Key Points
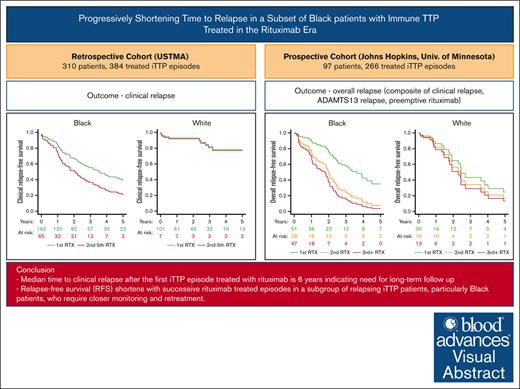
RFS shortens with successive rituximab-treated episodes in a subgroup of relapsing patients with iTTP.
Progressively shortened time to relapse is most pronounced in Black patients with iTTP, who require closer monitoring and retreatment.
Visual Abstract
Immune thrombotic thrombocytopenic purpura (iTTP) is a chronically relapsing disorder caused by autoantibody-mediated deficiency of ADAMTS13. Rituximab is frequently administered to prevent relapses, but whether the durability of rituximab effect is maintained with subsequent treatment courses has not been studied. Using the United States Thrombotic Microangiopathy Consortium (USTMA) retrospective iTTP registry, we evaluated clinical relapse-free survival (RFS) with subsequent courses of rituximab treatment in multiply relapsing patients. Separately, we evaluated overall RFS (composite of time to clinical relapse, ADAMTS13 relapse, or preemptive rituximab) in a prospective iTTP cohort from the Johns Hopkins University and the University of Minnesota. In the USTMA registry, median clinical RFS was shorter after the second or subsequent rituximab-treated episode than the first (2.1 vs 6.0 years; P = .04). White patients’ clinical relapse risk after the second and subsequent rituximab courses was not significantly different compared with the first (hazard ratio [HR], 1.86; 95% confidence interval [CI], 0.22-15.80; P = .57), whereas for Black patients, clinical relapse risk was significantly higher after the second or subsequent courses (HR, 2.82; 95% CI, 1.52-5.24; P = .001). In the prospective cohort, overall RFS progressively shortened after each episode of rituximab treatment with the first episode having the longest RFS (2.8 years; interquartile range, 2.0-6.0) and this loss of response durability was most pronounced in Black patients. The durability of rituximab’s effect declines with subsequent treatments, which is more pronounced in Black patients, who may benefit from closer monitoring and alternative immunomodulatory approaches such as maintenance rituximab and consideration of other agents.
Introduction
Immune thrombotic thrombocytopenic purpura (iTTP) is a rare hematologic disorder caused by an autoantibody-mediated deficiency of ADAMTS13 that presents with life-threatening episodes of thrombocytopenia, microangiopathic hemolytic anemia, and ischemic organ injury.1 Treatment with plasma exchange and immunosuppression has reduced mortality of acute iTTP episodes from >90% to <10%.2,3 However, over a third of patients still experience relapses of iTTP.4 Rituximab, an anti-CD20 monoclonal antibody targets B cells and suppresses pathogenic anti-ADAMTS13 antibodies leading to improvement in ADAMTS13 activity. Previously reserved for relapsed or refractory iTTP,5-8 rituximab is now commonly used in the management of iTTP even at first presentation, primarily because of its effect on prolonging relapse-free survival (RFS),9,10 which is supported by the recent International Society on Thrombosis and Hemostasis guidelines.11 Rituximab is increasingly used preemptively to prevent clinical relapse in patients who develop ADAMTS13 relapse (ADAMTS13 activity of <20% in the absence of thrombocytopenia or other iTTP symptoms).12,13 Although rituximab prolongs RFS in iTTP, there is clearly heterogeneity in rituximab response and duration.14 We recently reported that Black patients (compared to White patients) have a shorter response duration and shorter RFS after rituximab therapy for iTTP.15 Our clinical experience suggests that RFS may shorten with subsequent episodes in a subset of patients, particularly Black patients, with multiply relapsing iTTP, but this has not been formally studied. Previous studies of relapse in iTTP mostly considered only clinical relapse as an outcome.4,9,10,16 It is now well established that ADAMTS13 deficiency in clinical remission predicts relapse,17,18 and that preemptive therapy with rituximab wards off >50% of clinically overt relapses.12,13 Thus, in the current era in which preemptive therapy is increasingly common, ADAMTS13 relapse, defined as ADAMTS13 activity of <20% in the absence of thrombocytopenia or clinical symptoms of iTTP,19 is recognized as an important clinical end point.
