Key Points
Ruxolitinib therapy was associated with improvements in lung function for participants with BOS.
Clinical responses were more commonly observed in participants with mild or moderate BOS.
Visual Abstract
Bronchiolitis obliterans syndrome (BOS) occurring after allogeneic hematopoietic cell transplantation (HCT) is a high-risk manifestation of chronic graft-versus-host disease. In this prospective, multicenter phase 2 trial, adult participants with BOS were treated with ruxolitinib 10 mg twice daily, continuously in 28-day cycles for up to 12 cycles. Participants enrolled into newly diagnosed (<6 months since BOS diagnosis, cohort A) or established (≥6 months since BOS diagnosis, cohort B) disease cohorts. The primary objective was to evaluate the early treatment effect of ruxolitinib, assessed by the change in forced expiratory volume in 1 second (FEV1) at 3 months compared with enrollment. The primary end point differed according to cohort (cohort A, improvement, defined as ≥10% increase in FEV1; cohort B, stabilization, defined as an absence of ≥10% decrease in FEV1). Between 2019 and 2022, 49 participants meeting the criteria for BOS were enrolled and treated (cohort A, n = 36; cohort B, n = 13). The primary end point was achieved by 27.8% of participants with new BOS and 92.3% of participants with established BOS. According to the 2014 National Institutes of Health Consensus Criteria, the best lung-specific overall response rate on ruxoltinib for the 49 participants was 34.7% (16.3% complete response and 18.4% partial response), with most responses occurring in mild or moderate disease. Noninfectious severe (grade ≥3) treatment-emergent adverse events were infrequent. Nine severe infectious events occurred and were largely respiratory in nature. These results support the use of ruxolitinib in the management of BOS after allogeneic HCT. This trial was registered at www.ClinicalTrials.gov as #NCT03674047.
Introduction
Chronic graft-versus-host disease (GVHD) is a multiorgan immunological complication of allogeneic hematopoietic cell transplantation (HCT), characterized by tissue injury in target organs.1 As such, chronic GVHD is a major determinant of morbidity, mortality, and impaired quality of life in long-term HCT survivors.2,3 Although rare, pulmonary involvement of chronic GVHD is considered a highly morbid disease manifestation that is associated with an increased risk of death.4 Bronchiolitis obliterans syndrome (BOS), the only formally recognized form of pulmonary chronic GVHD, develops as a result of an immune-mediated attack on the small airways, which ultimately leads to fibrotic narrowing and obliteration of respiratory bronchioles.5 Treatment options for BOS have traditionally consisted of inhaled therapies and standard GVHD therapies, such as systemic corticosteroids and immunosuppressive agents, which can limit disease progression but rarely improve pulmonary function or symptoms.6-8 Thus, clinical trials designed to investigate novel and established agents in BOS are of high priority.9,10
In recent years, the treatment landscape for chronic GVHD has expanded with multiple targeted agents receiving approval from the US Food and Drug Administration for the treatment of refractory disease.11 Ruxolitinib selectively inhibits Janus kinases (JAKs) 1 and 2, and is now frequently used in the treatment of refractory acute and chronic GVHD, after the US Food and Drug Administration approval for these indications. However, the clinical efficacy of ruxolitinib as a therapeutic agent for patients with BOS is not well characterized outside of small reports.12-15 In a randomized, phase 3 trial of ruxolitinib compared with best available therapies in the treatment of steroid-refractory chronic GVHD, 74 participants receiving ruxolitinib were identified as having lung involvement at baseline, including those with moderate (n = 24) and severe (n = 14) disease. At week 24, the lung-specific response rate was just 8.6%, although pulmonary function tests (PFTs) and spirometry were rarely performed on this trial.16 Nonetheless, there is biological rationale for the use of ruxolitinib in BOS, because the initial epithelial damage in the small airways is thought to be amplified by proinflammatory cytokines.17 Ruxolitinib may be able to inhibit this aberrant inflammation by interfering with cytokine sensing and production.18
We conducted a phase 2 trial of ruxolitinib in participants with BOS after allogeneic HCT. The primary objective was to evaluate the early treatment effect of ruxolitinib in BOS, with key secondary objectives consisting of safety, changes in corticosteroid dosing, and evaluation of patient-reported outcomes (PROs). The trial incorporated frequent formal evaluations of pulmonary function to enhance longitudinal assessment of organ-specific response in this rare, high-risk population.
