Key Points
The CHK1/WEE1-axis is a therapeutic target in mature T-cell neoplasms.
Dual WEE1- and JAK-inhibition shows activity in defined subtypes including T-PLL.
Visual Abstract
Mature T-cell leukemias and lymphomas (mTCL) comprise a clinically and genetically heterogeneous group of lymphoid malignancies. Most subtypes of peripheral T-cell lymphomas and leukemic T-cell malignancies show an aggressive clinical course and poor prognosis. Thus, these diseases urgently require novel therapeutic strategies. Taking advantage of recent progress deciphering the genetic basis of mTCL, we generated a comprehensive database of genetic alterations from 1825 patients with mTCL and utilized bioinformatic methodology developed to support treatment decisions in molecular tumor boards to identify novel potential therapeutics. Based on the results of this approach, we performed a systematic in vitro drug screen in molecularly characterized cell lines of mTCL to assess the activity of potential therapeutics. Of these, the cell cycle regulator WEE1 stood out as a novel therapeutic target in a genetically defined subgroup of mTCL. Indeed, WEE1 kinase inhibitors potently induced replication stress, premature mitotic entry, accumulation of DNA damage and induction of apoptosis in mTCL cell lines. Building on the single-agent activity of clinical grade WEE1 inhibitors, we next explored potential drug combination strategies through mechanistic studies. With this, we identified strong synergistic effects of combined WEE1 and JAK inhibition in genetically defined subtypes of mTCL, including primary patient samples of T-cell prolymphocytic leukemia. In summary, our study provides a comprehensive overview of the genetic landscape in mTCL and potential therapeutics in genetically defined subtypes. As such, we identified synergistic effects of dual WEE1 and JAK inhibition in cases with altered JAK/STAT signaling as a blueprint for clinical testing.
Introduction
The World Health Organization classification of lymphoid tumors currently defines 30 distinct entities of mature T-cell leukemias and lymphomas (mTCL) and natural killer (NK)-cell neoplasms.1 Across this clinically heterogenous group of diseases, most subtypes of nodal and extranodal peripheral T-cell lymphomas as well as the leukemic adult T-cell leukemia/lymphoma and T-cell prolymphocytic leukemia (T-PLL) are still associated with very poor prognosis.2-4 According to current treatment recommendations, anthracyclin-based chemotherapy remains the backbone of front-line therapy for most subtypes of nodal and extranodal mTCL and alemtuzumab-based treatments for T-PLL, respectively.5-7 However, these approaches provide cure for only a minority of patients and prognosis in relapsed and refractory disease remains extremely poor.3,8
During the past decade, comprehensive molecular profiling has significantly enhanced our understanding of the biology and pathogenesis of mTCL.9,10 Indeed, molecular signatures increasingly inform diagnosis, classification and prognostication in several subtypes of mTCL.11-14 Based on the improved understanding of the pathobiology of mTCLs, targeted therapeutic approaches including epigenetically modifying drugs and inhibitors of crucial oncogenic signaling pathways in mTCL show promise.15-17 Still, identification of molecularly informed treatment strategies, prognostic biomarkers and rational combination strategies remain an ongoing challenge.
To employ molecularly matched targeted therapies against specific oncogenic genetic alterations across cancer types and implement precision oncology, molecular tumor boards (MTBs) were established around the globe over the past decade. These multidisciplinary panels of physicians, pathologists, geneticists and bioinformaticians review and interpret molecular profiling results to facilitate biomarker-directed therapies.18
To unravel potential previously unappreciated therapeutics matched to genetic alterations across different subtypes of mTCL, we here applied bioinformatic methodology developed at the University Medical Center Goettingen to characterize genetic alterations of a given tumor, provide evidence for targeted therapeutics and support treatment decisions in MTBs (hereafter referred to as MTB-Report tool).19,20 With this MTB-Report tool, we analyzed genetic data from 1825 patients with mTCL compiled from recent landmark publications. Based on the results of the MTB-Report tool, a broad in vitro drug screening was performed to assess the activity of potential therapeutics in genetically and transcriptionally characterized cell lines of TCL and identify potential biomarkers of response and resistance. This screening confirmed previously established therapeutic strategies but also identified novel targetable vulnerabilities in mTCL. Of those, the DNA damage response (DDR) pathways and particularly the CHK1/WEE1 axis was identified as promising targets for clinical-grade inhibitors.
Methods
Database of genetic alterations and employment of a bioinformatic reporting tool for MTBs
A database of genetic alterations in T- and NK-cell lymphoma was built based on previous landmark publications, published between 2014 and 2021 (supplemental Table 1). This genomic data set, as well as mutational data of well-established T/NK-cell lymphoma cell lines and patient-derived xenograft-models, was annotated using the MTB-Report tool to obtain potential treatment options for matching single-nucleotide variants, copy number variants and gene fusions.19-21 The MTB-Report supports therapy selection by systematically analyzing and annotating genomic variants and linking them to actionable treatment options based on an evidence-based framework. The tool integrates public knowledge from databases such as CIViC, GDKD, OncoKB, and TARGET to identify and classify actionable variants, including treatment options, with respect to the associated tumor tissue.22-24 All entries are supported by an associated publication indicating the source of the information. MTB-Report matches variants to database entries by first determining whether the altered gene is listed with a predicted therapeutic effect. Next, it filters variants by the type of genetic alteration, such as missense mutations, copy number changes or fusions. For variants of unknown significance, loss-of-function or frameshift mutations, it lists all therapeutic actionable mutations that occur in the same gene. Based on this, supplemental Table 2 was generated and further annotated with manual drug classification and VUS (variant of unknown significance) analysis using VUS-Predict and DBSNP (single nucleotide polymorphism database) and ClinVar.25-27
Animals and in vivo drug testing
Transgenic NOD-Rag1null IL2rgnull mice were obtained from the animal facility at the University of Göttingen or from The Jackson Laboratory (Bar Harbor, ME, IMSR_JAX:007799). All animal experimentation was carried out in accordance with the European Council Directive. Experiments were approved by the Lower Saxony Federal State Office for Consumer Protection and Food Safety, Germany (33.19-42502-04-22-00135).