In this setting, the aim of our study was to test the hypothesis that clinical RFS and overall RFS (a composite of time to clinical relapse, ADAMTS13 relapse, or treatment with preemptive rituximab) after rituximab are progressively shortened with successive episodes of treatment, and that this effect is more pronounced in Black patients who comprise the majority of patients with iTTP in the United States.
Methods
Participant cohorts
To examine whether successive rituximab courses are associated with progressively shorter clinical RFS, we performed an analysis of patients with confirmed iTTP treated with rituximab from the United States Thrombotic Microangiopathies (USTMA) Consortium retrospective iTTP registry, which includes adult patients with iTTP treated at 15 high-volume academic centers between 1995 to 2020. The registry includes data on clinical presentation, treatments, and outcomes, including clinical relapse from 645 patients with iTTP. The USTMA retrospective registry does not include data on serial measurement of ADAMTS13 activity in remission so does not allow assessment of ADAMTS13 relapse. To evaluate the end point of overall RFS (composite of time to clinical relapse, ADAMTS13 relapse, or preemptive rituximab) that also accounts for ADAMTS13 relapse, a clinically relevant end point in the current treatment landscape, we performed a second analysis of data of adult patients with iTTP treated at the Johns Hopkins University (JHU) and University of Minnesota between January 2004 and March 2023. In addition to data on clinical presentation, treatments, and outcomes of acute iTTP episodes, these prospective registries also collected data on serial ADAMTS13 measurements during clinical remission (allowing determination of ADAMTS13 relapse), as well as preemptive rituximab therapy. We did not evaluate clinical RFS separately in this cohort because preemptive rituximab therapy for ADAMTS13 relapse was commonly prescribed, which is expected to prevent clinical relapse. Thus, censoring at the point of ADAMTS13 relapse, is likely informative censoring that would bias results.
For both the retrospective USTMA cohort and the combined prospective JHU and University of Minnesota cohorts, iTTP diagnosis was based on thrombocytopenia (platelet count of <100 × 109/L), microangiopathic hemolytic anemia (hemoglobin below the lower limit of normal, with schistocytes on the peripheral blood smear), and either ADAMTS13 activity of <10% or ADAMTS13 activity of <20% with an inhibitor or antibody. Only patients who received rituximab for the treatment of at least 1 iTTP episode and had at least 1 year follow-up were included. For this study, a course or episode of rituximab therapy is defined as a standard course of rituximab 375 mg/m2 for 4 weekly doses. All treatments with rituximab including preemptive and maintenance rituximab were recorded. Preemptive rituximab was defined as administration of rituximab (most commonly 375 mg/m2 per week for 4 doses) in patients presenting with ADAMTS13 relapse (ADAMTS13 of <20% without clinical relapse), whereas maintenance rituximab was defined as patients who received a single dose of rituximab at scheduled 3-month, 6-month, or other fixed intervals because of concern for relapse. Episodes of maintenance rituximab therapy were excluded from analysis. Time before starting maintenance rituximab was included in the observation period and patients were censored at the point of starting maintenance rituximab. Participants were followed up from first course of rituximab until date of death or last contact.
Analysis plan
The primary outcomes of interest were clinical relapse and overall relapse. Clinical relapse was defined as a decrease in platelets of ≤150 × 109/L with or without clinical evidence of ischemic organ injury, confirmed by documentation of severe ADAMTS13 deficiency and occurring after having achieved clinical remission.19 Overall relapse was a composite end point of clinical relapse, ADAMTS13 relapse, and preemptive rituximab therapy. ADAMTS13 relapse was defined as a decrease in ADAMTS13 activity to of <20% after having achieved partial or complete ADAMTS13 remission. Although preemptive rituximab is most commonly used for ADAMTS13 relapse, we included preemptive rituximab in the composite outcome because some patients may receive preemptive rituximab before they meet strict criteria for ADAMTS13 relapse (ADAMTS13 of <20%), for example at higher ADAMTS13 levels such as 30% based on the slope of ADAMTS13 decline, their relapse history, and clinician judgment. Others may not receive preemptive rituximab for some time after they meet criteria for ADAMTS13 relapse either because of patient choice, or logistical and scheduling reasons.
