytometry by time-of-flight analysis showed that TQ reduced expression of proteins including A8, A9, and myeloperoxidase, while increasing expression of Growth Factor Independence 1 (GFI1), p21, and cleaved Poly(ADP-ribose) polymerase (PARP). Cotreatment with TQ and ruxolitinib or Bromodomain and extraterminal domain (BET) inhibitor induced synergistic lethality in advanced MPN-BP cells. Monotherapy with TQ significantly improved survival of immune-depleted NOD scid gamma (NSG) mice engrafted with PD xenograft (PDX) cells of MPN-AML. Notably, cotreatment with TQ and ruxolitinib or OTX015 induced significantly greater survival than treatment with single agents in the NSG mice engrafted with the PDX cells. These findings clearly demonstrate the preclinical efficacy of TQ in advanced MPN-BP cells and create the rationale to further interrogate the efficacy of TQ-based combinations with the current, frontline therapies or novel agents in advanced MPNs with excess blasts.
Introduction
Hematopoietic stem/progenitor cells in advanced myeloproliferative neoplasms (MPNs) express pathogenetic, disease-defining, driver mutations in the JAK2, c-MPL, or calreticulin (CALR) genes.1 They also exhibit constitutive activation of JAK-STAT5/3 and NF-κB signaling, which is likely responsible for the aberrant biology, phenotype, and clinical features of MPNs.1-3 These include development of myelofibrosis (MF), extramedullary hematopoiesis, splenomegaly, and inflammatory cytokine-driven constitutional symptoms.4 Dysregulated inflammatory cytokines are the key pathogenic drivers of MPNs, most notably MF, in which elevated interleukin-2 receptor (IL-2R), IL-8, IL-12, IL-15, and IL-10 are significantly associated with poor survival.5 Elevations in IL-8 also predict leukemia-free survival in advanced MPNs.5 Additionally, cytokine elevations have also been found to be predictive of leukemic transformation, that is, post-MPN secondary AML (sAML),6-8 supporting the notion that inflammation is a key pathogenic driver of disease evolution. The connection between systemic inflammation and disease initiation/progression in MPNs is both intrinsic, as a direct result of driver mutations (eg, in JAK2) which upregulate expression of inflammatory cytokines, as well as due to the extrinsic factors mediated by proinflammatory milieu in the bone marrow (BM), promoting mutagenesis.2,5,6
The alarmins, S100A8 (A8) and S100A9 (A9), are low molecular weight proteins belonging to the S100 protein family.9 They are encoded by a locus on chromosome 1q21. A8 and A9 can form homodimers or heterodimers, also called calprotectins.9 A8 and A9 contain 2 EF-hand motifs, each made of 2 α-chains, separated by a peptide loop that binds Ca2+.9,10 A8 and A9 are expressed at relatively low levels in myeloid progenitors but are highly expressed in the cytoplasm of mature granulocytes and monocytes.11 A8 and A9 are secreted into the extracellular space and plasma, in which they interact with Toll-like receptor 4 (TLR4), receptor for RAGE (receptor for advanced glycation and end-product), and CD33.11,12 Intracellularly, they act as Ca2+ biosensors and activate reduced NAD phosphate oxidase, stimulating reactive oxygen species production, which activates the NOD-, LRR-, and pyrin domain–containing protein 3 inflammasome.9,10 A8 and A9 also amplify the inflammatory response through TLR4, as well as by inducing inflammatory cytokines, including tumor necrosis factor α (TNF-α) and IL-6, through activation of NF-κB and MAP kinase pathways.13 A8 and A9 are highly expressed in acute myeloid leukemia (AML), especially myelomonocytic and monocytic AML, which is associated with poor prognosis.11 They are also present on the surface of AML blasts and secreted by them into plasma. Blocking A8 impairs progression of AML in the mouse H9M1 (HOXA9 and MEIS1) model.11 In AML cells, A9 treatment activates extracellular signal-regulated kinase 1/2 (ERK1/2) and JNK signaling through TLR4.10,11 Recently, sensitivity of AML cells to venetoclax was negatively correlated with A8 and A9 expressions.14 High A8 and A9 expression was also associated with resistance to venetoclax in the BEAT AML data set.15 Bromodomain and extraterminal domain (BET) inhibitor treatment has been shown to inhibit the expression of A8 and A9 in AML cells.16 In a recent report, the alarmin heterocomplex A8/A9 was highly expressed in myeloid and stromal cells in the single-cell RNA-sequencing (scRNA-seq) analysis of BM niches of primary myelofibrosis (PMF) samples.17 This increase in A8/A9 expression in MPNs marked progression of BM fibrosis in PMF.17 In the same report, to determine whether A8/A9 is a therapeutic target in PMF, the effect of tasquinimod (TQ; Active Biotech) was determined in the murine JAK2-V617F–driven PMF model.17 TQ is a quinoline-3-carboxamide linomide analog, orally active, immune-modulatory, anti-angiogenic and allosteric inhibitor of histone deacetylase 4 (HDAC4). TQ was shown to bind A8/A9 alarmin heterocomplex, inhibiting its interaction with TLR and RAGE receptors.17,18 Notably, treatment with TQ reduced myeloproliferation and splenomegaly as well as significantly reduced PMF in the JAK2-V617F mice.17 There is no standard therapy for advanced MPN in the blastic phase (BP) and intensive chemotherapy, DNA methyltransferase inhibitor, and/or venetoclax-based regimens, or JAK inhibitor (ruxolitinib)–based regimens, do not improve the dismal outcome in these patients who have a median survival of <6 months.19-23 This highlights the significant and unmet need to develop novel therapies and combinations for the treatment of advanced MPN. In these studies, we determined in vitro and in vivo efficacy of TQ against cellular models of advanced MPN and patient-derived (PD) CD34+ BP (>5% blasts in the peripheral blood [PB]) MPN cells. Our findings show that treatment with TQ induced significant loss of viability in the MPN-AML SET2, HEL92.1.7 cell lines and PD MPN-BP cells, but not in normal CD34+ progenitor cells. We also performed transcriptome and proteome analysis in post-MPN sAML cells. In PD MPN-AML cells, RNA-seq analysis showed that exposure to TQ led to negative enrichment of the gene sets of MYC and E2F targets, of TORC1 and IL-6–JAK/STAT3 signaling, as well as of inflammatory response in PD MPN-AML cells. Mass spectrometry of PD post-MPN sAML cells after TQ treatment demonstrated depletion of proteins involved in signal transduction, cytokine signaling, and transcription. Cytometry by time-of-flight (CyTOF) analysis on PD post-MPN sAML cells showed that in phenotypically defined CD34+ stem/progenitor cells, with high levels of CLEC12A, CD99, and CD123 but low expression of CD11b, TQ treatment reduced the protein expressions of A8, A9, phosphorylated ERK, myeloperoxidase (MPO), CXCR4, cyclin D1, Purine-rich box1 (PU.1), and Ki67, whereas the levels of GFI1, p21, and cleaved PARP were increased. Importantly, cotreatment with TQ and ruxolitinib or BET inhibitor OTX015 or pelabresib (CPI0610), induced synergistic lethality in advanced MPN-BP cells. Furthermore, monotherapy with TQ in NSG mice engrafted with MPN-AML cells (mutant CALR and mutant Telomerase reverse transcriptase [TERT]) significantly improved survival without inducing host toxicity. Compared with single agent treatment, cotreatment with TQ and ruxolitinib or OTX015 also significantly improved survival of the NSG mice. Our findings demonstrate the preclinical efficacy of TQ and/or JAK or BET inhibitors or with novel agents identified here in advanced MPN and MPN-AML cells. They also create the rationale to further interrogate the preclinical efficacy of the TQ-based combinations against cellular models of advanced MPN.