For xenotransplantation, 1 × 107 MyLa cells were mixed with Corning Matrigel Extracellular Matrix (Corning, NY) and serum-free RPMI media (volume-to-volume ratio 1:1) to a total volume of 100 μL. Then, cell suspensions were injected subcutaneously into the right flank. After 6 days of engraftment, tumor volumes were determined, and mice were randomized for treatment (= day 0). Length (L) and width (W) of tumors were measured and tumor volumes were calculated using the equation V = 0.5 × L × W2. Treatment was performed by daily oral gavage on 5 consecutive days. Ruxolitinib 90 mg/kg twice daily (10% dimethyl sulfoxide [DMSO], 40% PEG300, 5% Tween80, 45% saline), adavosertib 50 mg/kg daily (5% DMSO, 40% PEG300, 5% Tween80, 50% saline), or the combination of both. DMSO controls were treated accordingly. On day 7 upon initiation of treatment, tumor volumes were reassessed and mice were euthanized.
Ex vivo drug testing in primary patient cells
This study enrolled 13 samples from patients with T-PLL with available cryopreserved peripheral blood mononuclear cells, diagnosed according to consensus criteria.2 Blood samples were obtained from patients under institutional review board–approved protocols following written informed consent according to the Declaration of Helsinki. Collection and use have been approved for research purposes by the ethics committees of the University Hospital of Cologne (19-1089) and the University of Helsinki (303/13/03/01/2011). In addition, blood samples from 3 healthy volunteers were included. All patients and healthy volunteers signed informed consent for biobanking, research and publication of research data within this study. Based on cell line drug testing, primary samples from T-PLL patients were treated with ruxolitinib (5 μM), adavosertib (1 μM), and ZN-C3 (1 μM) as single-drug treatments, as well as combinations of ruxolitinib with each of the WEE1 inhibitors for up to 72 hours at 37°C and 5% CO2 in a 384-well format. As readout, the RealTime-Glo MT Cell Viability Assay was applied following the manufacturer’s instructions and luminescence was measured after 72 hours of treatment.
Results
Database of recurrent genetic alterations in mTCL
To uncover potentially targetable genetic alterations in mTCL, we generated a comprehensive data set of genetic alterations across different subtypes of mTCL. This data set comprises a total of 1825 patients covering 10 different subtypes of mTCL (supplemental Table 1; supplemental Figure 1A-C). Across all subtypes of mTCL, mutations of the TET2 gene were most prominent with an overall frequency of 19% and enriched in cases of angioimmunoblastic T-cell lymphoma and peripheral T-cell lymphoma, not otherwise specified. In these subtypes, TET2 mutations are often accompanied by alterations affecting RHOA, DNMT3A, and IDH2 (Figure 1A). Additional subtype-enriched alterations include mutations of PLCG1 in cases of adult T-cell leukemia/lymphoma and frequent alterations affecting ATM in cases of T-PLL. Besides TET2, mutations and copy number losses affecting TP53 were the second-most alteration across subtypes. Additionally, alterations affecting the JAK/STAT signaling pathway, including STAT3, STAT5B, JAK1, and JAK3, frequently occur across various subtypes of mature T-cell leukemias and lymphomas (Figure 1A). Together, this data set mirrors previously published frequencies of recurrent genetic alterations in specific subtypes of mTCL.
Targetable genetic alterations in mTCL. (A) Oncoprint of the 30 most recurrently altered genes across different subtypes of mature T-cell neoplasms among 1825 patients. (B-C) Results of the MTB-Report tool: drugs with proposed sensitivity (B) or resistance (C) based on genetic alterations of the indicated, affected genes. Shown in panels B-C are suggestions that occurred in at least 5% of the patients. AITL, angioimmunoblastic T-cell lymphoma; ALK– ALCL, anaplastic large cell lymphoma without ALK translocation; ALK+ ALCL, anaplastic large cell lymphoma with ALK translocation; ATLL, adult T-cell leukemia/lymphoma; CTCL, cutaneous T-cell lymphoma; EATL, enteropathy-associated T-cell lymphoma; HS-TCL, hepatosplenic T-cell lymphoma; NKT, NK/T-cell lymphoma; PTCL-NOS, peripheral T-cell lymphoma not otherwise specified.
Targetable genetic alterations in mTCL. (A) Oncoprint of the 30 most recurrently altered genes across different subtypes of mature T-cell neoplasms among 1825 patients. (B-C) Results of the MTB-Report tool: drugs with proposed sensitivity (B) or resistance (C) based on genetic alterations of the indicated, affected genes. Shown in panels B-C are suggestions that occurred in at least 5% of the patients. AITL, angioimmunoblastic T-cell lymphoma; ALK– ALCL, anaplastic large cell lymphoma without ALK translocation; ALK+ ALCL, anaplastic large cell lymphoma with ALK translocation; ATLL, adult T-cell leukemia/lymphoma; CTCL, cutaneous T-cell lymphoma; EATL, enteropathy-associated T-cell lymphoma; HS-TCL, hepatosplenic T-cell lymphoma; NKT, NK/T-cell lymphoma; PTCL-NOS, peripheral T-cell lymphoma not otherwise specified.
Identification of molecularly informed targeted therapies
Applied to the mTCL data set, the MTB-Report tool identified genetic alterations of potential therapeutic relevance and reported evidence for potential therapeutics for 60% of the patients (supplemental Figure 1D-F; supplemental Table 2). The MTB-Report tool recommended a total of 438 distinct compounds, which were grouped into 76 drug classes according to their pharmacological targets (supplemental Table 3).
Therapeutics targeting multiple tyrosine kinases, were suggested for 31% of the patients. Anthracyclines, a cornerstone of chemotherapeutic frontline regimen to treat mTCL, were suggested for a considerable proportion of patients (29%) (Figure 1B). Furthermore, the MTB-Report tool suggested additional—and meanwhile clinically validated—therapeutic approaches including demethylating agents and inhibitors of the PI3K/mTOR (phosphatidylinositol 3-kinase/mammalian target of rapamycin) and the JAK/STAT pathway, based on genetic alterations affecting epigenetic modulators or pathway components of the PI3K/mTOR or JAK/STAT pathways, respectively (Figure 1B-C).16,17,28 Notably, multiple drugs targeting components of DDR pathways were suggested as potential therapeutics by the MTB-Report tool. Of these, inhibitors of WEE1, an important regulator of cell cycle progression and DNA damage repair, stood out as potential therapeutics across all subtypes of mTCL included in this study (Figure 1B).
