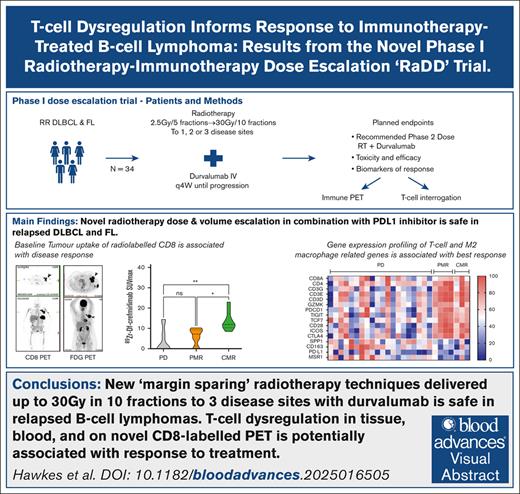
Key Points
Delivering RT to multiple disease sites in combination with immunotherapies such as PD-L1 inhibitors is safe.
T-cell dysregulation identified on tissue, blood, and CD8 PET imaging is potentially associated with response to immunotherapy.
Visual Abstract
Diffuse large B-cell lymphoma (DLBCL) and follicular lymphoma (FL) are highly radiosensitive with immune-driven abscopal responses reported. Programmed cell death 1/programmed cell death ligand 1 (PD-L1) inhibitors are relatively ineffective in DLBCL/FL; however, evidence suggests synergy with radiotherapy (RT), but no clear biomarkers. This phase 1 study examined the safety of escalating RT dose and treated volumes with durvalumab (PD-L1 inhibitor) in 34 adults with relapsed/refractory DLBCL and relapsed/refractory FL, and the role of immune-cell subsets on outcomes. Patients received external-beam RT (2.5-30 Gray [Gy], 5 or 10 fractions up to 3 target sites) plus durvalumab from RT day 2, until progression. Novel positron emission tomography (PET) biodistribution studies of 89Zr-durvalumab and CD8 T-cell minibody-89Zr-Df-crefmirlimab were incorporated. The RT recommended phase 2 dose was 10 Gy/5 fractions and 30 Gy/10 fractions to 3 sites for FL and DLBCL, respectively. The most common grade 3 to 4 toxicities included anemia (9%), neutropenia (11%), and liver dysfunction (5%). Overall response was 60% in FL (3/5; complete response, 40% [2/5]), and 14% in DLBCL (4/27; complete response, 7% [2/27]). Distinct peripheral blood and tumor T-cell features, including CD8 PET–determined intratumoral CD8 T-cells, correlated with response (P < .05). RT-durvalumab with 30 Gy/10 fractions of RT to 3 disease sites is safe, and offers promising responses in FL. Intratumoral and peripheral blood CD8 T-cell dysregulation correlate with treatment response. This trial was registered at www.clinicaltrials.gov as #NCT03610061.
Introduction
Tumor microenvironment (TME) immune cell composition and function heavily dictate the outcomes in both diffuse large B-cell lymphoma (DLBCL) and follicular lymphoma (FL).1,2 Immune checkpoint inhibitors have revolutionized solid tumor treatment, and most commonly block programmed cell death 1 (PD-1) and its ligand (PD-L1), which are upregulated to facilitate immune evasion in lymphoma.3-5 However, PD-1 and PD-L1 inhibitors (PD-1i and PD-L1i) have elicited variable responses in lymphoma, benefiting only a minority of patients with non-Hodgkin lymphoma, particularly in the relapsed setting.6
Augmenting immunotherapy responses with established immunogenic agents has improved outcomes. Radiotherapy (RT) is one such therapy, causing DNA damage in target tissue within the treated volume, resulting in apoptotic cell death and tumor neoantigen release with subsequent activation of innate and adaptive immune systems resulting in abscopal effects in distant disease.7 Exposure to larger RT volumes potentially increases both the quantity and breadth of circulating tumor neoantigens, further stimulating immunotherapy-driven cancer cell death. DLBCL and FL are highly radiosensitive, and effective RT doses offer tumor antigen release and immune stimulation with minimal toxicity.8
In addition to therapeutic knowledge gaps in patients with immunotherapy-treated lymphoma, robust predictive biomarkers are lacking. Comprehensive studies of imaging, tissue and blood-based biomarkers in the same population are scant. Multimodality biomarker studies embedded in early-phase studies are critical to drug development and future patient selection.
This study tested the hypothesis that increasing both dose and treatment volumes of low-moderate dose RT with PD-L1i (durvalumab) enhances immune augmentation in DLBCL and FL, and evaluated the safety of this approach. The primary end point was RT recommended phase 2 dose and treatment volumes. An extensive preplanned proof-of-concept exploratory biomarker, positron emission tomography (PET) radiomics, and novel immunoPET program was undertaken.
Methods
The “RADD” study (ClinicalTrials.gov identifier: NCT03610061) was an investigator-initiated multicenter, phase 1b RT dose- and treated volume-escalation trial with a standard 3+3 design (supplemental Figure 1).
Eligible adult patients provided written informed consent, and had histologically proven DLBCL or FL according to World Health Organization classification,9 were relapsed/refractory to at least 1 prior systemic therapy, transplant ineligible, Eastern Cooperative Oncology Group performance status 0 to 2, with adequate organ function, at least 3 disease sites that were 18F-fluorodeoxyglucose (FDG)-PET-avid, measurable and amenable to RT. Prior chimeric antigen receptor-T cell therapy was allowed. Key exclusions were prior immune checkpoint inhibitors, concurrent cancer treatment, active autoimmune disease, systemic immunosuppression, interstitial lung disease, central nervous system involvement, active hepatitis B or C, and positive HIV serology. Study data management used the REDCap electronic data capture tool.10
Treatment
All patients received at least 5 RT fractions (#) to between 1 and 3 treated sites, and the cumulative dose ranged between 2.5 Gy and 30 Gy across 6 dose cohorts (supplemental Figure 1). Durvalumab started on day 2 of RT (1500 mg fixed-dose intravenous [IV] infusion), continuing every 28 days until disease progression or unacceptable toxicities. The timing ensured that RT-induced tumor antigen release and inflammation commenced 24 hours prior to PD-L1i administration.