Data were summarized as counts (and percentages) and means (standard deviations) for categorical and continuous variables, respectively. We used mixed-effects Cox regression to evaluate factors associated with clinical relapse and overall relapse while accounting for participants with >1 episode. Covariates in both models were selected based on biologic plausibility and included age, sex, race, and number of previous rituximab courses, in addition to random effect terms for participant and institution. Adjusted survival curves were generated to report RFS after the first rituximab-treated episode vs subsequent courses of rituximab while adjusting for the covariates listed above using the G-formula or corrected-group-prognosis method.20 Based on the findings from the initial Cox model that race was a strong risk factor for relapse, RFS estimates by number of rituximab treatment courses were also examined separately for Black and White patients. Analyses were conducted using R version 4.2.2 including the coxme package version 2.2-18.1.21 A 2-sided P < .05 was considered significant.
The studies were approved by the institutional review boards of the respective institutions.
Results
Cohort characteristics
USTMA registry cohort
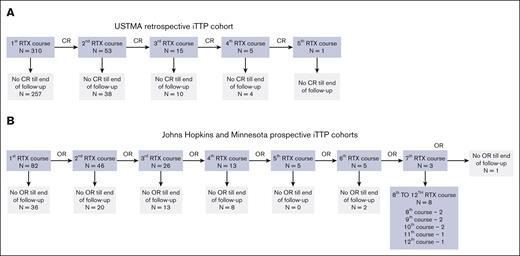
The USTMA Consortium registry includes data from 645 unique individuals with iTTP. We identified 310 unique individuals who received treatment with rituximab between 2004 and 2019. We excluded patients who received immunosuppression other than rituximab or corticosteroids. Of 384 total courses of treatment with rituximab, 310 were first episodes, 53 second courses, and 21 were third and subsequent courses (Figure 1A). Because of the small number of episodes in the latter categories, we analyzed first vs subsequent courses of treatment. Table 1 demonstrates demographic and clinical characteristics of rituximab-treated patients from the USTMA registry.
Patient cohorts for analysis. The primary outcome analyzed in the USTMA retrospective registry cohort (2004-2019) (A) was clinical relapse (CR) defined as a decrease in platelets of ≤150 × 109/L with or without clinical evidence of ischemic organ injury, confirmed by documentation of severe ADAMTS13 deficiency and occurring after having achieved clinical remission.19 The prospective Johns Hopkins and University of Minnesota cohort (2004-2023) (B) also included data on serial ADAMTS13 assessments and the primary outcome analyzed in this cohort was overall relapse defined as a composite end point of CR, ADAMTS13 relapse, and preemptive rituximab (RTX) therapy. OR, overall relapse.
Patient cohorts for analysis. The primary outcome analyzed in the USTMA retrospective registry cohort (2004-2019) (A) was clinical relapse (CR) defined as a decrease in platelets of ≤150 × 109/L with or without clinical evidence of ischemic organ injury, confirmed by documentation of severe ADAMTS13 deficiency and occurring after having achieved clinical remission.19 The prospective Johns Hopkins and University of Minnesota cohort (2004-2023) (B) also included data on serial ADAMTS13 assessments and the primary outcome analyzed in this cohort was overall relapse defined as a composite end point of CR, ADAMTS13 relapse, and preemptive rituximab (RTX) therapy. OR, overall relapse.