Methods
This prospective, open-label, multicenter phase 2 trial was conducted at 7 clinical sites. The Massachusetts General Hospital Coordinating Center monitored all sites to ensure protocol compliance and data accuracy. The study was approved by the institutional review board at each center. Informed consent was obtained from enrolled participants. The trial was conducted according to the Declaration of Helsinki and registered at ClinicalTrials.gov (identifier: NCT03674047).
Participants
Participants were adults (aged 18-75 years) with a diagnosis of BOS occurring after allogeneic HCT. Participants had not received ruxolitinib for the treatment of chronic GVHD and were ≥4 weeks out from the start of their most recent GVHD therapy. Concurrent therapy with systemic corticosteroids, calcineurin inhibitors, sirolimus, mycophenolate mofetil, or FAM (inhaled corticosteroids, azithromycin, and montelukast) was permitted during the trial. Key exclusion criteria included disease relapse, inadequate marrow function (absolute neutrophil count <1 x 103/μL, platelets <50 x 109/μL, or hemoglobin <8 g/dL), or organ dysfunction (uncontrolled cardiac disease, calculated creatinine clearance <40 mL/min, alanine transaminase or aspartate aminotransferase >5 times the upper limit of normal, or total bilirubin >3 times the upper limit of normal).
BOS was defined by the 2014 National Institutes of Health (NIH) Consensus Criteria or by atypical BOS criteria. The NIH definition of BOS required the following 4 criteria to be met: (1) forced expiratory volume in 1 second (FEV1)/vital capacity (VC) <0.7 or the fifth percentile of predicted; (2) FEV1 <75% of predicted with ≥10% absolute decline over <2 years; (3) absence of active infection in the respiratory tract; and (4) evidence of air trapping by expiratory computed tomography (CT) scan or PFTs or small airway thickening or bronchiectasis by high-resolution chest CT scan.19 The atypical BOS definition required the following 4 criteria to be met: (1) FEV1 <80% of predicted with ≥10% absolute decline over <2 years; (2) VC <80% of predicted; (3) FEV1/VC >0.7; and (4) absence of active infection in the respiratory tract.20
Procedures
All participants were treated with ruxolitinib 10 mg twice daily for up to 12 cycles (28 days per cycle), with dose adjustments allowed during treatment for concurrent strong CYP3A4 inhibitors (eg, fluconazole), the development of cytopenias, or nonhematologic adverse events. The dose of ruxolitinib was reduced by 50% when administered with a strong CYP3A4 inhibitor. If grade ≥3 thrombocytopenia (platelets <50 x 109/μL) or anemia (hemoglobin <8 mg/dL) occurred, ruxolitinib was held until cytopenias recovered to grade ≤2 and then resumed at 50% reduced dose. If cytopenias did not reoccur after 2 weeks on the reduced dose, the original dose could be resumed. Participants were allowed to concurrently receive systemic corticosteroids, inhaled therapies, and supplemental oxygen for BOS while on trial. Key criteria for removing participants off protocol therapy with ruxolitinib included disease relapse, initiation of new systemic therapy for chronic GVHD or BOS, and prolonged treatment-related adverse events.