In vitro screening of potential drug candidates
Based on the results of the MTB-Report tool (supplemental Tables 2 and 3), 70 drug candidates covering 56 pharmacological drug classes were chosen for an in vitro screening across 22 genetically characterized mTCL cell lines of 7 different mTCL subtypes. The selection of compounds was guided by their mechanism of action, clinical applicability and overlapping drug recommendations of the MTB-Report tool in the patients’ cohort and preclinical models (supplemental Figure 1G-H). With the in vitro drug screening, we covered 91% of the drugs suggested in the patient cohort (supplemental Figure 1H).
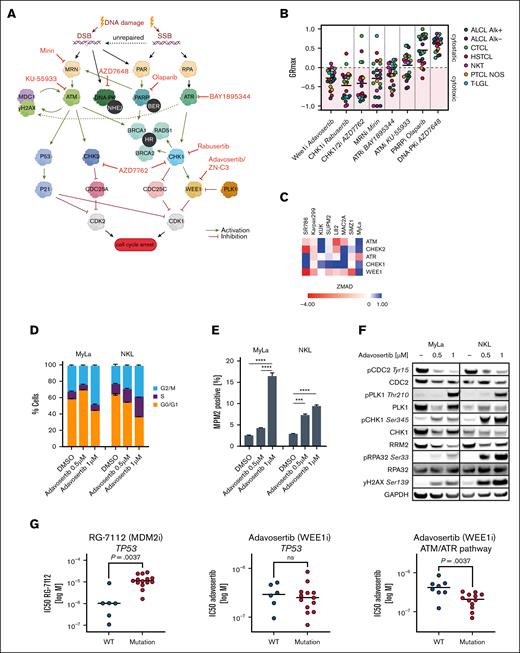
The in vitro drug screening revealed highly heterogeneous patterns of response across the tested therapeutics and individual cell lines (Figure 2; supplemental Figure 2). Strikingly, the anaplastic lymphoma kinase inhibitor crizotinib showed dominant activity in ALK+ anaplastic large cell lymphoma cell lines. Besides subtype-specificity, some compounds exhibited a particularly heterogenous response pattern, such as the JAK inhibitor ruxolitinib, suggesting distinct mechanisms of sensitivity and resistance. In line with the suggestions of the MTB-Report tool applied to the initial patient cohort, the in vitro drug screening also showed activity of inhibitors targeting components of the DDR machinery. To further dissect druggable vulnerabilities within the DDR machinery, we assessed the cytotoxicity of small molecules selectively inhibiting components of DDR pathways using the GRmax method (Figure 3A-B).29 Notably, inhibitors of WEE1 (adavosertib aka AZD1775) and CHK1 (rabusertib) showed broad cytotoxic effects (20/22 cell lines), as assessed by GRmax values of ≤0 (Figure 3B), a finding that was further corroborated by a previously performed genome-wide CRISPR screen (Figure 3C).21
Systematic in vitro drug screening. Fifty percent inhibitory concentration (IC50) values of 72 drug compounds (x-axis) tested across 22 cell lines (y-axis) of 7 T-cell lymphoma subtypes after 72-hour treatment. Illustrated IC50 values are mean values from at least 3 independent experiments. T-LGL, T-cell large granular lymphocytic leukemia.
Systematic in vitro drug screening. Fifty percent inhibitory concentration (IC50) values of 72 drug compounds (x-axis) tested across 22 cell lines (y-axis) of 7 T-cell lymphoma subtypes after 72-hour treatment. Illustrated IC50 values are mean values from at least 3 independent experiments. T-LGL, T-cell large granular lymphocytic leukemia.
Therapeutic targeting of DDR pathways. (A) Schematic representation of the ATM/Chk2 and ATR/Chk1 pathways in response to different types of DNA damage and the investigated compounds targeting different components within these pathways. (B) GRmax values of 8 compounds targeting different components of the DDR across 22 TCL cell lines after 72-hour treatment. GRmax >0 indicate partial cytostatic response, GRmax = 0 indicate full cytostatic response, GRmax <0 indicate partial cytotoxic response, GRmax = −1 indicate full cytotoxic effect. GR50 values represent mean values from at least 3 independent experiments. (C) ZMAD scores of ATM and ATR pathway genes obtained from a previously performed genome-wide CRISPR screen, with negative ZMAD scores (red colors) indicating a high and positive ZMAD scores (blue color) indicating low genetic dependency. (D-F) Cellular effects of WEE1 inhibition after 24 hours treatment with adavosertib: (D) Cell cycle analysis after adavosertib treatment. (E) MPM2 positivity by flow cytometry after adavosertib treatment. Significance was assessed with a 2-way analysis of variance (ANOVA) test after Tukey using correction for multiple comparison. (F) Immunoblot of proteins affected by WEE1 inhibition. (G) Drug response of TCL cell lines measured in relative IC50 values vs mutation status. Left: IC50 values of the MDM2i RG-7112 in TP53 wild type vs TP53 mutated cell lines. Middle: IC50 values of the WEE1 inhibitor (WEE1i) adavosertib in TP53 wild type vs TP53 mutated cell lines. Right: IC50 values of the WEE1i adavosertib in cell lines harboring loss of function alterations in the ATM/ATR pathways (ATM, ATR, Chek1, RPA1/2, and NBN) vs cell lines without such mutations. Significance was assessed with an unpaired 2-tailed t test. IC50 values are mean values from at least 3 independent experiments. ∗∗∗P ≤ .001, ∗∗∗∗P ≤ .0001. BER, base excision repair; DSB, DNA double-strand break; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; HR, homologous recombination; MDM2i, MDM2 inhibitor; NHEJ, nonhomologous end joining; ns, nonsignificant; SSB, DNA single-strand breaks; WT, wild type.