Materials and methods
Reagents
TQ for in vitro and in vivo studies was obtained through a material transfer agreement with Active Biotech AB (Lund, Sweden). Navitoclax and DT-2216 for in vitro studies as well as OTX015 and ruxolitinib for in vitro and in vivo studies were obtained from MedChem Express (Monmouth Junction, NJ).
Cell lines and cell culture
SET-2 (catalog no. ACC 608; RRID:CVCL_2187) cells were obtained from the German Collection of Microorganisms and Cell Cultures GmbH (DSMZ) (Braunschweig, Germany). HEL92.1.7 (catalog no. TIB-180; RRID:CVCL_2481) and HS5 (catalog no. CRL-3611; RRID:CVCL_3720) cells were obtained from American Type Culture Collection (Manassas, VA). HEK-293T (RRID:CVCL_0063) cells were obtained from the Characterized Cell Line Core Facility at MD Anderson Cancer Center (Houston, TX). All experiments with cell lines were performed within 6 months after thawing or obtaining from American Type Culture Collection or DSMZ.
Assessment of percentage nonviable cells
After designated treatments (72-96 hours), cultured cell lines or PD sAML blast cells, were washed with 1× phosphate-buffered saline, stained with TO-PRO-3 iodide and analyzed by flow cytometry on a BD Accuri CFlow-6 flow cytometer (BD Biosciences, San Jose, CA). We used matrix dosing of agents in combinations to allow synergy assessment using the SynergyFinder V2/V3 online web application tool and delta synergy scores by Zero Interaction Potency (ZIP) method.24,25 We also assessed cell viability using a CellTiter-Glo assay following the manufacturer’s protocol. Luminescence was measured for 1 second on a BioTek Synergy H1 hybrid multimode microplate reader (RRID:SCR_019748).
Statistical analysis
Significant differences between values obtained in sAML cells treated with different experimental conditions compared with dimethyl sulfoxide–treated control cells were determined using the Student t test in GraphPad version 10 (RRID:SCR_002798). For the in vivo mouse models, a 2-tailed, unpaired t test was used for comparing total bioluminescent flux differences between vehicle and single-agent–treated or between single-agent– and combination-treated mice. For survival analysis, a Kaplan-Meier survival plot and a Mantel-Cox log-rank test was used for comparisons of different cohorts. P values of <.05 were assigned significance.
Primary PB and/or BM aspirate samples were obtained with informed consent from patients with sAML transformed from high risk (score of ≥3) MF (according to the International Prognostic Scoring System) under an Institutional Review Board (IRB)-sanctioned protocol. All in vivo studies were approved by and conducted in accordance with the guidelines of the Institutional Animal Care and Use Committee (IACUC) at the MD Anderson Cancer Center, an Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC)-accredited facility.
Detailed methods for RNA-seq, mass spectrometry, depositing data in the ProteomeXchange26 and CyTOF analyses are provided in the supplemental Materials and methods.
Results
Treatment with TQ induced loss of viability in post-MPN sAML cells while sparing BM stromal cells and normal CD34+ hematopoietic stem/progenitor cells
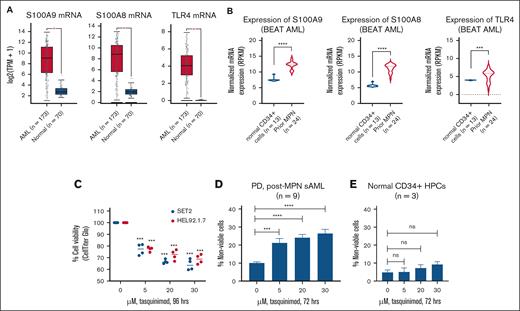
We first determined the expression of S100A8 and S100A9 messenger RNA (mRNA) in 173 patients for whom RNA-seq data were available in The Cancer Genome Atlas AML data set.27 Using a z-score threshold of 1.4, we observed that 12% of patients had high expression of S100A9, whereas 11% had high expression of S100A8 (supplemental Figure 1A). We also compared the mRNA expression of the 173 TCGA samples with 70 normal cell samples. As shown in Figure 1A, the mRNA expression of S100A8, S100A9, and TLR4 is significantly higher in PD AML cells than in normal cells.28 The expression levels of S100A8, S100A9 and TLR4 were also noted to be significantly higher in AML samples arising from a previous MPN (n = 24 previous MPN vs 13 normal CD34+ samples) in the BEAT AML data set (Figure 1B).15 We next determined the lethal activity of TQ in human post-MPN sAML cells. Treatment with TQ dose-dependently and significantly (P < .05) reduced the percentage of viable HEL92.1.7 and SET-2 cells as measured by a CellTiter-Glo assay, as shown in Figure 1C). Importantly, TQ also induced significant (P < .005) loss of cell viability in 9 samples of PD post-MPN sAML cells (Figure 1D). The oncoplot of the next-generation sequencing–detected mutations in the PD post-MPN sAML cells is shown in supplemental Figure 1B. Additionally, shown in supplemental Figure 1C and Figure 1E, TQ treatment did not inhibit the viability of HS5 BM stromal cells and was relatively sparing of normal CD34+ progenitor cells and induced <10% loss of cell viability at a 30 μM dose.
Treatment with TQ induced loss of viability in cultured and PD sAML cells while sparing normal CD34+ HPCs. (A) mRNA expression of S100A9, S100A8, and TLR4 in TCGA AML samples (n = 173) vs 70 normal samples from the GEPIA server. ∗P < .05. (B) mRNA expression of S100A9, S100A8, and TLR4 in post-MPN sAML cells (n = 24) compared with normal CD34+ cells (n = 13) from the BEAT AML data set. ∗∗∗P < .005; ∗∗∗∗P < .001. (C) SET-2 and HEL92.1.7 cells were treated with the indicated concentrations of TQ for 96 hours. At the end of treatment, relative cell viability was determined using a CellTiter-Glo assay. The percentage of viable cells in each condition was normalized relative to the untreated control cells. Columns, mean of 2 independent experiments performed in duplicate; bars, standard error of the mean (SEM). (D) PD post-MPN sAML cells (n = 9) were treated with the indicated concentrations of TQ for 72 hours. After this, the cells were washed with 1× phosphate-buffered saline (PBS) and stained with TO-PRO-3 iodide. The percentage of TO-PRO-3 iodide–positive, nonviable cells were determined by flow cytometry. Columns, mean of 9 samples; bars, SEM. ∗∗∗P < .005; ∗∗∗∗P < .001, compared with the control cells. (E) Normal CD34+ HPCs were treated with the indicated concentrations of TQ for 72 hours. After this, the cells were washed with 1× PBS and stained with TO-PRO-3 iodide. The percentage of TO-PRO-3 iodide–positive, nonviable cells was determined by flow cytometry. HPC, hematopoietic stem/progenitor cell; ns, not significant; TPM, transcripts per million.