Pulmonary function was assessed by PFTs or spirometry at up to 6 time points (baseline, before cycles 2, 4, 7, and 10, and at the end of treatment). Comprehensive chronic GVHD assessments were performed at each study visit. PRO measures were collected at up to 5 time points (baseline, before cycles 4, 7, and 10, and at the end of treatment). To assess the overall chronic GVHD symptom burden, we used the Lee Symptom Scale (LSS), a 30-item questionnaire with scores ranging from 0 to 100.21 Higher scores correspond to worse GVHD symptoms. A 7-point change in the LSS is considered clinically meaningful. To assess the severity of BOS symptoms, we used the lung subscale of the LSS; scores range from 0 to 100, with higher scores signifying worse BOS symptoms. LSS questions pertaining to breathing assess the following: (1) frequency of cough; (2) presence of colored sputum; (3) shortness of breath with exercise; (4) shortness of breath at rest; and (5) need for oxygen use.
For biological correlative studies, whole blood samples were collected at up to 3 time points (baseline and before cycles 2 and 4) for the analysis of GVHD or BOS-associated biomarkers. Patient serum was processed and stored by the Immune Monitoring Laboratory of the Massachusetts General Hospital Cancer Center. Serum samples were tested in duplicate using custom multiplex Luminex assays (R&D Systems), run on the FlexMap 3D (Bio-Techne), for the following analytes: tumor necrosis factor α (TNFα), interleukin-6 (IL-6), interleukin-33R (IL-33R), IL-10, vascular endothelial growth factor (VEGF), CD163, CD138, CC motif chemokine ligand 15 (CCL15), IL-4, IL-17, B-cell activating factor (BAFF), CD25, IL-8, CXCL9, regenerating islet-derived protein 3 alpha (REG3α), interferon gamma (IFN-γ), CXCL11, tumor necrosis factor receptor 1 (TNFR1), CXCL10, osteopontin, and HGF. Matrix metalloproteinase-3 (MMP-3) was quantified using a Quantikine ELISA kit (R&D Systems), performed according to the manufacturer’s instructions.
Study design
This was a single-stage, single-arm study of 2 parallel cohorts based on the duration of the disease: newly diagnosed (<6 months since BOS diagnosis, cohort A) or established disease cohorts (≥6 months since BOS diagnosis, cohort B). The rationale for these cohorts was that clinical response rates and toxicity profiles may differ when comparing new BOS with long-standing BOS. Additionally, the cohorts were defined by the time since diagnosis; previous therapy for BOS was allowed in both cohorts. Each cohort had an initial accrual goal of 25 participants. The primary objective was to evaluate the treatment effect of ruxoltinib for BOS. In cohort A (newly diagnosed), the primary end point was the proportion of participants with improvement in disease, defined as ≥10% increase in absolute FEV1 in liters after 3 months of ruxolitinib, compared with baseline. The null and alternative hypotheses were 10% and 30%, respectively, with a decision rule. This design gives 90% power at 1-sided type 1 error rate of 10%. In cohort B (established disease), the primary end point was the proportion of participants with stabilization of disease, defined as those without ≥10% decrease in absolute FEV1 in liters after 3 months of ruxolitinib, compared with baseline. This cohort was largely exploratory with a focus on gathering preliminary data for future studies.
Secondary objectives included lung-specific response rates (overall response rate [ORR], partial response [PR], and complete response [CR]) according to NIH response criteria, characterization of treatment-emergent toxicities and infections, longitudinal changes in corticosteroid dosing, exploratory evaluations of PROs, and exploratory evaluations of GVHD and BOS-associated biomarkers. The severity of chronic GVHD was graded according to the 2014 NIH consensus criteria.19 Treatment response was defined using an organ-specific chronic GVHD response assessment, as defined by the 2014 NIH Consensus Criteria, with the modification that lung-specific responses were assessed solely on the evaluation of the percentage of predicted FEV1 value (%FEV1) and without substitution of NIH lung symptom score in the absence of %FEV1.22
Statistical analysis
Baseline characteristics were reported descriptively and compared using Fisher exact test, χ2 test, or Wilcoxon rank-sum test, as appropriate. Univariable logistic regression analysis was performed to investigate clinical factors (listed in Table 1) that were associated with clinical response. Because the time to response was not of primary interest, we treated response as a fixed event that occurred during the treatment period. Multivariable analysis was not performed due to the limited event size. Correlation analysis was performed to assess the correlation among cytokines. Overall survival (OS) and progression-free survival (PFS) were measured from the start of treatment to death (OS) or disease progression or death (PFS). OS and PFS were estimated using the Kaplan-Meier method. For measurements of percent predicted FEV1, cytokines, and PRO over time, repeated measures analysis was performed using mixed model to characterize trajectories of changes and to explore the differences in outcomes between responders and nonresponders. In these models, subjects were included in the random effect term, and an interaction between response and time (cycle) was examined. Before modeling, normality was checked, and cytokine values were log-transformed. Due to the limited sample size, other covariates were not included. All analyses were performed using SAS 9.3 (SAS Institute Inc, Cary, NC) and R version 3.5.2 (the CRAN project).