Therapeutic targeting of DDR pathways. (A) Schematic representation of the ATM/Chk2 and ATR/Chk1 pathways in response to different types of DNA damage and the investigated compounds targeting different components within these pathways. (B) GRmax values of 8 compounds targeting different components of the DDR across 22 TCL cell lines after 72-hour treatment. GRmax >0 indicate partial cytostatic response, GRmax = 0 indicate full cytostatic response, GRmax <0 indicate partial cytotoxic response, GRmax = −1 indicate full cytotoxic effect. GR50 values represent mean values from at least 3 independent experiments. (C) ZMAD scores of ATM and ATR pathway genes obtained from a previously performed genome-wide CRISPR screen, with negative ZMAD scores (red colors) indicating a high and positive ZMAD scores (blue color) indicating low genetic dependency. (D-F) Cellular effects of WEE1 inhibition after 24 hours treatment with adavosertib: (D) Cell cycle analysis after adavosertib treatment. (E) MPM2 positivity by flow cytometry after adavosertib treatment. Significance was assessed with a 2-way analysis of variance (ANOVA) test after Tukey using correction for multiple comparison. (F) Immunoblot of proteins affected by WEE1 inhibition. (G) Drug response of TCL cell lines measured in relative IC50 values vs mutation status. Left: IC50 values of the MDM2i RG-7112 in TP53 wild type vs TP53 mutated cell lines. Middle: IC50 values of the WEE1 inhibitor (WEE1i) adavosertib in TP53 wild type vs TP53 mutated cell lines. Right: IC50 values of the WEE1i adavosertib in cell lines harboring loss of function alterations in the ATM/ATR pathways (ATM, ATR, Chek1, RPA1/2, and NBN) vs cell lines without such mutations. Significance was assessed with an unpaired 2-tailed t test. IC50 values are mean values from at least 3 independent experiments. ∗∗∗P ≤ .001, ∗∗∗∗P ≤ .0001. BER, base excision repair; DSB, DNA double-strand break; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; HR, homologous recombination; MDM2i, MDM2 inhibitor; NHEJ, nonhomologous end joining; ns, nonsignificant; SSB, DNA single-strand breaks; WT, wild type.
WEE1 inhibition causes premature mitotic entry and replicative stress
Based on the frequent recommendation of WEE1 inhibition in our patient cohort and encouraging in vitro efficacy adavosertib, we next studied the distinct mechanism of action. Treatment with the WEE1 inhibitor for 24 hours resulted in premature mitotic entry, as indicated by an accumulation of cells in the G2/M phase (Figure 3D) as well as an increase in the mitotic index as shown by increased MPM2 positive cells (Figure 3E). Additionally, western blot studies revealed a decreased Tyr15 phosphorylation of CDC2 and an increased PLK1 phosphorylation, markers for mitotic entry.30 We also observed an increase of γH2AX, a marker for DNA damage and replicative stress, and of phosphoRPA2/32 (Ser33), an indicator for single-stranded DNA accumulation and of replicative stress (Figure 3F).31 Furthermore, we identified a decrease in RRM2 (Figure 3F), which plays a critical role in replication fork progression.32 In summary, WEE1 inhibition caused replication stress, DNA damage and premature entry into mitosis.
WEE1i response is independent of the TP53 mutation status but correlates with DDR pathway alterations in mTCL cells
To examine the correlation of drug sensitivity and genetic alterations as potential biomarkers, we first focused on the TP53 mutation status, since TP53 mutations were recently identified as high-risk events for patients with peripheral TCL33 and WEE1 inhibitors were suggested as potential therapeutics in TP53 mutated cases of mTCL (Figure 1B). Indeed, although cell lines harboring inactivating mutations of TP53 were relatively resistant to the MDM2 inhibitor RG-7112 (P = .0037), the activity of the WEE1 inhibitor adavosertib was not affected by TP53 mutations (Figure 3G). Notably, damaging alterations in DDR-related genes (ATM, NBN, ATR, CHEK1, RPA1, and RPA2) were significantly associated with sensitivity toward adavosertib (P = .0037, Figure 3G).
Adavosertib synergizes with chemotherapeutic agents and ruxolitinib
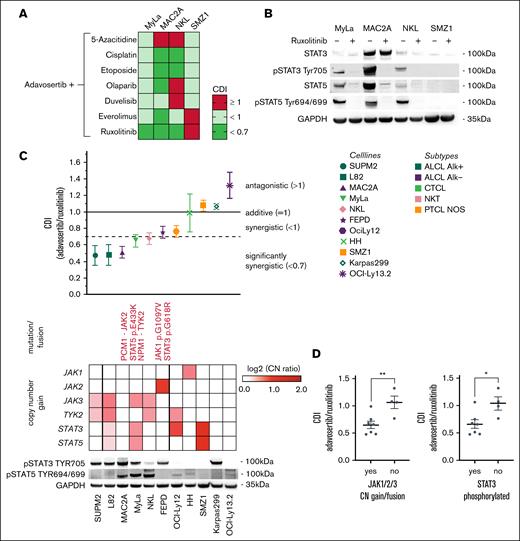
To enhance the single drug effect of adavosertib, rational drug combination approaches were investigated in cell lines representing 4 common subtypes of mTCL. The combination partners were chosen based on previously described synergistic effects with WEE1 inhibition, including PARP inhibitors or DNA-damaging chemotherapeutic agents. Furthermore, therapeutics including 5-azacitidine and inhibitors of the PI3K- (duvelisib), mTOR- (everolimus) and JAK/STAT signaling pathway (ruxolitinib) were applied (Figure 4A; supplemental Figure 3A). The combinatory treatment of WEE1 inhibition with cisplatin and etoposide induced significant synergy coefficient of drug interaction (CDI < 0.7) in 2 of 4 cell lines. Remarkably, among the investigated compounds, the most robust synergistic effect was evident with the JAK1/2 inhibitor ruxolitinib. This interaction yielded significant synergy in 3 out of the 4 tested cell lines (Figure 4A). Importantly, the 3 cell lines showing synergistic activity of WEE1 + JAK inhibition (MyLa, MAC2A, and NKL) exhibited an activation of the JAK/STAT pathway by phosphorylated STAT3 and/or STAT5, whereas SMZ1 cell did not. In MyLa, MAC2A and NKL cells, ruxolitinib potently reduced STAT3/STAT5 phosphorylation (Figure 4B).