Treatment with TQ induced loss of viability in cultured and PD sAML cells while sparing normal CD34+ HPCs. (A) mRNA expression of S100A9, S100A8, and TLR4 in TCGA AML samples (n = 173) vs 70 normal samples from the GEPIA server. ∗P < .05. (B) mRNA expression of S100A9, S100A8, and TLR4 in post-MPN sAML cells (n = 24) compared with normal CD34+ cells (n = 13) from the BEAT AML data set. ∗∗∗P < .005; ∗∗∗∗P < .001. (C) SET-2 and HEL92.1.7 cells were treated with the indicated concentrations of TQ for 96 hours. At the end of treatment, relative cell viability was determined using a CellTiter-Glo assay. The percentage of viable cells in each condition was normalized relative to the untreated control cells. Columns, mean of 2 independent experiments performed in duplicate; bars, standard error of the mean (SEM). (D) PD post-MPN sAML cells (n = 9) were treated with the indicated concentrations of TQ for 72 hours. After this, the cells were washed with 1× phosphate-buffered saline (PBS) and stained with TO-PRO-3 iodide. The percentage of TO-PRO-3 iodide–positive, nonviable cells were determined by flow cytometry. Columns, mean of 9 samples; bars, SEM. ∗∗∗P < .005; ∗∗∗∗P < .001, compared with the control cells. (E) Normal CD34+ HPCs were treated with the indicated concentrations of TQ for 72 hours. After this, the cells were washed with 1× PBS and stained with TO-PRO-3 iodide. The percentage of TO-PRO-3 iodide–positive, nonviable cells was determined by flow cytometry. HPC, hematopoietic stem/progenitor cell; ns, not significant; TPM, transcripts per million.
Treatment with TQ negatively enriched gene sets for MYC targets, E2F targets, inflammatory response, and JAK-STAT3 signaling in post-MPN sAML cells
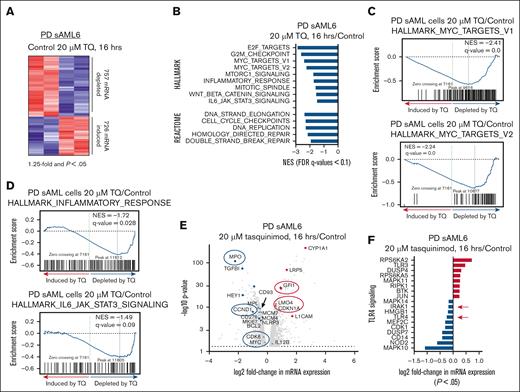
We next performed RNA-seq analysis on PD CD34+ sAML cells treated with TQ. After 16 hours of treatment, 757 mRNAs were depleted whereas 726 were induced >1.25-fold, with a P value <.05 (Figure 2A). Gene set enrichment analysis of TQ-treated cells demonstrated negative enrichment for HALLMARK and REACTOME gene sets including MYC targets, E2F targets, inflammatory response, and IL-6–JAK/STAT3 signaling (Figure 2B-D).29 A heat map of selected MYC targets and volcano plots of genes involved in the inflammatory response and JAK-STAT3 signaling are shown in supplemental Figure 2A-B. Figure 2E shows a volcano plot of the log2 fold-change in selected mRNAs downregulated or upregulated by TQ treatment. These included MPO, MPL, CCND1, BCL2, CDK6, MYC, GFI1, and CDKN1A (Figure 2E). Notably, TQ treatment also significantly depleted expression of mRNAs involved in TLR4 signaling including TLR4 and IRAK1 and NF-κB target genes (Figure 2F; supplemental Figure 2C). We also conducted RNA-seq in SET-2 cells treated with 20 μM of TQ. As shown in supplemental Figure 2D, after 16 hours of treatment, 324 mRNAs were depleted whereas 252 were induced >1.25-fold with a P value <.05. The Venn diagrams in supplemental Figure 2E show that 91 mRNAs were commonly upregulated and 94 mRNAs were commonly downregulated between SET2 and the PD sAML cells treated with TQ. The bar graphs in supplemental Figure 2F show the log2 fold-change of specific mRNAs within the REACTOME_IMMUNE_SYSTEM and REACTOME_SIGNAL_TRANSDUCTION gene sets. Similar gene sets and mRNA expression alterations were also observed in a separate PD MF-MPN sample treated with TQ and analyzed by RNA-seq (supplemental Figure 2G-H).
Treatment with TQ depletes mRNA expression and causes negative enrichment of MYC targets, inflammatory response and IL-6–JAK/STAT signaling pathways in post-MPN sAML cells. (A) PD post-MPN (JAK2-V617F) sAML cells (number 6 on the oncoplot) were treated with 20 μM of TQ for 16 hours as biologic replicates. Total RNA was isolated and used for RNA-seq analysis. The heat map shows the number of mRNAs depleted or induced >1.25-fold with a P value <.05. (B) Gene set enrichment analysis of TQ-treated sAML cells compared with HALLMARK and REACTOME pathways. All q-values are <0.1. (C-D) Gene set enrichment plots for HALLMARK_MYC_TARGETS, INFLAMMATORY RESPONSE, and IL-6_JAK_STAT3_SIGNALING. (E) Volcano plot of log2 fold-change vs –log10P value for all mRNA with >1.25-fold change up or down and a P value <.05. (F) Log2 fold-change (P < .05) for TLR4 signaling genes in the RNA-seq analysis from the PD sAML cells (biologic replicates) treated with 20 μM of TQ for 16 hours. FDR, false discovery rate; NES, normalized enrichment score.
Treatment with TQ depletes mRNA expression and causes negative enrichment of MYC targets, inflammatory response and IL-6–JAK/STAT signaling pathways in post-MPN sAML cells. (A) PD post-MPN (JAK2-V617F) sAML cells (number 6 on the oncoplot) were treated with 20 μM of TQ for 16 hours as biologic replicates. Total RNA was isolated and used for RNA-seq analysis. The heat map shows the number of mRNAs depleted or induced >1.25-fold with a P value <.05. (B) Gene set enrichment analysis of TQ-treated sAML cells compared with HALLMARK and REACTOME pathways. All q-values are <0.1. (C-D) Gene set enrichment plots for HALLMARK_MYC_TARGETS, INFLAMMATORY RESPONSE, and IL-6_JAK_STAT3_SIGNALING. (E) Volcano plot of log2 fold-change vs –log10P value for all mRNA with >1.25-fold change up or down and a P value <.05. (F) Log2 fold-change (P < .05) for TLR4 signaling genes in the RNA-seq analysis from the PD sAML cells (biologic replicates) treated with 20 μM of TQ for 16 hours. FDR, false discovery rate; NES, normalized enrichment score.