Demographics and clinical characteristics of USTMA cohort
| Variable . | Total cohort . | Black . | White . | Other . |
|---|---|---|---|---|
| Total individuals, n | 310 | 192 | 101 | 17 |
| Total episodes, n | 384 | 257 | 108 | 19 |
| Age at episode, median (IQR), y | 45 (34-55) | 46 (36-55) | 43 (31-55) | 44 (36-55) |
| Female sex, n (%) | 211 (68%) | 124 (65%) | 73 (72%) | 14 (82%) |
| De novo episodes | 136 (35%) | 78 (30%) | 50 (46%) | 8 (42%) |
| Relapse episodes | 248 (65%) | 179 (70%) | 58 (54%) | 11 (58%) |
| Total follow-up time, median (IQR), y | 1.9 (0.5-4.0) | 2.1 (0.6-4.4) | 1.5 (0.4-3.4) | 1.7 (1.0-3.4) |
| Variable . | Total cohort . | Black . | White . | Other . |
|---|---|---|---|---|
| Total individuals, n | 310 | 192 | 101 | 17 |
| Total episodes, n | 384 | 257 | 108 | 19 |
| Age at episode, median (IQR), y | 45 (34-55) | 46 (36-55) | 43 (31-55) | 44 (36-55) |
| Female sex, n (%) | 211 (68%) | 124 (65%) | 73 (72%) | 14 (82%) |
| De novo episodes | 136 (35%) | 78 (30%) | 50 (46%) | 8 (42%) |
| Relapse episodes | 248 (65%) | 179 (70%) | 58 (54%) | 11 (58%) |
| Total follow-up time, median (IQR), y | 1.9 (0.5-4.0) | 2.1 (0.6-4.4) | 1.5 (0.4-3.4) | 1.7 (1.0-3.4) |
JHU and University of Minnesota prospective cohort
The Johns Hopkins and Minnesota cohort included 97 individuals (86 from JHU and 11 from the University of Minnesota) with a total of 266 episodes of treatment for iTTP. Twenty-three de novo acute iTTP episodes not treated with rituximab, 3 episodes with no follow-up, and 52 episodes of maintenance rituximab were excluded from analysis. Time before starting maintenance rituximab was included in the observation period and patients were censored at the point of starting maintenance rituximab. The remaining 188 episodes include 82 first courses of rituximab, 46 second courses, and 60 third and subsequent courses (Figure 1B). Table 2 summarizes demographic and clinical characteristics of the Johns Hopkins and Minnesota prospective cohort, categorized by race.
Demographics and clinical characteristics of JHU and University of Minnesota prospective cohorts
| Variable . | Total cohort . | Black . | White . | Other . |
|---|---|---|---|---|
| Total individuals, n | 97 | 60 | 36 | 1 |
| Total episodes, n | 188 | 127 | 59 | 2 |
| Total number of RTX-treated iTTP episodes per patient, median (IQR) | 1 (1-2) | 1 (1-3) | 1 (1-2) | 2 (2-2) |
| Age at episode, median (IQR), y | 45 (34.5-55.5) | 45 (36-55) | 44 (30-60) | 41 (41-41) |
| Female sex, n (%) | 71 (73.2) | 43 (71.7) | 27 (75.0) | 1 (100) |
| De novo episodes | 51 (27.1) | 32 (25.2) | 18 (30.5) | 1 (50) |
| Relapse episodes | 137 (72.9) | 95 (74.8) | 41 (69.5) | 1 (50) |
| Any ADAMTS13 relapse | 18 (18.6) | 15 (25.0) | 13 (36.1) | 0 (0.0) |
| Any preemptive RTX received | 19 (19.5) | 9 (15.0) | 10 (27.8) | 0 (0.0) |
| Total follow-up time, median (IQR), y | 2.6 (0.7-5.9) | 3.0 (0.7-6.0) | 1.7 (0.7-6.0) | 0.9 (0.9-0.9) |
| Variable . | Total cohort . | Black . | White . | Other . |
|---|---|---|---|---|
| Total individuals, n | 97 | 60 | 36 | 1 |
| Total episodes, n | 188 | 127 | 59 | 2 |
| Total number of RTX-treated iTTP episodes per patient, median (IQR) | 1 (1-2) | 1 (1-3) | 1 (1-2) | 2 (2-2) |
| Age at episode, median (IQR), y | 45 (34.5-55.5) | 45 (36-55) | 44 (30-60) | 41 (41-41) |
| Female sex, n (%) | 71 (73.2) | 43 (71.7) | 27 (75.0) | 1 (100) |
| De novo episodes | 51 (27.1) | 32 (25.2) | 18 (30.5) | 1 (50) |
| Relapse episodes | 137 (72.9) | 95 (74.8) | 41 (69.5) | 1 (50) |
| Any ADAMTS13 relapse | 18 (18.6) | 15 (25.0) | 13 (36.1) | 0 (0.0) |
| Any preemptive RTX received | 19 (19.5) | 9 (15.0) | 10 (27.8) | 0 (0.0) |
| Total follow-up time, median (IQR), y | 2.6 (0.7-5.9) | 3.0 (0.7-6.0) | 1.7 (0.7-6.0) | 0.9 (0.9-0.9) |
RTX, rituximab.