RT planning
All participants underwent baseline computed tomography (CT)-guided RT planning, with appropriate consideration of respiratory and visceral motion where required (4-dimensional CT or fluoroscopic). Treated sites were selected using a preference list to minimize concurrent toxicities (eg, sites such as lung and gastrointestinal tract were deprioritized due to risk of potential additive toxicity) (supplemental Table 1; supplemental Methods). For each of 1 to 3 sites, the gross tumor volume was treated, with a margin for setup uncertainty and organ motion, and with no margin for subclinical spread due to the noncurative intent of the RT (supplemental Methods). Cohorts 1 to 5 received doses that escalated from 2.5 Gy/5# to 20 Gy/5#, the latter a typical palliative dose with response rates >80%.11 RT dose was capped at 10 Gy/5# for FL due to established superior radiosensitivity,12 and prognosis of FL and potential second malignancies favoring the risk/benefit ratio of this dose. Cohort 6 used 30 Gy/10# to reduce the risk of potential tumor lysis with large fraction sizes in anticipation of potential heavy tumor burden.12 The dose to 90% of the volume was 100%, with a maximum point dose of 110%, using 3-dimensional conformal RT or intensity-modulated RT. Quality assurance was performed by the Trans-Tasman Radiation Oncology Group, consisting of several credentialing cases, pretreatment plan and contour peer review for the first 2 cases, and posttreatment quality assurance thereafter.
PET
The FDG-PET/CTs were performed at baseline, after durvalumab cycle 2, and then every 8 weeks until progressive disease (PD) or consent withdrawal, on Australian Radiopharmaceutical Trials Network accredited scanners,13 with a low-dose, noncontrast CT (supplemental Methods).
All scans were reviewed centrally according to Lugano criteria and quantitative PET parameters.14 Analysis was performed using MIM Encore software (version 7.3.4). Lesions were delineated using a “Lesion ID” workflow, a semiautomated preselection of FDG-avid structures defined by a threshold level of standardized uptake value (SUV) ≥4. Nontumor lesions were manually deleted, and any lymphoma lesions that were not delineated by the automated workflow were manually added. Extranodal and splenic involvement was included only if focal hypermetabolism was demonstrated.
The PET/CTs were reviewed by 2 experienced nuclear medicine physicians for abnormal uptake in any lesions. Together with a radiation oncologist, these lesions were correlated with RT plans to determine location according to RT treatment volumes. This information was not used to modify gross tumor volume, which was based on standard planning. For response assessments, lesions were designated as “in-treated volume” or “out-of-treated volume” disease response. For each FDG-PET, the following metabolic parameters were determined: maximum SUV (SUVmax); mean SUV (SUVmean); total metabolic tumor volume (TMTV); and total lesion glycolysis, defined as the sum of MTV × SUVmean. Quantitative analysis was performed on all PET/CTs, and the percentage reduction of each metric in relation to baseline (Δ) was calculated.
ImmunoPET
In a subset of patients, exploratory PET/CTs using investigational PET tracers zirconium-89-labeled-durvalumab (89Zr-durvalumab) and zirconium-89-Df-crefmirlimab (89Zr-Df-crefmirlimab [formerly IAB22M2C] an anti–cluster of differentiation [CD]8 minibody) (ImaginAb Inc)15 were undertaken. 89Zr-Df-crefmirlimab PET/CTs were performed 6 days before RT and 50 days after treatment commencement (D50). 89Zr-durvalumab PET/CT was performed a maximum of 14 days prior to RT commencement. For these specific PET/CTs, biodistribution and dosimetry of the injected tracer were also analyzed. The PET/CTs were acquired at predefined sequences after injection of 89Zr-durvalumab or 89Zr-Df-crefmirlimab, respectively (supplemental Methods). Regions of interest for all known tumor locations were manually defined on the 24-hour PET/CT scan using the same MIM software, providing quantitative PET metrics (supplemental Figure 2).
Biomarker assessment
The DLBCL cell-of-origin was determined by gene expression profiling (GEP) where possible or, if not, according to Hans algorithm immunohistochemistry.16,17 Epstein-Barr virus-in situ hybridization was performed on all cases. Preplanned research biospecimen collection included tumor tissue (baseline, C1D8, and PD) and peripheral blood sampling timed with FDG-PET/CT response assessments where practical.
GEP was performed on 48 formalin-fixed paraffin-embedded tissue biopsy samples (31 samples obtained at baseline, 12 on C1D8-D12, and 5 at progression). Four patients were not analyzed due to consent withdrawal (1), or insufficient tissue (3). RNA was extracted using Qiagen AllPrep DNA/RNA formalin-fixed paraffin-embedded kit according to the manufacturer’s guidelines, and quantified by Qubit RNA High Sensitivity Assay Kit. RNA expression was digitally quantified using the NanoString PanCancer Immune panel (730 immune-related genes plus 40 housekeeping genes). Data were normalized as per standard NanoString Advanced Analysis protocols, and analyzed using the Rosalind platform. The T-cell activation score was estimated using the geometric mean of normalized gene expression of 11 available genes, as previously published.18,19 The immune ratio of T-cell infiltration to PD-L1 expression and M2 macrophages (CD4∗CD8/(CD163:CD68[M2])∗PD-L1) was calculated to determine the effect of overall immune balance to response.18
Flow cytometry analysis was performed on a subset of patients’ peripheral blood mononuclear cells pretreatment (baseline), and on-treatment (C1D8) where available (3 patients with complete metabolic response [CMR]; 2 with partial metabolic response [PMR]; 8 with PD, as evaluated at best response). Expression of immune checkpoints (PD-1 and T-cell immunoreceptor with immunoglobulin and immunoreceptor tyrosine-based inhibitory motif domains [TIGIT]) and transcription factor 7 (TCF7), a marker of reversible T-cell exhaustion, and heightened T-cell response to checkpoint blockade, were examined on T-cell subsets.20 Cell subsets were defined by gating on forward and side scatter, singlets and live/dead staining before specific immune subset gating. CD3+CD4+ T cells, CD3+CD8+ T cells, and CD3+CD4+CD25+ regulatory T cells were evaluated, and were further divided into central memory T cells, effector memory T cells, terminally differentiated effector memory T cells, and naïve T cells based on CCR7 and CD45RA expression. One sample was excluded from analysis due to insufficient T cells. Data were acquired using a FACSymphony A5, and analyzed using FlowJo software. Supplemental Table 2 outlines full details of antibodies used, and supplemental Figure 3 represents the gating strategy.