Development of rational drug combinations. (A) CDI of different drug combinations with adavosertib across 4 mTCL cell lines. CDIs of adavosertib (WEE1i) with 5-azacitidine (demethylating agent), cisplatin (platinum), etoposide (topoisomerase inhibitor), olaparib (PARP inhibitor), everolimus (mTOR inhibitor), duvelisib (PI3K inhibitor), and ruxolitinib (JAK1/2 inhibitor) were calculated after 24-hour treatment. CDI values are mean values of 3 independent experiments. (B) Impact of JAK1/2 inhibition on STAT3/5 phosphorylation. Western blot data of 4 cell lines after treatment with 5 μM ruxolitinib for 24 hours. (C) Response to WEE1/JAK inhibitor combination treatment and genetic JAK/STAT signaling background of TCL cell lines. Treatment combination of the WEE1i adavosertib and JAK1/2 inhibitor (JAK1) ruxolitinib of 11 TCL cell lines showed a wide range of CDIs and an increased occurrence of activating JAK/STAT signaling alterations (single-nucleotide variants, fusions, or copy number gains), resulting in constitutive STAT3 (Tyr705) and STAT5 (Tyr694/699) phosphorylation in cells with lower CDI values. (D) Cell lines holding activating alterations of JAK1, JAK2 or JAK3 or phosphorylation of STAT3 demonstrated significantly lower CDI values than cell lines without such alterations. Significance was calculated using an unpaired 2-tailed t test. CDI values represent mean values of at least 3 independent experiments. ∗P ≤ .05, ∗∗P ≤ .01.
Development of rational drug combinations. (A) CDI of different drug combinations with adavosertib across 4 mTCL cell lines. CDIs of adavosertib (WEE1i) with 5-azacitidine (demethylating agent), cisplatin (platinum), etoposide (topoisomerase inhibitor), olaparib (PARP inhibitor), everolimus (mTOR inhibitor), duvelisib (PI3K inhibitor), and ruxolitinib (JAK1/2 inhibitor) were calculated after 24-hour treatment. CDI values are mean values of 3 independent experiments. (B) Impact of JAK1/2 inhibition on STAT3/5 phosphorylation. Western blot data of 4 cell lines after treatment with 5 μM ruxolitinib for 24 hours. (C) Response to WEE1/JAK inhibitor combination treatment and genetic JAK/STAT signaling background of TCL cell lines. Treatment combination of the WEE1i adavosertib and JAK1/2 inhibitor (JAK1) ruxolitinib of 11 TCL cell lines showed a wide range of CDIs and an increased occurrence of activating JAK/STAT signaling alterations (single-nucleotide variants, fusions, or copy number gains), resulting in constitutive STAT3 (Tyr705) and STAT5 (Tyr694/699) phosphorylation in cells with lower CDI values. (D) Cell lines holding activating alterations of JAK1, JAK2 or JAK3 or phosphorylation of STAT3 demonstrated significantly lower CDI values than cell lines without such alterations. Significance was calculated using an unpaired 2-tailed t test. CDI values represent mean values of at least 3 independent experiments. ∗P ≤ .05, ∗∗P ≤ .01.
Based on this result, we evaluated this therapeutic approach on an expanded panel of 11 cell lines, representing 5 different subtypes of mTCL. The treatment responses exhibited a spectrum ranging from pronounced synergistic effects (CDI of 0.5) to instances of putative antagonism (CDI of 1.3). Notably, the combination strategy led to a synergistic effect in two-thirds of the tested cell lines. Cell lines exhibiting synergistic responses to the combination treatment frequently carry activating single nucleotide variants, gene fusions, or amplifications affecting genes within the JAK/STAT pathway and contributing to the activation of STAT3 and STAT5 proteins (Figure 4C). Indeed, cell lines exhibiting activating alterations of JAK1, JAK2, or JAK3 or phosphorylation of STAT3 showed significantly enhanced synergistic CDIs compared to cell lines lacking such genetic alterations or phosphorylation of STAT3 (Figure 4D).
The impact of adavosertib and ruxolitinib on cell cycle progression and DNA replication
Based on the synergistic effects of adavosertib and ruxolitinib, we next explored underlying mechanisms of dual WEE1- and JAK1/2-inhibition. Considering the dominant effect of the drug combination in cell lines harboring constitutive activation of the JAK/STAT pathway, we first performed RNA sequencing of 3 different cell lines (MAC2A, MyLa, NKL) treated with ruxolitinib. As expected, single drug treatment of ruxolitinib led to an inhibition of STAT3 and STAT5 phosphorylation (Figure 4B) and downregulation of hallmark gene sets involved in JAK/STAT signaling as assessed by gene set enrichment analyses. Notably also gene sets related to cell cycle progression, DNA replication and DDR were consistently and significantly downregulated following ruxolitinib treatment (Figure 5A). To further dissect the impact of WEE1- and JAK1/2-inhibition on cell cycle progression and DNA replication, we first performed cell cycle analyses, confirming an increased G2/M fraction in cells treated with adavosertib (Figure 5B). Notably, ruxolitinib induced expression of p27 (Figure 5E) and a partial G1 arrest, still leaving cycling cells (Figure 5B,F). For cycling cells, RNA sequencing analyses suggested increased replication stress as indicated by the Repstress gene set (Figure 5C).34 Indeed, EdU (5-ethynyl-2'-deoxyuridine) staining identified a moderate, but significantly higher proportion of EdU-negative S-phase cells compared to untreated cells, suggesting impaired active DNA replication (Figure 5D). Immunoblotting of single drug and combination treated cells further corroborated the aforementioned findings and showed an inhibitory effect of ruxolitinib as single agent and in combination with adavosertib on JAK downstream targets including dephosphorylation of STAT3/5, suppression of SOCS3 and induction of p27 (Figure 5E). Inhibition of WEE1 induced replication stress and triggered activation of the DDR pathways, as indicated by elevated phosphorylation of RPA32/2, Chk1, and γH2AX. Combined WEE1 and JAK inhibition further enhanced the effect of WEE1 inhibition on CDC2 Tyr15 phosphorylation and decreased overall CDC2 and RRM2 protein expression. Furthermore, combination treated cells showed an increase in γH2AX phosphorylation (Figure 5E). In summary, our studies imply differential effects of ruxolitinib, adavosertib, and the combination of both drugs on cell cycle progression and DNA replication. We, thus, explored cell cycle–dependent cell death in response to adavosertib, ruxolitinib, and the combination. To this end, cells were costained with Hoechst33342 to define the cell cycle and propidium iodide (PI) to identify dead cells following 24-hour treatment of single-agent or combined treatment with adavosertib and ruxolitinib. Indeed, flow cytometry analysis revealed an accumulation of dead cells during cell cycle progression, notably including an increase in cell death, dominantly during G1- and S-phase of the cell cycle (Figure 5F; supplemental Figure 4).