Clinical RFS shortens with each episode of rituximab treatment
In the USTMA registry cohort, presenting with relapsed iTTP (hazard ratio [HR], 2.10; 95% confidence interval [CI], 1.24-3.58), Black race (HR, 3.12; 95% CI, 1.68-5.80) and second or subsequent rituximab course (HR, 2.80; 95% CI, 1.53-5.10, first course as reference category) were associated with clinical relapse in a Cox regression model that also included age, sex, peak lactate dehydrogenase level, and occurrence of exacerbations (Table 3). Longitudinal depiction of relapses over time for each individual patient in the USTMA cohort is shown in supplemental Figure 1.
Predictors of clinical relapse in the USTMA retrospective iTTP cohort
| Variable . | HR . | 95% CI . |
|---|---|---|
| Second or subsequent rituximab course | 2.80 | 1.53-5.10 |
| Relapsed iTTP | 2.10 | 1.24-3.58 |
| Black race | 3.12 | 1.68-5.80 |
| Age: 18-33 y (Q1) | Reference | |
| Age: 33.1-42.2 y (Q2) | 0.55 | 0.30-0.98 |
| Age: 42.4-54.8 y (Q3) | 0.64 | 0.38-1.07 |
| Age: 54.9-81.0 y (Q4) | 0.54 | 0.30-0.95 |
| Female sex | 1.23 | 0.81-1.89 |
| Peak LDH level | 1.67 | 0.86-3.28 |
| Exacerbation present | 1.16 | 0.74-1.82 |
| Variable . | HR . | 95% CI . |
|---|---|---|
| Second or subsequent rituximab course | 2.80 | 1.53-5.10 |
| Relapsed iTTP | 2.10 | 1.24-3.58 |
| Black race | 3.12 | 1.68-5.80 |
| Age: 18-33 y (Q1) | Reference | |
| Age: 33.1-42.2 y (Q2) | 0.55 | 0.30-0.98 |
| Age: 42.4-54.8 y (Q3) | 0.64 | 0.38-1.07 |
| Age: 54.9-81.0 y (Q4) | 0.54 | 0.30-0.95 |
| Female sex | 1.23 | 0.81-1.89 |
| Peak LDH level | 1.67 | 0.86-3.28 |
| Exacerbation present | 1.16 | 0.74-1.82 |
LDH, lactate dehydrogenase; Q, quartile.
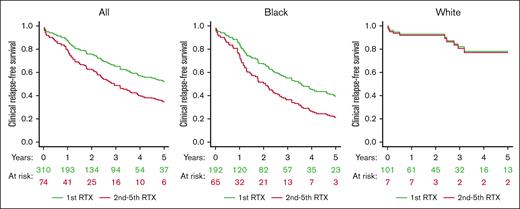
Median clinical RFS was shorter after the second or subsequent rituximab course than the first rituximab-treated course (2.1 vs 6.0 years; P = .04; Figure 2) Because race was significantly associated with clinical RFS in the Cox regression model, we evaluated clinical RFS after first and subsequent rituximab courses separately for Black and White participants. For White patients, clinical relapse risk after the second and subsequent rituximab courses was not significantly different compared with the first course (HR, 1.86; 95% CI, 0.22-15.80; P = .57). For Black patients, clinical relapse risk was higher after the second and subsequent rituximab courses than the first treatment course (HR, 2.82; 95% CI, 1.52-5.24; P = .001; Figure 2).