Baseline demographics and disease characteristics
| Characteristic . | Total . | Newly diagnosed BOS (cohort A) . | Established BOS (cohort B) . | P value . |
|---|---|---|---|---|
| Number of participants | 49 | 36 | 13 | |
| Median age, y (range) | 63 (21-71) | 64 (30-77) | 60 (21-71) | .37 |
| Sex, n (%) | .53 | |||
| Female | 22 (45) | 15 (42) | 7 (54) | |
| Male | 27 (55) | 21 (58) | 6 (46) | |
| Ethnicity, n (%) | .32 | |||
| Hispanic | 4 (8) | 3 (8) | 1 (8) | |
| Non-Hispanic | 34 (69) | 23 (64) | 11 (85) | |
| Unknown | 11 (22) | 10 (28) | 1 (8) | |
| Race, n (%) | .89 | |||
| White | 42 (86) | 30 (83) | 12 (92) | |
| Asian | 1 (2) | 1 (3) | 0 (0) | |
| American Indian or Alaskan Native | 1 (2) | 1 (3) | 0 (0) | |
| >1 race | 1 (2) | 1 (3) | 0 (0) | |
| Unknown | 4 (8) | 3 (8) | 1 (8) | |
| Maximum chronic GVHD severity before enrollment, n (%) | .53 | |||
| Moderate | 27 (55) | 21 (58) | 6 (46) | |
| Severe | 22 (45) | 15 (42) | 7 (54) | |
| Median number of organs with GVHD involvement at enrollment (range) | 3 (1-6) | 3 (1-6) | 3 (1-6) | .92 |
| Median time from BOS diagnosis to start of ruxolitinib, mo (range) | 1.4 (0.1-111.7) | 0.9 (0.1-4.9) | 14.6 (6.7-111.7) | NA |
| BOS classification, n (%) | 1.00 | |||
| Atypical phenotype criteria | 16 (33) | 12 (33) | 4 (31) | |
| NIH diagnostic criteria | 33 (67) | 24 (67) | 9 (69) | |
| NIH lung score at enrollment, n (%) | .67 | |||
| Mild (%FEV1 60%-79%) | 15 (31) | 12 (33) | 3 (23) | |
| Moderate (%FEV1 41%-59%) | 26 (53) | 19 (53) | 7 (54) | |
| Severe (%FEV1 <40%) | 8 (16) | 5 (14) | 3 (23) | |
| Receiving systemic corticosteroids at enrollment, n (%) | .50 | |||
| Yes | 34 (73) | 26 (72) | 8 (62) | |
| No | 13 (27) | 10 (28) | 5 (38) | |
| Median daily dose of prednisone at enrollment (range)∗ | 25 (5-80) | 40 (5-80) | 10 (5-30) | .017 |
| Receiving inhaled therapies at enrollment, n (%) | .71 | |||
| Yes | 39 (80) | 28 (78) | 11 (85) | |
| No | 10 (20) | 8 (22) | 2 (15) | |
| Use of supplemental oxygen at enrollment, n (%) | .22 | |||
| Yes | 9 (18) | 5 (14) | 4 (31) | |
| No | 40 (82) | 31 (86) | 9 (69) |