Mechanisms of ruxolitinib combined with adavosertib. (A) Downregulation of DDR/replication stress gene sets after JAK1/2 inhibition. Various GSEA (gene set enrichment analysis) gene sets regulating cell cycle, DDR and replication were significant downregulated (FDR q value < 0.001) after 24-hour ruxolitinib treatment in the NKL cell line (left). (B) Cell cycle analyses indication G0/G1, S, and G2/M phases in after 24 hours of treatment with DMSO, 500 nM adavosertib, 5 μM ruxolitinib, or the combination of adavosertib and ruxolitinib. (C) Multiple genes from the Repstress gene set (Takahashi et al34) were significant downregulated (P < .05) after treatment (right). (D) Assessment of EdU negative S phase cells through adavosertib, ruxolitinib and combination treatment. Illustrated are mean values of 3 independent experiments. (E) Immunoblotting shows protein expression changes of proteins involved in JAK/STAT signaling, DNA damage repair, replication stress and cell cycle regulation. (F) Accumulation of cell death during cell cycle progression through combined WEE1/JAK inhibition. Significances were assessed with a 2-way ANOVA after Dunnett correction. Bar graphs illustrate mean values of 3 independent experiments. ∗∗∗P ≤ .001, ∗∗∗∗P ≤ .0001. FDR, false discovery rate; NES, normalized enrichment score.
Mechanisms of ruxolitinib combined with adavosertib. (A) Downregulation of DDR/replication stress gene sets after JAK1/2 inhibition. Various GSEA (gene set enrichment analysis) gene sets regulating cell cycle, DDR and replication were significant downregulated (FDR q value < 0.001) after 24-hour ruxolitinib treatment in the NKL cell line (left). (B) Cell cycle analyses indication G0/G1, S, and G2/M phases in after 24 hours of treatment with DMSO, 500 nM adavosertib, 5 μM ruxolitinib, or the combination of adavosertib and ruxolitinib. (C) Multiple genes from the Repstress gene set (Takahashi et al34) were significant downregulated (P < .05) after treatment (right). (D) Assessment of EdU negative S phase cells through adavosertib, ruxolitinib and combination treatment. Illustrated are mean values of 3 independent experiments. (E) Immunoblotting shows protein expression changes of proteins involved in JAK/STAT signaling, DNA damage repair, replication stress and cell cycle regulation. (F) Accumulation of cell death during cell cycle progression through combined WEE1/JAK inhibition. Significances were assessed with a 2-way ANOVA after Dunnett correction. Bar graphs illustrate mean values of 3 independent experiments. ∗∗∗P ≤ .001, ∗∗∗∗P ≤ .0001. FDR, false discovery rate; NES, normalized enrichment score.
Functional apoptosis profiling reveals enhanced apoptotic priming upon dual WEE1 and JAK inhibition
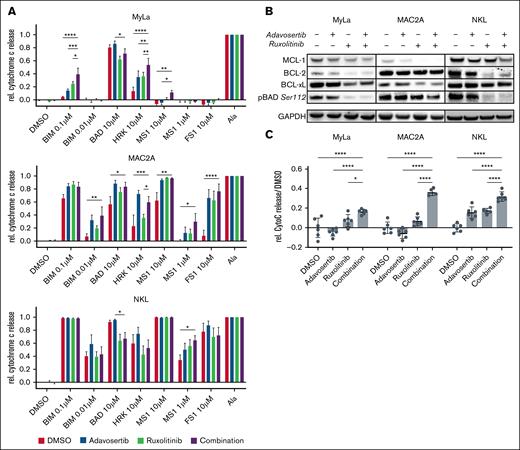
To further study mechanisms, through which dual inhibition of WEE1 and JAK induces cell death, we focused on apoptosis and employed dynamic BH3 profiling upon treatment with adavosertib, ruxolitinib, and the combination to characterize the impact of these drugs and their combination on the mitochondrial apoptosis pathway. BH3 profiling revealed enhanced apoptotic priming of the cells after combination treatment, as indicated by enhanced mitochondrial cytochrome c release upon stimulation with the proapoptotic activator BCL-2 Interacting Mediator of cell death (BIM) and proapoptotic sensitizers such as BAD, HRK, MS1, and FS1 (Figure 6A). Notably, MyLa cells exhibited an increased BCL-xL-specific vulnerability under WEE1 and JAK1/2 inhibition, which was further elevated in the combination treatment. MAC2A cells showed a broad increase in apoptotic priming, overcoming antiapoptotic BCL-xL and MCL-1. NKL cells showed a more specifically enhanced vulnerability toward MCL-1 and a notable reduction of functional BCL-2 dependence (Figure 6A). These findings are further supported by immunoblots showing a reduction of antiapoptotic BCL-2 family members and dephosphorylation of the proapoptotic sensitizer protein BAD (Figure 6B). Based on these proapoptotic changes, we next directly measured mitochondrial cytochrome c release as a marker for induction of apoptosis in response to the drugs. Strikingly, the combination of adavosertib and ruxolitinib induced a significantly stronger cytochrome c release than single-agent treatment or the DMSO control (Figure 6C).