TQ treatment concordantly depleted RNA and protein expressions in bulk PD post-MPN sAML cells as well as in sAML stem/progenitor cells
We next performed RNA-seq and whole-proteome mass spectrometry analysis on cells from the same JAK2-V617F–expressing sAML replicate samples treated with TQ or dimethyl sulfoxide. The volcano plot in Figure 3A shows that TQ treatment significantly depleted expression of MPO, CD34, CCND1, CSF1R, and MPL with concomitant induction of TXNIP, DDIT3, and BCL2L11. TQ treatment also caused significant alterations in protein expressions determined by tandem mass tag mass spectrometry (Figure 3B-C). Using a threshold of 1.25-fold change up or down and P value <.05, TQ significantly depleted protein expressions including MPO, CD93, and CD34, with concomitant induction of proteins including HSF1, FBXW7, GADD45A, and cytochrome p450 1A1 (Figure 3C). We also analyzed the number of TQ-mediated concordant mRNA and protein expression changes. The scatterplot in Figure 3D (complete table in supplemental Figure 3) shows that TQ treatment resulted in concordant expression alterations with 48 depleted and 13 induced mRNA and proteins in the PD JAK2-V617F–expressing sAML cells.30 We next conducted CyTOF analyses to determine the effects of TQ on protein expression in post-MPN sAML stem/progenitor cells. Figure 3E shows that in 2 PD post-MPN JAK2-V617F–expressing sAML samples phenotypically defined as stem/progenitor cells by high expression of CLEC12A, CD99, CD123, and CD33 but low expression of CD11b,31,32 TQ treatment reduced expression of S100A8 and S100A9, MPO, PU.1, KI67, and CDK6 while concomitantly upregulating the expression of PUMA, MCL1, and GFI1.
Treatment with TQ induced concordant alterations in the transcriptome and proteome of post-MPN sAML cells and reduced protein expression of S100A8 and S100A9 in phenotypically defined post-MPN sAML stem/progenitor cells. (A) PD (JAK2-V617F) sAML cells (sample 13 on the oncoplot) were treated with 20 μM of TQ for 16 hours in biologic duplicates. After this, total RNA was isolated and used for RNA-seq analysis. The volcano plot shows the log2 fold-change vs –log10P value for all mRNA with >1.25-fold change up or down with a P value <.05. (B) PD (JAK2-V617F) sAML cells (sample 13 on the oncoplot) were treated with the indicated concentration of TQ for 48 hours in biologic duplicates. At the end of treatment, cells were harvested and used for whole-proteome tandem mass spectrometry. The volcano plot shows the log2 fold-change vs –log10P value for all proteins with >1.2-fold change up or down with a P value <.05. (C) Log2 fold-change in selected protein expressions in TQ-treated PD post-MPN sAML cells compared with dimethyl sulfoxide control cells. Threshold is proteins with greater than a 1.2-fold change up or down and a P value <.05. (D) Scatterplot of TQ-induced, concordant mRNA and protein expression changes in PD post-MPN sAML 13 cells. (E) PD (JAK2-V617F) sAML cells (9 and 14 from the oncoplot) were treated with 20 μM of TQ for 48 hours. Cells were used for CyTOF analysis with a cocktail of rare metal–tagged antibodies to define stem/progenitor cells and other sAML-relevant oncoproteins. Panel shows a heat map of log2 fold-change in protein expression from cells treated with 20 μM of TQ for 48 hours compared with the control cells. Hi, high; Lo, low.
Treatment with TQ induced concordant alterations in the transcriptome and proteome of post-MPN sAML cells and reduced protein expression of S100A8 and S100A9 in phenotypically defined post-MPN sAML stem/progenitor cells. (A) PD (JAK2-V617F) sAML cells (sample 13 on the oncoplot) were treated with 20 μM of TQ for 16 hours in biologic duplicates. After this, total RNA was isolated and used for RNA-seq analysis. The volcano plot shows the log2 fold-change vs –log10P value for all mRNA with >1.25-fold change up or down with a P value <.05. (B) PD (JAK2-V617F) sAML cells (sample 13 on the oncoplot) were treated with the indicated concentration of TQ for 48 hours in biologic duplicates. At the end of treatment, cells were harvested and used for whole-proteome tandem mass spectrometry. The volcano plot shows the log2 fold-change vs –log10P value for all proteins with >1.2-fold change up or down with a P value <.05. (C) Log2 fold-change in selected protein expressions in TQ-treated PD post-MPN sAML cells compared with dimethyl sulfoxide control cells. Threshold is proteins with greater than a 1.2-fold change up or down and a P value <.05. (D) Scatterplot of TQ-induced, concordant mRNA and protein expression changes in PD post-MPN sAML 13 cells. (E) PD (JAK2-V617F) sAML cells (9 and 14 from the oncoplot) were treated with 20 μM of TQ for 48 hours. Cells were used for CyTOF analysis with a cocktail of rare metal–tagged antibodies to define stem/progenitor cells and other sAML-relevant oncoproteins. Panel shows a heat map of log2 fold-change in protein expression from cells treated with 20 μM of TQ for 48 hours compared with the control cells. Hi, high; Lo, low.
TQ treatment attenuated inflammatory cytokine release in post-MPN sAML cells
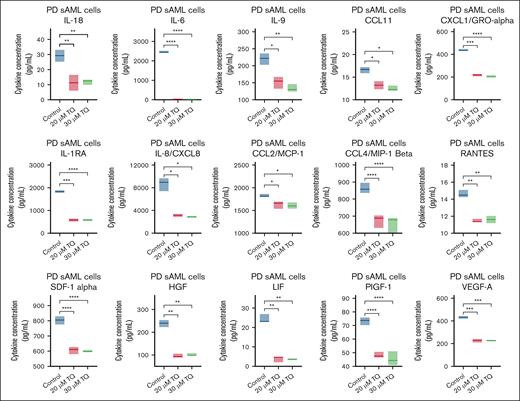
MPN and post-MPN sAML cells exhibit increased inflammatory signaling. Moreover, chronic inflammation is a hallmark of MPN and plays an integral role in its biology as well as progression.33 Increased levels of macrophage inflammatory protein 1β (MIP-1β; CCL4/MIP-1β), IL-1Rα, IL-6, and TNF-α are partly responsible for the inflammatory state in MPN and post-MPN sAML cells.5,6,34,35 S100A8 and S100A9 also induce inflammatory cytokines, including TNF-α and IL-6.13 We next determined the effects of TQ treatment on cytokines secreted from PD post-MPN sAML cells using a Luminex assay measuring 45 cytokines. As shown in Figure 4 and supplemental Figure 4A-B, TQ significantly reduced the expression levels of many inflammatory cytokines including IL-6, IL-1Rα, IL-8/CXCL8, CXCL1/Growth regulated protein alpha (GRO-α), CCL4/MIP-1β, Hepatocyte growth factor (HGF), and Leukemia Inhibitory Factor (LIF). TQ treatment also modestly lowered the levels of S100A8/A9 dimer (active form) in the supernatants from 3 post-MPN sAML samples (supplemental Figure 4C).