End points and statistical analysis
The primary end point was RT recommended phase 2 dose (and treatment volume; RT-RP2D) combined with durvalumab. Secondary end points were toxicity using common terminology criteria for adverse events (AEs) (v4.03), overall response rate, progression-free survival, overall survival (OS), and RT maximum tolerated dose.
Patient characteristics, AEs and response proportions were reported using descriptive statistics. The Wilson interval was used to calculate 95% confidence intervals (CIs) due to sample size.
Statistical analysis of survival and PET parameters were performed using Graph Pad Prism (Version 10). Progression-free survival and OS were evaluated using Kaplan-Meier methodology with censoring at time of completion of follow-up or data lock with minimum follow-up 12 months from disease progression, or until the date of the first response assessment on the next treatment line.21 Analysis of mean differences in baseline TMTV of in-treated vs out-of-treated volume was conducted by a 2-tailed, paired t-test. Analysis of variance between patients in uptake of 89Zr-Df-crefmirlimab was compared across substudy participants using a nonparametric Kruskal-Wallis test.22 A pairwise analysis of mean uptake was undertaken using the Tukey multiple comparison test.23 Differences in GEP were determined using the nonparametric Mann-Whitney test.24 This study complied with the Declaration of Helsinki and was approved by an independent ethics committee (HREC/17/Austin/478).
Results
Patients
Between November 2018 and September 2021, 34 patients were enrolled across 3 Australian institutions (Figure 1A). Table 1 summarizes patient characteristics.
Treatment outcomes and response. (A) Consort diagram. (B) Treatment duration and follow-up (F/U) postcompletion according to disease and RT dose level. Durvalumab treatment indicated by colored solid bars. Triangles represent best response, circle represents PD. Black arrows illustrate participants who were alive at data lock. Participants in the PET substudy are denoted by colored arrows according to tracer. (C) TMTV change in lesions according to RT dose level and in-treated volume vs out-of-treated volume responses. X represents participants who did not have a F/U PET/CT. CNS, central nervous system; MRI, magnetic resonance imaging; CMR, complete metabolic response; PMR, partial metabolic response; SD, stable disease; PD, progressive disease.
Treatment outcomes and response. (A) Consort diagram. (B) Treatment duration and follow-up (F/U) postcompletion according to disease and RT dose level. Durvalumab treatment indicated by colored solid bars. Triangles represent best response, circle represents PD. Black arrows illustrate participants who were alive at data lock. Participants in the PET substudy are denoted by colored arrows according to tracer. (C) TMTV change in lesions according to RT dose level and in-treated volume vs out-of-treated volume responses. X represents participants who did not have a F/U PET/CT. CNS, central nervous system; MRI, magnetic resonance imaging; CMR, complete metabolic response; PMR, partial metabolic response; SD, stable disease; PD, progressive disease.
Patient characteristics
| Patient characteristic . | n (%) . | Patients with DLBCL N = 29 n (%) . | Patients with FL N = 5 n (%) . |
|---|---|---|---|
| Age, median (range) | 74 (28-87) | ||
| Sex | |||
| Male | 17 (50) | 15 (51) | 2 (40) |
| Female | 17 (50) | 14 (48) | 3 (60) |
| Histology | |||
| FL | 5 (14) | ||
| DLBCL | 29 (85) | ||
| GCB | 18 (62) | ||
| Non-GCB | 8 (27) | ||
| EBV | 1 (3) | ||
| Unknown (not reported) | 2 (7) | ||
| Ann Arbor stage | |||
| II | 4 (14) | 1 (20) | |
| III | 5 (17) | 2 (40) | |
| IV | 20 (69) | 2 (40) | |
| B symptoms | 4 (12) | 4 (14) | 0 (0) |
| Extranodal disease | 23 (68) | 23 (80) | 0 (0) |
| LDH elevated | 22 (65) | 20 (70) | 2 (40) |
| Prior treatment lines | |||
| 1 | 10 (29) | 6 (21) | 4 (80) |
| 2 | 6 (17) | 6 (21) | 0 (0) |
| 3+ | 18 (53) | 17 (58) | 1 (20) |
| Prior treatment details | |||
| Chemotherapy/R-chemotherapy | 34 (100) | 29 (100) | 5 (100) |
| Immunotherapy | 0 (0) | 0 (0) | 0 (0) |
| Bispecific therapy | 4 (12) | 3 (10) | 1 (20) |
| CAR T cell | 3 (9) | 3 (10) | 0 (0) |
| Other | |||
| Autologous stem cell transplant | 1 (3) | 1 (3) | 0 (0) |
| Patient characteristic . | n (%) . | Patients with DLBCL N = 29 n (%) . | Patients with FL N = 5 n (%) . |
|---|---|---|---|
| Age, median (range) | 74 (28-87) | ||
| Sex | |||
| Male | 17 (50) | 15 (51) | 2 (40) |
| Female | 17 (50) | 14 (48) | 3 (60) |
| Histology | |||
| FL | 5 (14) | ||
| DLBCL | 29 (85) | ||
| GCB | 18 (62) | ||
| Non-GCB | 8 (27) | ||
| EBV | 1 (3) | ||
| Unknown (not reported) | 2 (7) | ||
| Ann Arbor stage | |||
| II | 4 (14) | 1 (20) | |
| III | 5 (17) | 2 (40) | |
| IV | 20 (69) | 2 (40) | |
| B symptoms | 4 (12) | 4 (14) | 0 (0) |
| Extranodal disease | 23 (68) | 23 (80) | 0 (0) |
| LDH elevated | 22 (65) | 20 (70) | 2 (40) |
| Prior treatment lines | |||
| 1 | 10 (29) | 6 (21) | 4 (80) |
| 2 | 6 (17) | 6 (21) | 0 (0) |
| 3+ | 18 (53) | 17 (58) | 1 (20) |
| Prior treatment details | |||
| Chemotherapy/R-chemotherapy | 34 (100) | 29 (100) | 5 (100) |
| Immunotherapy | 0 (0) | 0 (0) | 0 (0) |
| Bispecific therapy | 4 (12) | 3 (10) | 1 (20) |
| CAR T cell | 3 (9) | 3 (10) | 0 (0) |
| Other | |||
| Autologous stem cell transplant | 1 (3) | 1 (3) | 0 (0) |
CAR, chimeric antigen receptor; EBV, Epstein-Barr virus; GCB, germinal center B-cell-like; LDH, lactate dehydrogenase.