| Characteristic . | Total . | Newly diagnosed BOS (cohort A) . | Established BOS (cohort B) . | P value . |
|---|---|---|---|---|
| Number of participants | 49 | 36 | 13 | |
| Median age, y (range) | 63 (21-71) | 64 (30-77) | 60 (21-71) | .37 |
| Sex, n (%) | .53 | |||
| Female | 22 (45) | 15 (42) | 7 (54) | |
| Male | 27 (55) | 21 (58) | 6 (46) | |
| Ethnicity, n (%) | .32 | |||
| Hispanic | 4 (8) | 3 (8) | 1 (8) | |
| Non-Hispanic | 34 (69) | 23 (64) | 11 (85) | |
| Unknown | 11 (22) | 10 (28) | 1 (8) | |
| Race, n (%) | .89 | |||
| White | 42 (86) | 30 (83) | 12 (92) | |
| Asian | 1 (2) | 1 (3) | 0 (0) | |
| American Indian or Alaskan Native | 1 (2) | 1 (3) | 0 (0) | |
| >1 race | 1 (2) | 1 (3) | 0 (0) | |
| Unknown | 4 (8) | 3 (8) | 1 (8) | |
| Maximum chronic GVHD severity before enrollment, n (%) | .53 | |||
| Moderate | 27 (55) | 21 (58) | 6 (46) | |
| Severe | 22 (45) | 15 (42) | 7 (54) | |
| Median number of organs with GVHD involvement at enrollment (range) | 3 (1-6) | 3 (1-6) | 3 (1-6) | .92 |
| Median time from BOS diagnosis to start of ruxolitinib, mo (range) | 1.4 (0.1-111.7) | 0.9 (0.1-4.9) | 14.6 (6.7-111.7) | NA |
| BOS classification, n (%) | 1.00 | |||
| Atypical phenotype criteria | 16 (33) | 12 (33) | 4 (31) | |
| NIH diagnostic criteria | 33 (67) | 24 (67) | 9 (69) | |
| NIH lung score at enrollment, n (%) | .67 | |||
| Mild (%FEV1 60%-79%) | 15 (31) | 12 (33) | 3 (23) | |
| Moderate (%FEV1 41%-59%) | 26 (53) | 19 (53) | 7 (54) | |
| Severe (%FEV1 <40%) | 8 (16) | 5 (14) | 3 (23) | |
| Receiving systemic corticosteroids at enrollment, n (%) | .50 | |||
| Yes | 34 (73) | 26 (72) | 8 (62) | |
| No | 13 (27) | 10 (28) | 5 (38) | |
| Median daily dose of prednisone at enrollment (range)∗ | 25 (5-80) | 40 (5-80) | 10 (5-30) | .017 |
| Receiving inhaled therapies at enrollment, n (%) | .71 | |||
| Yes | 39 (80) | 28 (78) | 11 (85) | |
| No | 10 (20) | 8 (22) | 2 (15) | |
| Use of supplemental oxygen at enrollment, n (%) | .22 | |||
| Yes | 9 (18) | 5 (14) | 4 (31) | |
| No | 40 (82) | 31 (86) | 9 (69) |
NA, not applicable.
For participants receiving systemic corticosteroids at enrollment.