Treatment with TQ depleted IL-1Rα, IL-8/CXCL8, MIP-1β, and other cytokine expressions in PD post-MPN sAML cells. PD post-MPN (mutant CALR) sAML cells (sample 16 on the oncoplot) were treated in duplicate with the indicated concentrations of TQ for 48 hours. At the end of treatment, culture supernatant was harvested and frozen at −80°C. A 45-cytokine panel assay was used to determine the levels of cytokines including IL-1Rα, IL-8/CXCL8, MIP-1β, SDF1α, HGF, LIF, and CXCL1 in the untreated and TQ-treated samples. ∗P < .05; ∗∗P < .01; ∗∗∗P < .005; ∗∗∗∗P < .001.
Treatment with TQ depleted IL-1Rα, IL-8/CXCL8, MIP-1β, and other cytokine expressions in PD post-MPN sAML cells. PD post-MPN (mutant CALR) sAML cells (sample 16 on the oncoplot) were treated in duplicate with the indicated concentrations of TQ for 48 hours. At the end of treatment, culture supernatant was harvested and frozen at −80°C. A 45-cytokine panel assay was used to determine the levels of cytokines including IL-1Rα, IL-8/CXCL8, MIP-1β, SDF1α, HGF, LIF, and CXCL1 in the untreated and TQ-treated samples. ∗P < .05; ∗∗P < .01; ∗∗∗P < .005; ∗∗∗∗P < .001.
TLR4 knockdown in HS5 stromal cells induced CXCL8/IL-8, VEGF-A, LIF, and CXCL1 while TQ reduced CXCL1, CXCL10, RANTES, FGF2, and CCL2/MCP-1 and enriched TNF-α signaling via NF-κB
To determine the transcriptomic and cytokine expression effects of TQ treatment on the stromal microenvironment, we treated HS5 stromal cells with TQ, with and without TLR4 depletion by siRNA. supplemental Figure 5A shows that knockdown of TLR4 in HS5 depleted 65 mRNA expressions including TLR4 and TIMP3, while concomitantly inducing 91 mRNA expressions including CXCL3/MIP-2β, CXCL2, CXCL8 (IL-8), IL-1α and NF-κBIZ (IκB-ζ), which regulates inflammation and activates IL-6 production. TLR4 knockdown resulted in positive enrichment of gene sets involved in translation initiation and translation elongation as well as gene sets involved in cellular response to starvation and was associated with decreased proliferation of the TLR4 depleted cells (supplemental Figures 5B-C). TQ treatment also caused significant alterations in mRNA expressions in HS5 stromal cells determined by RNA-seq analysis (supplemental Figure 5D). Using a threshold of 1.20-fold change and P value <.05, TQ treatment significantly induced 283 mRNA expressions including IL-6, LIF, Vascular endothelial growth factor A (VEGF-A), CXCL2, IL-1A, CXCL8, as well as GADD45A and CDKN1A (p21). This was associated with positive enrichment of the TNF-α signaling via NF-κB pathway (Normalized Enrichment Score [NES] 1.95; q = 0.13; supplemental Figure 5E). Interestingly, in HS5 stromal cells with TLR4 knockdown, although treatment with TQ altered less mRNA expressions overall (47 mRNAs depleted and 161 mRNAs induced) expression of IL-6, LIF, VEGF-A, CXCL2, IL-1A, CXCL8 as well as GADD45A and BCL2L11 (BIM) were induced (supplemental Figure 5F). These expression alterations were negatively correlated with HALLMARK_MYC targets gene sets (supplemental Figure 5G). Additionally, knockdown of TLR4 led to induction of IL-8/CXCL8, VEGF-A, LIF and CXCL1 cytokine expression in the stromal cells (supplemental Figure 5H). In contrast, treatment with TQ depleted cytokine expression of CXCL1, CXCL10, RANTES, FGF2, CCL2/MCP-1 but induced LIF expression (supplemental Figure 5H). Furthermore, TQ treatment in cells with TLR4 knockdown exhibited significantly greater expression of GMCSF, VEGF-A, and LIF (supplemental Figure 5H).
Treatment with TQ-based combinations induced synergistic loss of viability in post-MPN sAML cells
In sAML, because TQ monotherapy would be unlikely to be curative, we next determined whether TQ would be synergistically lethal with ruxolitinib in post-MPN sAML cells. Indeed, as shown in Table 1 and supplemental Figure 6A, cotreatment with TQ and ruxolitinib induced synergistic loss of viability in 3 samples of post-MPN sAML, with delta synergy scores of >1.0 by the ZIP method at each concentration of the drugs. Additionally, because post-MPN sAML and MPN-BP cells are molecularly distinct from de novo AML and exhibit greater dependence on B-cell lymphoma-extra-large (BCL-xL) than B-cell lymphoma-2 (BCL2)25 (supplemental Figure 6B), we determined whether cotreatment with TQ and the BCL2/BCL-xL inhibitor navitoclax or the BCL-xL degrader DT-2216 would be synergistically lethal in post-MPN sAML cells. As shown in supplemental Figure 6C-D, cotreatment with TQ and navitoclax or DT-2216 induced synergistic loss of viability in PD post-MPN sAML samples, with delta synergy scores >1.0 by the ZIP method at each concentration of the drugs. We next conducted a CRISPR screen in SET-2 cells with and without TQ treatment to determine cellular dependencies and TQ-mediated codependencies in post-MPN sAML cells. As shown in supplemental Figure 6E, among the guide RNAs that dropped out at day-12 after transduction compared with day-2 in SET-2 cells was BRD4, representing a cellular dependency and druggable vulnerability. Due to the druggable nature of this target, we next determined the effects of cotreatment with TQ and BET inhibitor OTX015 or CPI0610 against post-MPN sAML cells. Table 1 and supplemental Figure 6F-G show that cotreatment with TQ and OTX015 or CPI0610 induced synergistic loss of viability in 3 samples of post-MPN sAML, with delta synergy scores of >1.0 by the ZIP method at each concentration of the drugs. Importantly, the domain-specific CRISPR screen in SET-2 cells also identified potentially druggable TQ treatment codependencies, including the arginine methyltransferase CARM1 and the histone demethylase JMJD6, as well as TQ treatment–associated coenrichments, including HDAC3 and NSD2, suggesting these also as potentially druggable targets for modulating TQ activity (supplemental Figure 6H). Notably, cotreatment with TQ and the HDAC3-specific inhibitor RGFP966 induced synergistic in vitro loss of viability of SET-2 and HEL92.1.7 cells supporting the results of the CRISPR screen in SET-2 cells (supplemental Figure 6I). We next determined the effects of BET inhibitor OTX015, or of ruxolitinib on the mRNA expression of S100A8 and S100A9 in 2 PD post-MPN sAML cell samples by quantitative polymerase chain reaction analysis. As shown in supplemental Figure 7A-B, treatment with OTX015 for 16 hours significantly depleted the mRNA expression of S100A8 and S100A9, as well as altered the expression of well-known BET inhibitor biomarkers of response including MYC, CD93, HEXIM1, and DCXR.36-38 Conversely, treatment with ruxolitinib had no notable effects on the expression of S100A8 or S100A9 in the sAML cells (supplemental Figure 7C-D). Treatment with TQ and OTX015 reduced the protein expression of pSTAT3, pIkB-α, c-Myc, CDK6, S100A9, TLR4, and HDAC4 in the cell lysates of 2 PD post-MPN sAML samples (supplemental Figure 7E-F).