Ten patients were enrolled in the PET substudy; 2 underwent 89Zr-durvalumab PET/CT, 7 89Zr-Df-crefmirlimab PET/CT, and 1 underwent both PET tracer scans at baseline; 6 patients underwent the second 89Zr Df-crefmirlimab PET/CT at D50.
Safety
All patients experienced at least 1 AE. Treatment-related AEs of ≥grade 3 occurred in 13 patients (38%), and are summarized in Table 2. One death occurred due to sepsis, and was considered unrelated to study interventions. Eight serious AEs occurred in 8 patients, with 3 therapy-related (thromboembolism, pleural effusion, odynophagia; supplemental Table 3).
Summary of AEs
| Toxicity grade . | Grade 1 . | Grade 2 . | Grade 3 . | Grade 4 . | Grade 5 . |
|---|---|---|---|---|---|
| All-cause AEs, n (%) | 34 (100) | 22 (64) | 15 (44) | 4 (12) | 1 (3) |
| Treatment related, n (%) | 20 (59) | 14 (41) | 10 (29) | 3 (9) | 0 (0) |
| Treatment-related adverse events | Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
| Hematological | |||||
| Neutrophil count decreased | 1 | 1 | 1 | 3 | |
| Platelet count decreased | 3 | 1 | |||
| Anemia | 1 | 3 | |||
| Gastrointestinal | |||||
| Nausea/vomiting | 3 | 2 | |||
| Diarrhea | 3 | 1 | |||
| Mucositis oral | 1 | ||||
| Liver enzyme derangement | 1 | 1 | 2 | ||
| Anorexia | 1 | 2 | |||
| Odynophagia | 3∗ | ||||
| Laryngeal inflammation | 1 | ||||
| Constipation | 1 | ||||
| Dysgeusia | 1 | ||||
| Abdominal pain | 1 | ||||
| Other | |||||
| Fatigue/lethargy | 6 | 3 | 1 | ||
| Infection | 5 | 1 | |||
| Pruritus | 4 | 1 | |||
| Squamous cell carcinoma | 3 | ||||
| Hyperthyroidism | 2 | ||||
| Hypothyroidism | 1 | ||||
| Fever | 2 | ||||
| Rash (undefined) | 2 | ||||
| Pleural effusion | 1∗ | ||||
| Hypercalcemia | 1 | ||||
| Hypocalcemia | 1 | ||||
| Hypomagnesemia | 1 | ||||
| Hypokalemia | 1 | ||||
| Hypophosphatemia | 1 | ||||
| Arthralgia | 1 | ||||
| Myalgia | 1 | ||||
| Itchy eyes | 1 | ||||
| Pain | 1 | ||||
| Paresthesia | 1 | ||||
| Thromboembolic event | 1∗ | ||||
| Headache | 1 |
| Toxicity grade . | Grade 1 . | Grade 2 . | Grade 3 . | Grade 4 . | Grade 5 . |
|---|---|---|---|---|---|
| All-cause AEs, n (%) | 34 (100) | 22 (64) | 15 (44) | 4 (12) | 1 (3) |
| Treatment related, n (%) | 20 (59) | 14 (41) | 10 (29) | 3 (9) | 0 (0) |
| Treatment-related adverse events | Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
| Hematological | |||||
| Neutrophil count decreased | 1 | 1 | 1 | 3 | |
| Platelet count decreased | 3 | 1 | |||
| Anemia | 1 | 3 | |||
| Gastrointestinal | |||||
| Nausea/vomiting | 3 | 2 | |||
| Diarrhea | 3 | 1 | |||
| Mucositis oral | 1 | ||||
| Liver enzyme derangement | 1 | 1 | 2 | ||
| Anorexia | 1 | 2 | |||
| Odynophagia | 3∗ | ||||
| Laryngeal inflammation | 1 | ||||
| Constipation | 1 | ||||
| Dysgeusia | 1 | ||||
| Abdominal pain | 1 | ||||
| Other | |||||
| Fatigue/lethargy | 6 | 3 | 1 | ||
| Infection | 5 | 1 | |||
| Pruritus | 4 | 1 | |||
| Squamous cell carcinoma | 3 | ||||
| Hyperthyroidism | 2 | ||||
| Hypothyroidism | 1 | ||||
| Fever | 2 | ||||
| Rash (undefined) | 2 | ||||
| Pleural effusion | 1∗ | ||||
| Hypercalcemia | 1 | ||||
| Hypocalcemia | 1 | ||||
| Hypomagnesemia | 1 | ||||
| Hypokalemia | 1 | ||||
| Hypophosphatemia | 1 | ||||
| Arthralgia | 1 | ||||
| Myalgia | 1 | ||||
| Itchy eyes | 1 | ||||
| Pain | 1 | ||||
| Paresthesia | 1 | ||||
| Thromboembolic event | 1∗ | ||||
| Headache | 1 |
There were no treatment-related deaths on the study.