Proapoptotic mechanisms of adavosertib and ruxolitinib. (A) Change in apoptotic dependencies after drug treatment assessed through dynamic BH3 profiling. hBIM is an activator of BAX and BAK, mBAD antagonizes BCL-2, BCL-xL, and BCL-w, HRK specifically antagonizes BCL-xL and MS1 specifically antagonizes MCL-1. DMSO served as a negative control, alamethicin served as a positive control. Significance was assessed with a 2-way ANOVA after Dunnett correction. Bar graphs illustrate mean values of 3 independent experiments. (B) Western blot data of single drug and combination treated cells. Three cell lines were treated for 24 hours with 500 nM adavosertib, 5 μM ruxolitinib, or the combination of both compounds. Immunoblotting shows protein expression changes of antiapoptotic BCL-2 family members and phosphorylation of the proapoptotic sensitizer BAD. Illustrated is a representative example of 3 independent experiments. (C) Drug-induced mitochondrial cytochrome c release in cell lines treated for 24 hours with 500 nM adavosertib, 5 μM ruxolitinib, or the combination of both compounds. Significance was assessed with a 1-way ANOVA and Bonferroni correction. ∗P ≤ .05; ∗∗P ≤ .01, ∗∗∗P ≤ .001, ∗∗∗∗P ≤ .0001. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Proapoptotic mechanisms of adavosertib and ruxolitinib. (A) Change in apoptotic dependencies after drug treatment assessed through dynamic BH3 profiling. hBIM is an activator of BAX and BAK, mBAD antagonizes BCL-2, BCL-xL, and BCL-w, HRK specifically antagonizes BCL-xL and MS1 specifically antagonizes MCL-1. DMSO served as a negative control, alamethicin served as a positive control. Significance was assessed with a 2-way ANOVA after Dunnett correction. Bar graphs illustrate mean values of 3 independent experiments. (B) Western blot data of single drug and combination treated cells. Three cell lines were treated for 24 hours with 500 nM adavosertib, 5 μM ruxolitinib, or the combination of both compounds. Immunoblotting shows protein expression changes of antiapoptotic BCL-2 family members and phosphorylation of the proapoptotic sensitizer BAD. Illustrated is a representative example of 3 independent experiments. (C) Drug-induced mitochondrial cytochrome c release in cell lines treated for 24 hours with 500 nM adavosertib, 5 μM ruxolitinib, or the combination of both compounds. Significance was assessed with a 1-way ANOVA and Bonferroni correction. ∗P ≤ .05; ∗∗P ≤ .01, ∗∗∗P ≤ .001, ∗∗∗∗P ≤ .0001. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Combined WEE1 and JAK inhibition shows activity in vivo and in primary patient samples of T-PLL
To confirm the promising in vitro results, the efficacy of combined WEE1 and JAK1/2 inhibition was evaluated in vivo using a cell line xenograft model and ex vivo using primary T-PLL patient samples. For in vivo testing, MyLa cells were injected subcutaneously in NRG mice. Upon engraftment, mice were treated with vehicle control, ruxolitinib, adavosertib or the combination of both for 5 days. On day 7, we reassessed tumor volumes and detected significantly impaired tumor growth in mice treated with the combination of ruxolitinib and adavosertib (Figure 7A).
In vivo xenograft study and ex vivo treatment of T-PLL primary samples vs healthy control samples. (A) Xenograft study indicating tumor volumes at day 0 and day 7 of NRG-mice engrafted with MyLa cells and treated with a vehicle control, adavosertib, ruxolitinib, or the combination of adavosertib and ruxolitinib. (B) Normalized viability measurement of 13 T-PLL primary samples and 3 healthy control samples after 72-hour single drug and combination treatment shows significant decreases in viability through combination treatment selectively in the T-PLL samples. Cell viability was measured with RealTime-Glo MT Cell Viability Assay and normalized to a DMSO-treated control. Significance was assessed with 1-way ANOVA after Tukey correction. ∗P ≤ .05, ∗∗P ≤ .01, ∗∗∗P ≤ .001, ∗∗∗∗P ≤ .0001. A, adavosertib; R, ruxolitinib; Z, ZN-C3.
In vivo xenograft study and ex vivo treatment of T-PLL primary samples vs healthy control samples. (A) Xenograft study indicating tumor volumes at day 0 and day 7 of NRG-mice engrafted with MyLa cells and treated with a vehicle control, adavosertib, ruxolitinib, or the combination of adavosertib and ruxolitinib. (B) Normalized viability measurement of 13 T-PLL primary samples and 3 healthy control samples after 72-hour single drug and combination treatment shows significant decreases in viability through combination treatment selectively in the T-PLL samples. Cell viability was measured with RealTime-Glo MT Cell Viability Assay and normalized to a DMSO-treated control. Significance was assessed with 1-way ANOVA after Tukey correction. ∗P ≤ .05, ∗∗P ≤ .01, ∗∗∗P ≤ .001, ∗∗∗∗P ≤ .0001. A, adavosertib; R, ruxolitinib; Z, ZN-C3.
For ex vivo validation, we utilized T cells from healthy donors and patients with T-PLL. T-PLL samples were chosen due to unique molecular characteristics of the disease, including altered DDR through frequent inactivating mutations or deletions of ATM alongside with activating alterations of the JAK/STAT pathway. Furthermore, active proliferation of T-PLL cells ex vivo supports their usefulness to assess cell cycle–dependent cytotoxicity (supplemental Figure 3). Thirteen T-PLL samples as well as 3 healthy control samples were treated for 72 hours with the various treatment strategies. Besides adavosertib, also the novel WEE1 inhibitor ZN-c3 was included to confirm the effectiveness of WEE1 inhibition and enhance the clinical perspective of the combination strategy. Notably, the synergistic effects of ZN-c3 combined with ruxolitinib significantly correlated with the effects of adavosertib and ruxolitinib (supplemental Figure 3B). In the ex vivo drug testing, the assessment of cell viability showed a marked reduction in the viability of T-PLL samples through single drug treatments compared to untreated controls (Figure 7B). The combination treatment resulted in a substantial additional decrease in cell viability compared to the single drug approaches in the primary T-PLL samples. Conversely, in healthy control samples, the applied drug treatments demonstrated no notable impact on cell viability, whether administered individually or in combination, indicating selective inhibition and synergy in the T-PLL cells (Figure 7B).