Cotreatment with TQ and ruxolitinib, OTX015, or pelabresib (CPI0610) exerted synergistic lethality against PD post-MPN sAML cells
| Combination agent . | TQ . | ||
|---|---|---|---|
| 5 μM . | 10 μM . | 20 μM . | |
| Ruxolitinib | |||
| 250 nM | 5.604 | 1.308 | 6.535 |
| 500 nM | 3.912 | 5.400 | 4.598 |
| 1000 nM | 8.245 | 5.736 | 9.062 |
| OTX015 | |||
| 50 nM | 2.562 | 3.206 | 4.540 |
| 100 nM | 1.603 | 2.629 | 2.806 |
| 250 nM | 4.041 | 3.840 | 3.838 |
| Pelabresib | |||
| 100 nM | 2.245 | 7.082 | 12.276 |
| 250 nM | 5.561 | 7.764 | 20.382 |
| 500 nM | 2.110 | 3.558 | 20.232 |
| Combination agent . | TQ . | ||
|---|---|---|---|
| 5 μM . | 10 μM . | 20 μM . | |
| Ruxolitinib | |||
| 250 nM | 5.604 | 1.308 | 6.535 |
| 500 nM | 3.912 | 5.400 | 4.598 |
| 1000 nM | 8.245 | 5.736 | 9.062 |
| OTX015 | |||
| 50 nM | 2.562 | 3.206 | 4.540 |
| 100 nM | 1.603 | 2.629 | 2.806 |
| 250 nM | 4.041 | 3.840 | 3.838 |
| Pelabresib | |||
| 100 nM | 2.245 | 7.082 | 12.276 |
| 250 nM | 5.561 | 7.764 | 20.382 |
| 500 nM | 2.110 | 3.558 | 20.232 |
Table shows delta synergy scores for the combination agents with TQ at the doses listed in 3 PD post-MPN sAML cells. Delta synergy scores (ZIP method) of TQ concentrations in PD sAML cells (n = 3).
Cotreatment with TQ and BET inhibitor reduced leukemia burden in mice transplanted with fulminant murine JAK2-V617F and TP53-KO AML
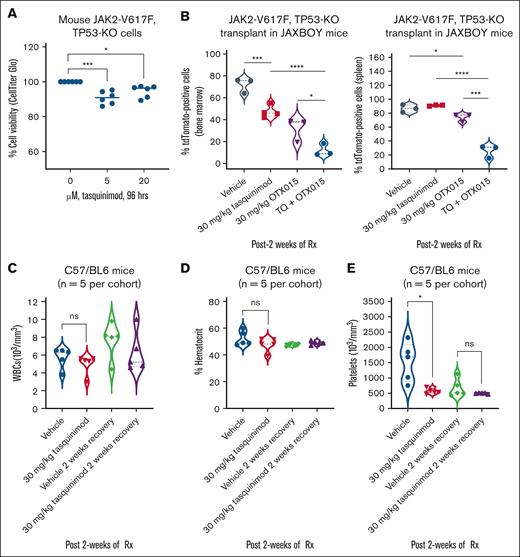
Using a previously developed and well-characterized murine model of erythroleukemia expressing JAK2-V617F with knockout of TP53 (TP53-KO),39,40 we first determined the in vitro effects of TQ treatment. Cells were treated for 96 hours and the effects on cell viability were assessed by CellTiter-Glo assay. As shown in Figure 5A, treatment with TQ modestly but significantly reduced the cell viability of these erythroleukemia cells despite loss of TP53. We next determined the in vivo effects of TQ alone and in combination with OTX015 on leukemia burden in the BM and spleen of JAXBOY mice transplanted with JAK2-V617F + TP53-KO erythroleukemia cells. As shown, treatment with TQ and/or OTX015 for 2 weeks significantly reduced the leukemia burden of this aggressive leukemia as measured by dTomato fluorescence and decreased spleen lengths (Figure 5B; supplemental Figure 7G). Despite a significant reduction in the leukemia burden in the mice, due to the highly aggressive nature of this murine model, there was no improvement in the median or overall survival of the mice treated with TQ alone or in combination with OTX015. Importantly, in nonleukemic, immunocompetent C57/BL6 mice, TQ treatment caused only modest and reversible effects on platelets without change in white blood cells or hematocrit after 2 weeks of treatment (Figure 5C-E).
Cotreatment with TQ and BET inhibitor OTX015 reduced leukemia burden greater than either agent alone in a murine BM transplant model. (A) Murine JAK2-V617F, TP53-KO cells were treated with the indicated concentrations of TQ for 96 hours. At the end of treatment, relative cell viability was determined using a CellTiter-Glo assay. The percentage of viable cells in each condition was normalized relative to the untreated control cells. All the data points from 2 independent experiments performed in triplicate are shown; bar, median loss of viability. ∗P < .05; ∗∗∗P < .005. (B) JAXBOY mice were infused with 1 × 103 murine JAK2-V617F, TP53-KO cells plus 1 × 106 total BM cells from JAXBOY donor mice. Mice were treated with the indicated doses of TQ and/or OTX015 for 2 weeks. The mice were euthanized, and the spleen and BM cells were collected. The isolated cells were analyzed for dTomato fluorescence by flow cytometry. ∗P < .05; ∗∗∗P < .005; ∗∗∗∗P < .001. (C-E) C57/BL6 mice (n = 5 mice per cohort) were treated with vehicle or TQ for 2 weeks. Mice were bled and complete blood counts (CBCs) were performed. The mice were allowed to recover with no treatment for 2 weeks and then bled for CBC analysis. ∗P < .05, compared with vehicle-treated mice. ns, not significant; Rx, treatment; WBCs, white blood cells.
Cotreatment with TQ and BET inhibitor OTX015 reduced leukemia burden greater than either agent alone in a murine BM transplant model. (A) Murine JAK2-V617F, TP53-KO cells were treated with the indicated concentrations of TQ for 96 hours. At the end of treatment, relative cell viability was determined using a CellTiter-Glo assay. The percentage of viable cells in each condition was normalized relative to the untreated control cells. All the data points from 2 independent experiments performed in triplicate are shown; bar, median loss of viability. ∗P < .05; ∗∗∗P < .005. (B) JAXBOY mice were infused with 1 × 103 murine JAK2-V617F, TP53-KO cells plus 1 × 106 total BM cells from JAXBOY donor mice. Mice were treated with the indicated doses of TQ and/or OTX015 for 2 weeks. The mice were euthanized, and the spleen and BM cells were collected. The isolated cells were analyzed for dTomato fluorescence by flow cytometry. ∗P < .05; ∗∗∗P < .005; ∗∗∗∗P < .001. (C-E) C57/BL6 mice (n = 5 mice per cohort) were treated with vehicle or TQ for 2 weeks. Mice were bled and complete blood counts (CBCs) were performed. The mice were allowed to recover with no treatment for 2 weeks and then bled for CBC analysis. ∗P < .05, compared with vehicle-treated mice. ns, not significant; Rx, treatment; WBCs, white blood cells.