Events meeting serious AE criteria defined by the protocol.
No dose-limiting toxicities occurred, thus a maximum tolerated dose was not identified. The RP2D based on toxicity data and the 3+3 design was 10 Gy/5# to 3 sites for FL, and 30 Gy/10# to 3 sites for DLBCL.
Efficacy
Treatment duration and response are presented in Figure 1B. The median number of durvalumab cycles was 2 (range 1-32; DLBCL median 2 cycles, range, 1-32; FL median 11 cycles, range, 4-30). The median follow-up was 7.1 months (range, 0.17-60 months). OS is presented in supplemental Figure 4.
Thirty-two patients (94%) were evaluable for response, with a 22% objective response rate (ORR; 7/32) and 12% CMR (4/32); the ORR in patients with FL was 60% (3/5), and was 14% (4/27) in patients with DLCBL. Two patients with DLBCL were not evaluable due to an early unrelated grade 5 AE, and withdrawal of consent prior to disease response assessment, respectively. Figure 1C and supplemental Figure 5 present disease-specific responses. The median response duration was 3.3 months (95% CI, 2-16) and >12 months in 50% (4/8). Twenty-six patients were evaluable for PET/CT radiomic assessments (baseline and on-treatment PET/CT). RT-treated volume responses were observed across all cohorts, with 100% of those receiving ≥10 Gy demonstrating marked TMTV reduction (Figure 1C) from baseline. Abscopal (out-of-treated volume) disease control was observed in 21% of patients (95% CI, 10-40), all in the ≥10 Gy dose levels (Figure 1C; supplemental Table 4). Full baseline PET-radiomic values are presented in supplemental Table 5.
Eleven patients had data available on the next therapeutic line, with 6 responding to the subsequent treatment (supplemental Table 6)
Biomarker analysis
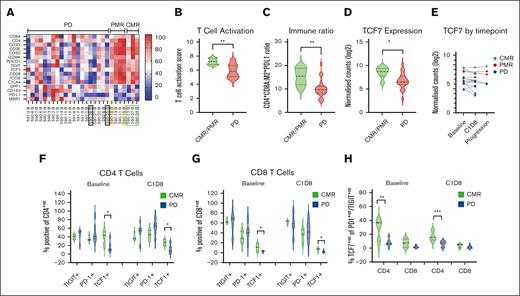
The ORR was significantly higher in patients with high levels of T-cell infiltration in baseline TME as assessed by GEP (Figure 2A) compared with those with lower T-cell infiltration. Multiple T-cell markers, including CD8A and cytotoxic T-cell markers, were enriched in responders with T-cell activation scores significantly higher in responders (P = .0013) vs nonresponders (Figure 2B). The immune ratio of T-cell infiltration to PD-L1 expression and M2 macrophages (Figure 2C) was significantly lower in patients with PD compared with responders (P = .0051). In addition, gene expression of TCF7 was significantly higher in responders (P = .0160) and this was maintained on serial biopsies when compared with patients who ultimately progressed during treatment (Figure 2D-E).
Biomarker analysis. Gene expression profiling in tumor biopsies was examined, and differentially expressed genes evaluated based on best response. (A) Heat map of T cells and M2 macrophage-related genes demonstrating differential gene expression according to best response. Participants who underwent CD8 PET are highlighted by color-coded boxes that correspond to Figure 3D results. (B-D) Expression of T-cell activation score (as described in “Methods”), immune ratio, and TCF7 expression based on best response are represented by panels B, C, and D, respectively. (E) Matched tumor biopsies from baseline (BL), C1D8-15 and progression were evaluated for TCF7 expression (3 CMR in green; 2 PMR in blue; and 7 PD in red). (F-G) Flow cytometry analysis of immune checkpoints (TIGIT and PD-1) and TCF7 was evaluated in CD4+ and CD8+ T cells at BL and C1D8 (CMR, n = 3; PD, n = 7) for panels F and G, respectively. (H) TCF7 expression was examined in PD1/TIGIT dual-positive cells for CD4+ and CD8+ cells (CMR, n = 3; PD, n = 7). ∗P < .05; ∗∗P < .01; ∗∗∗P = .009.
Biomarker analysis. Gene expression profiling in tumor biopsies was examined, and differentially expressed genes evaluated based on best response. (A) Heat map of T cells and M2 macrophage-related genes demonstrating differential gene expression according to best response. Participants who underwent CD8 PET are highlighted by color-coded boxes that correspond to Figure 3D results. (B-D) Expression of T-cell activation score (as described in “Methods”), immune ratio, and TCF7 expression based on best response are represented by panels B, C, and D, respectively. (E) Matched tumor biopsies from baseline (BL), C1D8-15 and progression were evaluated for TCF7 expression (3 CMR in green; 2 PMR in blue; and 7 PD in red). (F-G) Flow cytometry analysis of immune checkpoints (TIGIT and PD-1) and TCF7 was evaluated in CD4+ and CD8+ T cells at BL and C1D8 (CMR, n = 3; PD, n = 7) for panels F and G, respectively. (H) TCF7 expression was examined in PD1/TIGIT dual-positive cells for CD4+ and CD8+ cells (CMR, n = 3; PD, n = 7). ∗P < .05; ∗∗P < .01; ∗∗∗P = .009.
While PD-L1 gene expression in patients with available tissue for analysis was not associated with therapy response, there was a significant reduction in PD-L1 RNA levels in biopsies at time of progression, suggesting that downregulation of PD-L1 may be a mechanism of therapeutic resistance (P = .005 for all samples; P = .0091 for paired samples; supplemental Figure 6).