Clinical RFS by number of RTX courses. In the USTMA retrospective iTTP registry, clinical RFS, defined as time from first RTX dose in the treatment course to CR was longest for the first RTX treatment than subsequent treatment courses (median clinical RFS, 6.0 vs 2.1 years; P = .04). When analyzed separately by race, clinical RFS was shorter with second or subsequent for Black patients but not for White patents with iTTP. RTX indicates RTX. Survival curves are adjusted for age, sex, race, institution, and participant.
Clinical RFS by number of RTX courses. In the USTMA retrospective iTTP registry, clinical RFS, defined as time from first RTX dose in the treatment course to CR was longest for the first RTX treatment than subsequent treatment courses (median clinical RFS, 6.0 vs 2.1 years; P = .04). When analyzed separately by race, clinical RFS was shorter with second or subsequent for Black patients but not for White patents with iTTP. RTX indicates RTX. Survival curves are adjusted for age, sex, race, institution, and participant.
Overall RFS is progressively shorter after each episode of rituximab treatment
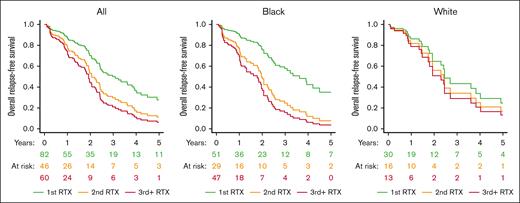
In the JHU and University of Minnesota prospective cohorts, overall RFS was longest after the first rituximab-treated episode (median, 2.8 years [interquartile range (IQR), 2.0-6.0]) followed by the second episode (median, 2.0 years [IQR, 1.2-3.6]), and third and subsequent episodes (median, 1.0 years [IQR, 0.5-2.5]; P = .0001; Figure 3). Longitudinal depiction of intervals between overall relapse events for individual patients is shown in supplemental Figure 2. Similar to clinical RFS, this finding is driven predominantly by a loss of response durability with later courses in Black patients; the median RFS in first rituximab-treated episode was 3.6 years for White patients and 2.7 years for Black patients; this decreased on multiply relapsed episodes to 1.5 years in White patients and 0.9 years in Black patients with third and more rituximab-treated iTTP relapse (Figure 3). In a Cox regression model the episode number of rituximab treatment (third or subsequent: HR, 2.47; 95% CI, 1.21-5.04; second episode: HR, 1.81; 95% CI, 0.87-3.75, with first rituximab treatment as reference category) was associated with overall RFS after adjusting for race, sex, and age (Table 4).
Overall RFS by number of RTX courses. Overall RFS was defined as a composite of time to CR, ADAMTS13 relapse, or treatment with preemptive RTX. In the combined Johns Hopkins and University of Minnesota prospective registries, overall RFS was longest after the first RTX-treated episode (median, 2.8 years) followed by the second episode (median, 2.0 years), and third and subsequent episodes episode (median, 1.0 year; P = .0001). The shortening of overall RFS was driven mostly by a loss of response durability in Black patients and was not significant in White patients with iTTP. RTX indicates RTX. Survival curves are adjusted for age, sex, race, institution, and participant.
Overall RFS by number of RTX courses. Overall RFS was defined as a composite of time to CR, ADAMTS13 relapse, or treatment with preemptive RTX. In the combined Johns Hopkins and University of Minnesota prospective registries, overall RFS was longest after the first RTX-treated episode (median, 2.8 years) followed by the second episode (median, 2.0 years), and third and subsequent episodes episode (median, 1.0 year; P = .0001). The shortening of overall RFS was driven mostly by a loss of response durability in Black patients and was not significant in White patients with iTTP. RTX indicates RTX. Survival curves are adjusted for age, sex, race, institution, and participant.