Treatment with TQ monotherapy or TQ-based combinations with BET inhibitor or ruxolitinib significantly improved survival of NSG mice engrafted with a PD xenograft (PDX) model of post-MPN sAML
We next asked whether TQ treatment would improve the survival of NSG mice engrafted with a luciferized HEL92.1.7 model. Mice were treated with vehicle or 30 mg/kg of TQ for 4 weeks. Figure 6A shows that, compared with vehicle-treated mice, mice treated with 30 mg/kg of TQ exhibited significantly greater median and overall survival (P < .05). We also determined the in vivo activity of TQ in NSG mice engrafted with a mutant CALR-expressing sAML PDX model (supplemental Figure 7H). Mice were treated with 10 and 30 mg/kg of TQ for 8 weeks. Figure 6B shows that mice treated with 10 and 30 mg/kg of TQ exhibited significantly greater survival than mice treated with vehicle. In a separate experiment, we also determined whether TQ-based combinations would reduce leukemia burden and further improve the survival of NSG mice engrafted with a post-MPN sAML PDX. Figure 6C-D show the reduction in spleen size after 6 weeks of treatment with TQ and/or ruxolitinib or OTX015. Cotreatment with TQ and ruxolitinib or OTX015 caused a greater reduction in spleen size than treatment with single agents. Additionally, mice treated with the combination of TQ and ruxolitinib or OTX015 exhibited significantly (P < .05) greater survival than mice treated with the single agents or vehicle control (Figure 6E).
Cotreatment with TQ and ruxolitinib or BET inhibitor OTX015 reduced spleen size and significantly improved survival of NSG mice bearing PDX models of post-MPN sAML. (A) NSG mice were infused with luciferized HEL92.1.7 cells and treated with TQ (30 mg/kg, daily, orally [gavage]) for 4 weeks. The survival of the mice is shown as a Kaplan-Meier survival plot. Significance was calculated by a Mantel-Cox log-rank test. ∗P < .05. (B) Kaplan-Meier survival plot of NSG mice (n = 6 per cohort) infused with a mutant CALR sAML PDX (CALR-E381 deletion, TERT A1062T mutation) and treated with vehicle or the indicated doses of TQ for 8 weeks. Significance was calculated by a Mantel-Cox log-rank test. (C) Spleen lengths of NSG mice infused with a mutant CALR sAML PDX and treated with vehicle, TQ and/or ruxolitinib, or OTX015 for 6 weeks. ∗P < .05; ∗∗P < .01. (D) Representative spleen images from panel C. (E) Kaplan-Meier survival plot of NSG mice infused with a mutant CALR sAML PDX (CALR-E381 deletion, TERT A1062T mutation) and treated with vehicle, the indicated doses of TQ and/or ruxolitinib, or OTX015 for 10 weeks. Significance was calculated by a Mantel-Cox log-rank test. ∗P < .05; ∗∗∗P < .005; ∗∗∗∗P < .001. GFP, green fluorescent protein; Luc, luciferase; mtCALR, mutant CALR; mtTERT, mutant TERT; RUX, Ruxolitinib; Rx, treatment.
Cotreatment with TQ and ruxolitinib or BET inhibitor OTX015 reduced spleen size and significantly improved survival of NSG mice bearing PDX models of post-MPN sAML. (A) NSG mice were infused with luciferized HEL92.1.7 cells and treated with TQ (30 mg/kg, daily, orally [gavage]) for 4 weeks. The survival of the mice is shown as a Kaplan-Meier survival plot. Significance was calculated by a Mantel-Cox log-rank test. ∗P < .05. (B) Kaplan-Meier survival plot of NSG mice (n = 6 per cohort) infused with a mutant CALR sAML PDX (CALR-E381 deletion, TERT A1062T mutation) and treated with vehicle or the indicated doses of TQ for 8 weeks. Significance was calculated by a Mantel-Cox log-rank test. (C) Spleen lengths of NSG mice infused with a mutant CALR sAML PDX and treated with vehicle, TQ and/or ruxolitinib, or OTX015 for 6 weeks. ∗P < .05; ∗∗P < .01. (D) Representative spleen images from panel C. (E) Kaplan-Meier survival plot of NSG mice infused with a mutant CALR sAML PDX (CALR-E381 deletion, TERT A1062T mutation) and treated with vehicle, the indicated doses of TQ and/or ruxolitinib, or OTX015 for 10 weeks. Significance was calculated by a Mantel-Cox log-rank test. ∗P < .05; ∗∗∗P < .005; ∗∗∗∗P < .001. GFP, green fluorescent protein; Luc, luciferase; mtCALR, mutant CALR; mtTERT, mutant TERT; RUX, Ruxolitinib; Rx, treatment.
Discussion
Although in other tumor settings, including prostate cancer, TQ has been reported to inhibit the occurrence of metastasis and delay tumor progression by modification of the tumor microenvironment and immunologic status,41,42 findings presented here demonstrate, to our knowledge, for the first time that TQ treatment directly exerts lethal activity in advanced MPN and post-MPN sAML cells. TQ also demonstrated significant in vivo activity against PDX models of sAML. The BEAT AML data showed that post-MPN sAML cells exhibited elevated levels of S100A8, S100A9, and TLR4 mRNA compared with normal CD34+ cells. This could explain why in our studies TQ treatment differentially induced loss of viability in post-MPN sAML cells while sparing the normal BM microenvironment cells, represented here by HS5 stromal cells. What gene expression perturbations were induced by TQ treatment that correlated with its differential cytotoxicity toward post-MPN sAML cells? Findings presented here also address this by showing that TQ treatment caused negative enrichment of mRNA gene sets of MYC targets, inflammatory response and IL-6–JAK/STAT3 signaling, as well as depleted multiple gene expressions within the TLR4 signaling pathway in post-MPN sAML cells. Concomitantly, after TQ treatment, the mass spectrometry analysis demonstrated depletion of proteins involved in signal transduction, cytokine signaling, and transcription in the PD post-MPN sAML cells. Importantly, the effects of TQ treatment extended to the phenotypically defined CD34+ stem/progenitor cells, in which, as demonstrated by CyTOF analysis, it reduced the protein expressions of A8, A9, p-ERK, MPO, CXCR4, cyclin D1, PU.1, and Ki67 whereas increasing the levels of GFI1, p21, and cleaved PARP. These TQ-induced protein expression alterations underpin the anti-sAML cytotoxicity due to TQ treatment in post-MPN sAML stem/progenitor cells.