In peripheral blood, there were no quantitative differences in T-cell subsets between responders and nonresponders at baseline or C1D8 (supplemental Figure 7A-C). However, patients achieving CMR (n = 3) had significantly higher levels of TCF7+ CD4+ and TCF7+ CD8+ T cells at baseline and day 8 after treatment (Figure 2F-G) compared with patients with PD (n = 7). In terms of immune checkpoint coexpression, responders had increased TCF7 expression in CD4+ T cells that coexpressed PD-1 and TIGIT (Figure 2H) at baseline (P = .01) and C1D8 (P = .009), suggestive of a progenitor, reversibly exhausted phenotype. A similar trend of increased TCF7 expression was seen in PD1+, TIGIT+ CD8+ T cells, but this did not reach statistical significance. In addition, there were significantly higher CD4+ and CD8+ T-effector memory cells that coexpressed TCF7 at baseline in patients who achieved a CMR (supplemental Figure 7D-E).
Overall, these data indicate that high levels of T-cell infiltration with reversible T-cell exhaustion and reduced immune suppression within the TME are associated with improved responses to immune checkpoint therapy and RT. Downregulation of PD-L1 gene expression may be a mechanism of therapeutic resistance. Furthermore, higher levels of TCF7 in T-cell subsets in peripheral blood and within the tumor are associated with improved responses to PD-L1i and RT on study.
CD8-PET and durvalumab-PET substudies
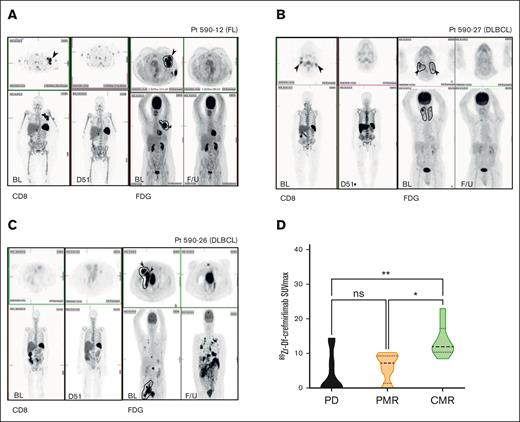
Eight patients underwent baseline 89Zr-Df-crefmirlimab-PET/CT, and 6 of 8 had a second D50 scan. Qualitative baseline tumor 89Zr-Df-crefmirlimab uptake was evident in all patients, although uptake varied between anatomical locations and known disease sites (Figure 3A-C).
Baseline 89Zr-Df-crefmirlimab (CD8 T-cell) uptake correlates with posttreatment tumor FDG-PET-CT response. (A-C) Representative baseline (BL) CD8 T-cell and paired FDG-PET imaging (and F/U scans ∼D50). (A) A 50-year-old female patient with stage II FL (at diagnosis): whole-body 89Zr-Df-crefmirlimab scan 24 hours postinjection (p.i.) (left) at BL and day 51 on treatment with paired FDG-PET (right). Axial slices of left axillary mass demonstrate intense uptake at BL and CMR on treatment. RT fields are illustrated on the FDG-PET image. (B) A 86-year-old male with DLBCL: whole-body 89Zr-Df-crefmirlimab scan 24 hours p.i. (left) at BL and F/U on treatment with paired FDG-PET (right). Axial slice of bilateral neck lesions demonstrate intense uptake at BL and CMR on treatment. RT fields are illustrated on the FDG-PET image. (C) A 76-year-old female with DLBCL: whole-body 89Zr-Df-crefmirlimab scan 24 hours p.i. (left) at BL and F/U on treatment with paired FDG-PET (right). On BL scans, axial slices of right external iliac mass demonstrate intense uptake of FDG pretreatment, but no BL 89Zr-Df-crefmirlimab uptake in tumor sites; however, some marrow uptake plus excretion of catabolized 89Zr in bowel is seen. Although the patient had a CMR in RT-treated volume, they had extensive PD out-of-treated volume on treatment. Radiotherapy fields are illustrated on the FDG-PET image. (D) Tumor uptake (SUVmax) of 89Zr-Df-crefmirlimab 24 hours p.i. at baseline was significantly higher in patients who achieved a CMR on study (P = .003; adjusted P values ∗P = .01, ∗∗P = .002). D51, 51 days after treatment commencement; ns, not significant; PD, progressive disease; PMR, partial metabolic response.
Baseline 89Zr-Df-crefmirlimab (CD8 T-cell) uptake correlates with posttreatment tumor FDG-PET-CT response. (A-C) Representative baseline (BL) CD8 T-cell and paired FDG-PET imaging (and F/U scans ∼D50). (A) A 50-year-old female patient with stage II FL (at diagnosis): whole-body 89Zr-Df-crefmirlimab scan 24 hours postinjection (p.i.) (left) at BL and day 51 on treatment with paired FDG-PET (right). Axial slices of left axillary mass demonstrate intense uptake at BL and CMR on treatment. RT fields are illustrated on the FDG-PET image. (B) A 86-year-old male with DLBCL: whole-body 89Zr-Df-crefmirlimab scan 24 hours p.i. (left) at BL and F/U on treatment with paired FDG-PET (right). Axial slice of bilateral neck lesions demonstrate intense uptake at BL and CMR on treatment. RT fields are illustrated on the FDG-PET image. (C) A 76-year-old female with DLBCL: whole-body 89Zr-Df-crefmirlimab scan 24 hours p.i. (left) at BL and F/U on treatment with paired FDG-PET (right). On BL scans, axial slices of right external iliac mass demonstrate intense uptake of FDG pretreatment, but no BL 89Zr-Df-crefmirlimab uptake in tumor sites; however, some marrow uptake plus excretion of catabolized 89Zr in bowel is seen. Although the patient had a CMR in RT-treated volume, they had extensive PD out-of-treated volume on treatment. Radiotherapy fields are illustrated on the FDG-PET image. (D) Tumor uptake (SUVmax) of 89Zr-Df-crefmirlimab 24 hours p.i. at baseline was significantly higher in patients who achieved a CMR on study (P = .003; adjusted P values ∗P = .01, ∗∗P = .002). D51, 51 days after treatment commencement; ns, not significant; PD, progressive disease; PMR, partial metabolic response.