Predictors of overall relapse in the combined Johns Hopkins and University of Minnesota iTTP cohorts
| Variable . | HR . | 95% CI . |
|---|---|---|
| First episode/rituximab course | Reference | |
| Second rituximab course | 1.81 | 0.87-3.75 |
| Third or subsequent rituximab course | 2.47 | 1.21-5.04 |
| Black race | 1.27 | 0.63-2.60 |
| Female sex | 1.36 | 0.66-2.80 |
| Age: 18-34 y (Q1) | Reference | |
| Age: 35-45 y (Q2) | 1.14 | 0.53-2.42 |
| Age: 45-55 y (Q3) | 1.48 | 0.66-3.36 |
| Age: 56-78 y (Q4) | 1.85 | 0.76-4.50 |
| Variable . | HR . | 95% CI . |
|---|---|---|
| First episode/rituximab course | Reference | |
| Second rituximab course | 1.81 | 0.87-3.75 |
| Third or subsequent rituximab course | 2.47 | 1.21-5.04 |
| Black race | 1.27 | 0.63-2.60 |
| Female sex | 1.36 | 0.66-2.80 |
| Age: 18-34 y (Q1) | Reference | |
| Age: 35-45 y (Q2) | 1.14 | 0.53-2.42 |
| Age: 45-55 y (Q3) | 1.48 | 0.66-3.36 |
| Age: 56-78 y (Q4) | 1.85 | 0.76-4.50 |
Discussion
Over the past decade, rituximab has been widely incorporated into the treatment of iTTP and forms the mainstay of relapse prevention.7,9,11,13,22-24 We demonstrate, to our knowledge, for the first time, a progressive shortening in time to relapse with successive courses of rituximab, particularly in a subset of Black patients with relapsing iTTP. This is, to our knowledge, the first study to also evaluate overall RFS, which incorporates time to ADAMTS13 relapse, in addition to clinical RFS (time to clinical relapse). ADAMTS13 relapse is a clinically relevant end point in the current era19 in which low ADAMTS13 activity in remission is frequently treated with preemptive immunosuppression24 because it is recognized as a risk factor for clinical relapse17 and adverse vascular outcomes.25,26
The mechanisms underlying the loss of durability of rituximab effect in a subset of patients need further study. Potential explanations include variability in B-cell reconstitution after therapy and production of anti-ADAMTS13 antibodies by plasmablasts and plasma cells27-29 that have also been implicated in other autoimmune blood disorders such as immune thrombocytopenia.30,31 Rituximab primarily targets CD20+ B cells, but does not directly target cells with low expression of CD20+ including progenitor B cells, plasmablasts, and long-lived plasma cells; these cells may provide a rituximab-resistant nidus of anti-ADAMTS13 production as has been demonstrated in other autoimmune disease settings.31-33 Long-lived plasma cells, in particular, persist for months to years in survival niches in the bone marrow and spleen and secrete autoantibodies independent from ongoing antigen stimulation.33 The “wearing off” of the relapse-preventing effects of rituximab is most prominent in a subgroup of Black patients with relapsed iTTP, which is in line with 2 recent studies showing racial differences in the efficacy of rituximab in preventing clinical relapses of iTTP.15,4 One postulated explanation is more robust B-cell reemergence in Black patients influenced by both genetic and environmental factors.34,35 In other autoimmune inflammatory diseases such as multiple sclerosis and lupus, Black patients appear to have earlier peripheral B-cell repletion after anti-CD20 infusion,34 and have higher circulating plasma blasts with increased B-cell activating markers.36-38 Finally, other non-ADAMTS13 factors that may influence relapse risk in Black patients need to be considered. Some studies indicate chronic low-grade elevations in inflammatory markers in Black patients,37,39 which has been attributed to adverse physiological responses to chronic stress, often termed allostatic load.40,41 The complex relationships between race, social determinants, and stress, and proinflammatory changes are an area of active investigation in multiple areas including autoimmunity and may be a key to understanding these disparities.39,42,43
Although the USTMA TTP registry is 1 of the largest iTTP databases in the world, there are limited data on later treatment courses because of loss to follow-up. For example, the reduction in RFS between first and subsequent courses of rituximab is striking. However, the difference between second, third, and later courses was less apparent, likely because of the small numbers of patients in these groups, a limitation of this study. Serial ADAMTS13 monitoring data are not available for the retrospective USTMA registry so we had to evaluate ADAMTS13 relapse and preemptive rituximab therapy in the smaller prospective cohorts from 2 institutions.