In advanced MPN and post-MPN sAML cells, the hematopoietic stem cells secrete and display enhanced proliferation in response to IL-8/CXCL8 stimulation. Previous studies have also shown that IL-8 levels are higher in the BM and PB of patients with MPN than in healthy control individuals.5,34,43 Furthermore, previous studies have shown that expression of IL-6 is important for regulating the immune response by promoting B- and T-cell differentiation.34 IL-6 also induces the activation of multiple signaling pathways including JAK/STAT, PI3K, and ERK, and has been reported to play a role in disease progression in advanced MPN and post-MPN sAML cells.6,34 By depleting the levels of IL-6, IL-8/CXCL8, and CCL2/MCP1, TQ treatment likely dampens the cytokine-mediated signaling through these pathways in advanced MPN cells. TQ treatment depleted the expression of MYC targets and c-Myc protein in PD post-MPN sAML cells as well as improved median and overall survival of mice with established disease due to post-MPN sAML cells. These findings are like those reported by Fan et al, who demonstrated that TQ treatment depleted MYC targets and c-Myc levels, leading to significantly decreased cell proliferation and colony formation by multiple myeloma cells accompanied by decreased tumor load and improved overall survival of multiple myeloma–bearing mice.44
Previous reports have also shown TQ-based combinations with lenalidomide as well as with proteasome inhibitors exhibit improved efficacy in multiple myeloma cellular models.44 Consistent with this, these studies demonstrate that the combination of TQ and ruxolitinib exhibited synergistic in vitro anti-sAML activity against PD advanced MPN and post-MPN sAML cells. Furthermore, the combination of TQ and BET inhibitor OTX015 or pelabresib (CPI0610) also exhibited synergistic in vitro lethality in advanced MPN and post-MPN sAML cells. Importantly, compared with the single agents, the combination of TQ with either ruxolitinib or the BET inhibitor OTX015 significantly reduced the in vivo post-MPN sAML burden, leading to improvement in the median and overall survival of the mice with established PDX models of post-MPN sAML cells. These results are of considerable translational importance considering the encouraging data from the recently updated phase 3 clinical trial of ruxolitinib and pelabresib. These data highlighted meaningful reductions in spleen volume, total symptom score, improvements in anemia, and BM fibrosis due to pelabresib, as compared with treatment with ruxolitinib and placebo.45 Overall, our findings create the rationale to further interrogate the efficacy of TQ monotherapy and TQ-based combinations with current, frontline drugs, including ruxolitinib, or with other novel therapeutic agents such as pelabresib in the therapy of advanced MPN in BP (sAML).
Acknowledgments
The authors thank the Advanced Technology Genomics Core and Flow Cytometry and Cellular Imaging Core Facility, which are supported by the MD Anderson Cancer Center support grant 5P30 CA016672-40. Next-generation sequencing studies performed using the NovaSeq6000 were supported by a grant from the National Institutes of Health (NIH; 1S10OD024977-01). The Baylor College of Medicine Mass Spectrometry Proteomics Core is supported by the Dan L. Duncan Comprehensive Cancer Center award (P30 CA125123), Cancer Prevention & Research Institute of Texas (CPRIT) Core Facility awards (RP170005 and RP210227), Intellectual Developmental Disabilities Research Center award (P50 HD103555), and NIH High End Instrument award (S10 OD026804; Orbitrap Exploris 480). K.N.B. was supported by a grant from the NIH (R01 CA255721). This research is supported, in part, by the MD Anderson Cancer Center Leukemia Specialized Programs of Research Excellence (SPORE) (P50 CA100632). Parts of research presented were performed under a financed Material Transfer Agreement (MTA) between Active Biotech and the MD Anderson Cancer Center and K.N.B.
Authorship
Contribution: K.N.B. designed the study, analyzed data, and wrote the manuscript; X.S. performed bioinformatics analyses; W.F., C.P.M., C.E.B., K.D., H.H., J.A.D., S.S., and S.L. performed research and analyzed the data; A.J. and A.M. performed the mass spectrometry analyses and analyzed the data; L.M., T.M., A.D., T.M.K., C.D.D., P.B., N.P., R.K.R., and M.T. contributed critical reagents; and W.F. also wrote the manuscript.
Conflict-of-interest disclosure: M.T. is a shareholder in, and an employee of, Active Biotech AB. K.N.B. has received research funding from Iterion, Foghorn, and Nurix Pharmaceuticals; and serves as a consultant for Iterion Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Kapil N. Bhalla, Department of Leukemia, The University of Texas MD Anderson Cancer Center, 1400 Holcombe Blvd, Unit 428, Houston, TX 77030; email: kbhalla@mdanderson.org.
References
Author notes
RNA-sequencing data sets have been deposited in Gene Expression Omnibus (accession number GSE301154). The mass spectrometry proteomics data have been deposited to the ProteomeXchange consortium via the PRoteomics IDEntifications Database (PRIDE) (data set identifier PXD053548).
Other data are available on request from the corresponding author, Kapil N. Bhalla (kbhalla@mdanderson.org).
The full-text version of this article contains a data supplement.


![Cotreatment with TQ and ruxolitinib or BET inhibitor OTX015 reduced spleen size and significantly improved survival of NSG mice bearing PDX models of post-MPN sAML. (A) NSG mice were infused with luciferized HEL92.1.7 cells and treated with TQ (30 mg/kg, daily, orally [gavage]) for 4 weeks. The survival of the mice is shown as a Kaplan-Meier survival plot. Significance was calculated by a Mantel-Cox log-rank test. ∗P < .05. (B) Kaplan-Meier survival plot of NSG mice (n = 6 per cohort) infused with a mutant CALR sAML PDX (CALR-E381 deletion, TERT A1062T mutation) and treated with vehicle or the indicated doses of TQ for 8 weeks. Significance was calculated by a Mantel-Cox log-rank test. (C) Spleen lengths of NSG mice infused with a mutant CALR sAML PDX and treated with vehicle, TQ and/or ruxolitinib, or OTX015 for 6 weeks. ∗P < .05; ∗∗P < .01. (D) Representative spleen images from panel C. (E) Kaplan-Meier survival plot of NSG mice infused with a mutant CALR sAML PDX (CALR-E381 deletion, TERT A1062T mutation) and treated with vehicle, the indicated doses of TQ and/or ruxolitinib, or OTX015 for 10 weeks. Significance was calculated by a Mantel-Cox log-rank test. ∗P < .05; ∗∗∗P < .005; ∗∗∗∗P < .001. GFP, green fluorescent protein; Luc, luciferase; mtCALR, mutant CALR; mtTERT, mutant TERT; RUX, Ruxolitinib; Rx, treatment.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/9/21/10.1182_bloodadvances.2025016898/1/m_blooda_adv-2025-016898-gr6.jpeg?Expires=1765292870&Signature=UX7ecLjd7UAJMmEHIB1zqNPSf-0rKQrv0MjFMM7fkzC2D12EGQ5Uu9nxXm6-dQn0ZckwvRQgKz3~tQReFWRu9xBhe-0dr00bW19sOySxOHG2yVb69zJrQaxY-chzYwcS9BCFczsEcLTaM~5yCsdlExFAVepMdcWdFOHf4AlyZ~7a6AAFMKI-Ky7bqLRYlYPCwQiuBZpEW4Tqyxv-oqfYmEllPpY1Z2~SeGtrvWwBhVcQWfWrM9~jV07KSGZ6p4u1jd9V0SlKzrKWf1VNsrOuNrKMIC3Gb8qqRgs-6hpSOPmf5rvF6Bq5N9ThauoEuJu0VXGP6tk7rDuQSj3tfpEh6Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)