Baseline 89Zr-Df-crefmirlimab uptake in disease sites (as determined by SUVmax) was higher in participants achieving CMR than those with partial metabolic responder or PD (P = .003; Figure 3D). GEP of CD8 T cells and M2 macrophage-related genes were variable in this subgroup (Figure 2A).
Three patients had baseline 89Zr-durvalumab-PET/CT. Uptake in tumor sites varied (supplemental Figure 8A-B). PD-L1 expression in baseline tumor (supplemental Figure 8D), and durvalumab distribution to disease sites as measured by SUVmax in 89Zr-durvalumab PET/CT (supplemental Figure 8C) did not correlate with response (supplemental Figure 8E).
Discussion
To our knowledge, this is the first study to escalate both absorbed RT dose and irradiated volumes in combination with PD-L1i with the goal of augmenting immune response using RT dual proinflammatory and cytotoxic properties. We demonstrated safe delivery of RT at doses of ≤30 Gy/10# to a maximum of 3 anatomically distinct sites with durvalumab from day 2 of RT. Although PD-1i/PD-L1i responses are as low as 3% to 4% in FL and DLBCL monotherapy studies, combination with incrementally higher RT absorbed doses and irradiated volumes appeared to elicit more abscopal responses in our study. Additionally, we demonstrated, to our knowledge, for the first time, the feasibility of a novel PET/CT substudy measuring CD8 T cells and PD-L1i biodistribution in patients with immunotherapy-treated lymphoma. Importantly, we identify key potential tumor immune characteristics associated with RT-durvalumab responses in lymphoma, including a higher presence of CD8 T cells on PET/CT measured by SUVmax, and high levels of certain T-cells in the TME of patient biopsies and peripheral blood.
Our demonstrated safety of RT with PD-L1i has broad applicability across different tumor types, and can be potentially extrapolated to RT combinations with newer T-cell engaging immunotherapies including bispecific antibodies. Some previous solid-tumor studies of combination RT-immunotherapy have reported increased toxicity. For example, in a randomized lung cancer study delivering RT specifically to lung tissue with or without durvalumab, RT-durvalumab yielded increased pneumonitis (34% vs 25%), although severe pneumonitis rates were similar. Our results demonstrate that the combination of low-dose, selective RT and durvalumab in a frail population is tolerable and did not reveal any safety signal suggestive of additive toxicity, even in patients with large volumes of nodal disease (maximum diameter 10 cm). Our data broadly reflect a similar safety profile to other single-arm trials of RT-immunotherapy.25
Our application of RT potentially impacts future combination trial designs by establishing the required RT dose to elicit FL and DLBCL in-field responses. Conventionally, RT volumes applied in this context tend to be regional rather than focal. Our approach treated involved nodes only, prioritized symptomatic or dominant disease with the rationale that it could overcome protracted time-to-maximal response seen with PD-L1i, and sites least likely to be affected by additive toxicity based on PD-L1i safety profiles. We observed consistently high in-field response rates at the ≥10 Gy/5# dose level for all tumors. DLBCL received the maximum dose of 20 to 30 Gy, resulting in the highest response rates with modest toxicity. Our approach of selective RT for dominant lesions and then “preferred” lesions may be also applicable in future trials that employ position RT as an immune modulator rather than a purely cytotoxic agent. The significant tolerability plus encouraging responses in FL led to our follow-on treatment-naïve FL study.26
The remarkable biomarker evidence we present provides a portrait of T-cell features associated with response across 3 key measures: CD8 T-cell imaging, GEP from tissue biopsies, and flow cytometry of peripheral blood. CD8 T-cell imaging showed that higher CD8 T-cell concentrations in tumors are powerfully associated with responsiveness to combined RT-PD-L1i, including out-of-treated volume sites of disease. Although the sample size is small, the possible correlation between SUVmax CD8 T cells and lymphoma tumor response is intriguing, and suggests that CD8 T-cell imaging should be further tested as a baseline predictive tool for T-cell enhancing immunotherapies.27 We are exploring this in ongoing next-generation immunotherapy studies.
Radiolabeled-durvalumab imaging provides information that traditional pharmacokinetic and pharmacodynamic studies cannot, especially when paired with FDG-PET/CT tumor assessments. Higher 89Zr-durvalumab uptake corresponded to disease FDG uptake, but unlike CD8 T-cell PET/CT, did not correlate with therapeutic response. Supporting these findings, our paired tissue analysis also demonstrated no correlation between baseline PDL1 levels and response. However, our provocative results, along with the notable decrease in PDL1 tissue levels after treatment, warrant further investigation.
Our baseline TME scrutiny of immune cell infiltrates, including CD8 T-cell infiltration and activation, and immune-suppressing macrophages, correlated strongly with responses in our small cohort, suggesting these may be predictive of response, although further studies are needed. The ratio of T-cell infiltration to PD-L1 expressing M2 macrophages was significantly higher in responders, consistent with prior literature in DLBCL where a lower immune ratio was associated with inferior OS in patients treated with chemoimmunotherapy.18 TCF7 expression was increased in baseline biopsies and in T-cell subsets in peripheral blood of patients achieving CMR to RT-durvalumab. TCF7 is a transcription factor that promotes T-cell memory function, and is upregulated in progenitor exhausted CD8 T-cell populations that are potentially tumor responsive after immune checkpoint blockade in contrast to terminally exhausted T cells that upregulate TOX.28,29 Higher TCF7 expression has been associated with immune checkpoint blockade response in melanoma, and TCF7+ tumor-infiltrating cells have been shown to be neoantigen specific in preclinical models.20 Taken together, the baseline presence of high levels of T-cell infiltration and reduced immune-suppressing macrophages within the TME could potentially select patients with B-cell lymphoma to prioritize for immunotherapeutic strategies, and monitoring for checkpoint downregulation is potentially warranted to identify reasons for treatment failure in future studies. Our preliminary findings on TCF7 need to be validated in larger cohorts with further analysis of T-cell phenotypes in tumors, including spatial localization of TCF7+ T-cells within the TME, and determining whether TCF7+ T-cells are neoepitope specific.