Our observations have important clinical implications. Patients with >2 episodes of iTTP (clinical or ADAMTS13 relapse) may need closer monitoring, earlier retreatment, or alternative immunomodulators such as those directed against T cells or plasma cells especially if they demonstrate either reduced or shorter lived responses to rituximab.28,44 Novel therapies such as B-cell activating factor inhibition,45,46 neonatal Fc receptor inhibition,47 and even chimeric antigen receptor T-cell therapies48 are being developed for autoimmune diseases and may find application in iTTP refractory to current immunosuppression strategies. Another insight from this study is that median time to first clinical relapse is as long as 6 years. In contrast, studies from the prerituximab era that suggested that most iTTP relapses occur within the first 2 years of the index episode,49 which informed the practice of monitoring patients with iTTP most closely in the first 2 years after diagnosis. Thus, even patients without a relapse in the first 2 years should continue longer term monitoring for clinical and ADAMTS13 relapse.50
In conclusion, we show that time to clinical relapse and overall relapse (clinical relapse, ADAMTS13 relapse, and need for preemptive rituximab) in iTTP is shortened with successive episodes treated with rituximab, especially for a subset of Black patients with known relapsing iTTP. Additional research is needed to identify clinical and laboratory biomarkers that identify patients likely to have absent or short-lived responses to rituximab, and to develop individualized strategies to treat these patients with relapsing iTTP.
Acknowledgments
This research was supported by the National Institutes of Health’s National Heart, Lung, and Blood Institute grants K99HL150594 and R00HL172303 (S.C.), R01HL157975-01A1 and R01HL164016-01A1 (X.L.Z.), and National Center for Advancing Translational Sciences grants UL1TR001111 and UL1TR002494 to the University of North Carolina, Chapel Hill and the University of Minnesota, for data maintenance and security of the REDCap databases used to house the data for this study; no industry support was provided.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health’s National Center for Advancing Translational Sciences.
Authorship
Contribution: A.F., S.C., and M.M. designed the study, accessed and verified data, analyzed, and interpreted the data, drafted the manuscript, and approved the final version of the manuscript contributed to the design of the study and data acquisition and interpretation, drafted the manuscript, and approved the final version of the manuscript; M.D.E. accessed and verified data, analyzed and interpreted the data, drafted the manuscript, and approved the final version of the manuscript; J.B. assisted with preparation of tables and figures; E.D., A.J., A.G.A., A.M.F., R.W., A.M., Y.A.P., G.d.R., B.G., R.S.K., D.K.L., S.E., F.A., T.C., L.B.K., K.R.M., H.V.U., M.Y.L., N.K.K., R.G., X.L.Z., J.S.R., C.M., M.S., R.S.G., and S.R.C. contributed to data acquisition and approved the final version of the manuscript; and J.E.S. contributed to data acquisition.
Conflict-of-interest disclosure: S.C. reports advisory board participation or consultancy for Alexion, Sanofi Genzyme, Novartis, Kyowa Kirin, Star, Sobi, and Takeda; steering committe participation for Takeda and Sobi; and her institution has received research funding on her behalf from Sanofi, Sobi, and Takeda. M.A.M. reports payment for lectures and travel by Sanofi Genzyme. X.L.Z. is a consultant for Alexion, Apollo, Argenx, BioMedica Diagnostics, GC Biopharma, Kyowa Kirin, Sanofi, and Takeda, and is a cofounder of Clotsolution. M.Y.L. reports advisory board participation for Alexion, Sanofi, and Takeda, and her institution has received research funding on her behalf from Sanofi and Sobi. The remaining authors declare no competing financial interests.
J. Evan Sadler died on 13 December 2018.
Correspondence: Shruti Chaturvedi, Division of Hematology, Johns Hopkins University School of Medicine, 720 Rutland Ave, Ross Research Bldg, Room 1025, Baltimore, MD 21205; email: schatur3@jhmi.edu.
References
Author notes
Original deidentified data are available on request from the authors, Shruti Chaturvedi (schatur3@jhmi.edu) or Marshall Mazepa (mmazepa@umn.edu). All requests will be reviewed for feasibility and priority.
The full-text version of this article contains a data supplement.