Discussion
Here, we report a novel approach to discover molecularly informed therapeutic strategies for mTCL, based on an MTB-Report tool, which identifies pathogenic genetic alterations of a given tumor and reports evidence for drug activity associated with distinct genetic profiles based on public databases such as CIViC, GDKD, OncoKB, and TARGET. Applying the MTB-Report tool to a cohort of 1825 patients with genetically characterized mTCL, we illustrate the genetic landscape of mTCL and report potential therapeutics associated with distinct genetic alterations. Notably, the approach suggested multiple therapeutics that are well established or currently undergo clinical testing in mTCL, including chemotherapeutic agents, epigenetic modifiers and inhibitors of crucial oncogenic signaling pathways such as the PI3K/mTOR- and JAK/STAT-pathway.16,17,28,35,36
Furthermore, the MTB-Report tool identified multiple alterations that provide a rational for therapeutic targeting of the DDR pathways (Figure 1B). This hypothesis is supported by recent preclinical studies suggesting increased efficacy of chemotherapeutic agents combined with ATM inhibition or targeted inhibition of ATR in a GATA3 mouse model of mTCL.37,38 We systematically dissected the cytotoxic activity of clinical grade compounds targeting specific components of the DDR pathways (Figure 3A) and identified dominant activity of drugs targeting the CHK1/WEE1 pathway (Figure 3B), which is in line with the prominent ranking of WEE1 inhibitors in the drugs suggested by the MTB-Report tool in the patient cohort (Figure 1B). WEE1 is a crucial G1/S- and G2/M-phase transition regulator and maintainer of genome stability. Inhibiting this kinase promotes cell cycle progression despite unrepaired DNA damage and replication stress and can lead to cell death through mitotic as well as replicative catastrophe.39-41 Targeting WEE1 previously showed promising preclinical activity in histone H3K36me3-deficient cancers by deoxynucleotide triphosphate (dNTP) starvation and was suggested as a therapeutic strategy for type II enteropathy–associated T-cell lymphoma, based on highly recurrent alterations affecting the histone methylase SETD2.42,43 Beyond SETD2 alterations, the MTB-Report tool also suggested activity in cases of mTCL harboring TP53 mutations (Figure 1B). Indeed, the in vitro activity of adavosertib in cell lines was broad and not impaired in cell lines harboring inactivating TP53 mutations (Figure 3G). Furthermore, we found significantly enhanced activity in cell lines harboring genetic alterations of the ATM or ATR pathways (Figure 3G), suggesting novel potential biomarkers of response beyond currently available evidence in databases queried by the MTB-Report tool.
Building on the single-agent activity of the WEE1 inhibitor adavosertib, we next explored potential combination strategies through systematic drug combination testing and mechanistic studies. In line with previous reports, we identified synergistic effects of adavosertib combined with chemotherapeutic agents including the topoisomerase II inhibitor etoposide or the alkylating drug cisplatin.44 Also, synergistic effects with the mTOR inhibitor everolimus through augmented replication stress were previously described in ovarian cancers.45 However, we here identified strong synergistic effects of adavosertib and the JAK1/2 inhibitor ruxolitinib (Figure 4A) in cell lines harboring either activating genetic alterations of the JAK/STAT signaling pathway or phosphorylated STAT3 (Figure 4B-D). This finding is very much in line with the results of a recent biomarker driven phase 2 study of ruxolitinib in patients with relapsed or refractory T-cell lymphomas showing clinical benefit particularly in patients with lymphomas harboring either activating genetic alterations of the JAK/STAT pathway or phosphorylation of STAT3.17 Furthermore, a case report and results of a preliminary study of ruxolitinib combined with venetoclax recently showed clinical activity in a cohort of patients with relapsed or refractory T-PLL, particularly in cases with mutations in the JAK/STAT pathway.46,47 Indeed, genetic alterations affecting the DDR machinery and activating alterations of the JAK/STAT pathway are a genetic hallmark of T-PLL.48-50 We, thus, explored the combination of ruxolitinib and WEE1 inhibitors in primary patient samples of T-PLL. To this end, we also included the novel clinical grade WEE1 inhibitor ZN-c3 that currently undergoes evaluation in clinical trials and with which we were able to confirm the in vitro results with adavosertib.51 Strikingly, the combination of ruxolitinib with either adavosertib or ZN-c3 showed significant activity exceeding monotherapy effects in all tested samples of T-PLL, but not in samples of healthy volunteers (Figure 7B).
In summary, we here built a comprehensive genetic database on genetic alterations across different subtypes of mTCL and applied bioinformatic methodology designed to identify molecularly informed therapeutic strategies. With this, we identified WEE1 inhibition as a potential therapeutic strategy in mTCL, including high-risk cases with mutated TP53. Furthermore, we developed a rational combination strategy of dual WEE1 and JAK inhibition in cases of mTCL with activated JAK/STAT signaling and show preclinical activity of this regimen on cases of T-PLL.
Acknowledgments
Flow cytometry was performed at the University Medical Center Göttingen flow cytometry core facility on a BD LSRFortessa X-20, supported by Deutsche Forschungsgemeinschaft (DFG)-project 442249343.
This work was supported by Deutsche Krebshilfe (Max-Eder grant 70113602 [R.K.]), the Volkswagen foundation (ZN3421-SP1 [J.D.]; ZN3421-SP2 [T.B. and K.K.]; and ZN3421-SP4 [R.K. and M.L.]). T.A. is supported by European Union’s Horizon 2020 Research and Innovation Programme (ERA PerMed JAKSTAT-TARGET and CLL-CLUE projects), Academy of Finland (grants 340141, 344698, and 345803), the Cancer Society of Finland, the Norwegian Cancer Society.
Authorship
Contribution: N.S., J.D., and R.K. designed and performed research, analyzed data, and wrote the manuscript; K.K., L.R., K.M., N.A.M.K., P.M., M.L., and T.B. designed and performed research and analyzed data; M.S. and G.S. performed research and analyzed data; A.D., A.I., M.H., T.A., and H.B. contributed reagents/samples and analyzed data; and B.C., T.B., and G.W. analyzed data and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Raphael Koch, Department of Hematology and Medical Oncology, University Medical Center Göttingen; Robert-Koch-Str. 40, 37075 Göttingen, Germany; email: raphael.koch@med.uni-goettingen.de.
References
Author notes
Original data are available on request from the corresponding author, Raphael Koch (raphael.koch@med.uni-goettingen.de).
The full-text version of this article contains a data supplement.