Our study has limitations. The nonrandomized design prevents assessment of the contribution of each treatment component to efficacy, and the applicability of our results to other diseases may be affected by our highest dose being only 30 Gy/10# and the conservative 10 Gy/5# for FL, when higher doses are used for limited-stage FL in curative contexts. Additionally, lymphoma favors nodal sites, and the safety of such doses and treatment volumes targeting extranodal disease in radiosensitive organs are unknown. Also, our biomarker studies are limited by small subgroups restricting formal statistical comparisons.
In conclusion, our unique phase 1 study escalating both RT dose and treatment volumes combined with durvalumab immunotherapy demonstrated safety with modest DLBCL efficacy, and promising FL efficacy. Although PD-L1i are not being widely developed in B-cell lymphomas, RT-PD-L1i is potentially of interest as chimeric antigen receptor-T cell therapy bridging or maintenance. Additionally, we are exploring RT with newer bispecific immunotherapies. Results from this comprehensive approach indicate that both baseline CD8 T-cell imaging with 89Zr-Df-crefmirlimab PET and PET-measured drug distribution, as well as GEP-determined TME T-cell infiltration plus peripheral blood T-cell composition should be evaluated in future lymphoma immunotherapy trials.
Acknowledgments
The positron emission tomography substudy imaging agent Df-crefmirlimab (formerly Df-IAB22M2C) was kindly provided by ImaginAb Inc. The authors thank the clinical trials teams at all sites for their work and care of the patients who have participated in the study.
This trial ONJ2017-DV-008259 was funded by the Victorian Cancer Agency grant TRP16-006. Drug and part funding was provided by AstraZeneca, Australia.
The sponsor (Olivia Newton-John Cancer Research Institute) oversaw study design, data collection, analysis, and the final manuscript. Additional drug and funding supporters (AstraZeneca, ImaginAb) approved the study protocol and final manuscript, but played no role in design, data collection, analysis, or interpretation.
Authorship
Contribution: E.A.H., R.K., M.M., C.K., J.S., and A.M.S. contribution to study design; and all authors contributed to writing the manuscript, reviewed the manuscript, approved of the final version of the manuscript before submission, and vouch for the data, analyses, and adherence of the study to the protocol.
Conflict-of-interest disclosure: E.A.H. has received research funding from Bristol Myers Squibb/Celgene, Merck KgaA, AstraZeneca, and F. Hoffmann-La Roche (all paid to institution); has acted as a consultant/advisor for F. Hoffmann-La Roche, Antengene, Bristol Myers Squibb, AstraZeneca, Novartis, Merck Sharpe Dohme, Specialised Therapeutics, Sobi, Regeneron, and Gilead; has acted as a speaker for Roche, AstraZeneca, Janssen, and Regeneron; and has received travel expenses from AstraZeneca. S.T.L. has received research funding from the Victorian Cancer Agency. J.S. reports receiving grant support, paid to Monash University, from Astex Pharmaceuticals; receiving consultancy fees from Bristol Myers Squibb, Mundipharma, Novartis, Otsuka, and Pfizer; receiving speaker bureau fees from Mundipharma and Novartis; serving as a Deputy Chair of the Australasia Leukaemia and Lymphoma Group, Scientific Advisory Committee; and serving on the board of directors for the Victorian Cancer Council. A.M.S. has acted as a consultant for Imagion; has received research funding from Telix, Curis, Isotope Technologies Munich, AdAlta, Fusion, AstraZeneca, EMD Serono, Cyclotek, Avid Radiopharmaceuticals/Lilly, Antengene, and Merck (all paid to institution); holds patents relating to antibodies to epidermal growth factor receptor (EGFR), human epidermal growth factor receptor 2 (HER2), platelet-derived growth factors (PDGFR)-CC, fibroblast growth factor-inducible 14 (FN-14), granulocyte-macrophage colony-stimulating factor, and EPH receptor A3; and is a board member of Australia and New Zealand Society of Nuclear Medicine and World Federation of Nuclear Medicine and Biology. C.K. has received honoraria from Roche, BeiGene, Karyopharm Therapeutics, and Gilead. G.C. has received research funding from Regeneron, HutchMed, Bristol Myers Squibb, Pharmacyclics, Bayer, AstraZeneca, Amgen, Seagen, Incyte, Roche, Dizal Pharma, Merck, and Innate Pharma; and acted as a consultant for Regeneron, Takeda, and Bristol Myers Squibb. The remaining authors declare no competing financial interests.
Correspondence: Eliza A. Hawkes, Olivia Newton-John Cancer Research Institute, L5 ONJ Building, Austin Health, 145 Studley Rd, Heidelberg, VIC 3084, Australia; email: eliza.hawkes@onjcri.org.au.
References
Author notes
∗A.M.S. and C.K. are joint senior authors.
Qualified researchers may request access to study documents including the clinical study report, approved protocol and amendments, deidentified patient-level data, and study results under a signed data access agreement. Requests should be made to the corresponding author, Eliza A. Hawkes (eliza.hawkes@onjcri.org.au). Requests for access to information regarding durvalumab should be directed to AstraZeneca. Requests for access to information regarding Df-crefmirlimab should be directed to ImaginAb Inc.
The full-text version of this article contains a data supplement.