Results
Baseline demographics and disease characteristics
Fifty participants were enrolled on the trial and received with ruxolitinib. One participant with a %FEV1 of 80% was mistakenly enrolled and treated; this participant is included in the safety and survival analyses but excluded from the efficacy analyses (supplemental Figure 1). The baseline demographics and clinical characteristics of the remaining 49 participants are shown in Table 1. Due to slow accrual in cohort B, the original accrual goal of 25 participants per cohort was removed. Thirty-six participants were enrolled in cohort A (newly diagnosed BOS) and 13 participants were enrolled in cohort B (established BOS). The median age at enrollment was 63 years (range, 21-77). Twenty-seven (55%) were male. The maximum severity of chronic GVHD before enrollment was either moderate (n = 27 [55%]) or severe (n = 22 [45%]). The median number of organs with chronic GVHD involvement at enrollment was 3 (range, 1-6). More participants were enrolled using the NIH criteria for BOS (n = 33 [67%]) than the atypical phenotype criteria (n = 16 [33%]). At enrollment, the NIH chronic GVHD lung score according to %FEV1 was mild (n = 15 [31%]), moderate (n = 26 [53%]), or severe (n = 8 [16%]). Most participants were receiving systemic corticosteroids (n = 36 [73%]) and inhaled therapies (n = 39 [80%]) at enrollment. Nine participants (18%) reported the use of supplemental oxygen. No significant differences in the key baseline characteristics between cohorts A and B were identified (Table 1).
Treatment duration, survival, adverse events, and infectious complications
At the time of data analysis, the median follow-up of survivors was 21 months (range, 2-38). The median number of cycles of ruxolitinib therapy received for the entire treatment population was 11 (range, 1-12). At the time of analysis, 17 participants remained on therapy. The reasons for ruxolitinib discontinuation included completion of planned therapy (n = 7), progression of BOS or GVHD (n = 6), underlying disease relapse (n = 6), physician discretion (n = 5), adverse events (n = 3), death (n = 2), participant withdrawal (n = 2), and lost to follow-up (n = 2). Four participants discontinued ruxolitinib before treatment response could be assessed. The 2 deaths on trial were cardiorespiratory in nature and considered not related to ruxolitinib therapy. The 1-year OS was 87% (95% confidence interval [CI], 73-94), and the 1-year PFS was 79% (95% CI, 64-88).
In the entire treatment population, noninfectious severe (grade ≥3) treatment-emergent adverse events were rare and included neutropenia (n = 3), anemia (n = 2), hypertension (n = 2), and thrombocytopenia (n = 1). Two noninfectious adverse events that led to the discontinuation of ruxolitinib were anemia (n = 1; treatment related) and hypertension (n = 1; treatment related). Nine severe (grade ≥3) infectious events occurred in participants while being treated with ruxolitinib, which consisted of upper respiratory infection (n = 6), pneumonia (n = 2), and urinary tract infection (n = 1). Of note, no fungal infections were observed. One infectious event (pneumonia) resulted in the discontinuation of ruxolitinib. There was no difference in the incidence of severe adverse events according to treatment cohort.
Treatment responses
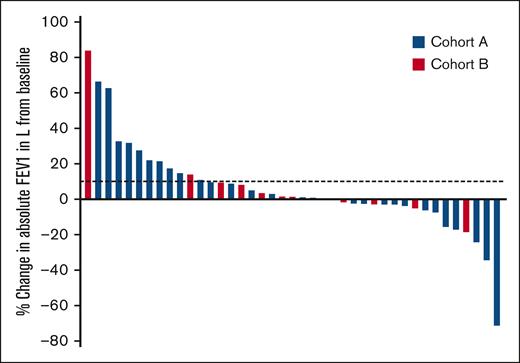
The primary end point of early treatment effect was evaluated in the 2 cohorts. According to the predefined end points, 10 of 36 participants (27.8%) in cohort A met the end point for improvement (≥10% increase in FEV1 in liters at 3 months), and 12 of 13 participants (92.3%) in cohort B met the end point of stabilization of disease (absence of ≥10% decline in FEV1 in liters at 3 months). Of note, there were 8 participants (7 in cohort A and 1 in cohort B) who came off the trial before the 3-month evaluation and were considered nonresponders. Forty-one participants had an evaluation of FEV1 after 3 months of ruxolitinib, compared with the baseline assessment, which is shown in Figure 1. For participants with newly diagnosed BOS, the early trajectory of lung function appears more dynamic, accounting for the largest changes in FEV1 (increase or decrease compared with baseline). Participants with established BOS had less significant changes in FEV1.
Early treatment effect of ruxolitinib in BOS after HCT. Change in FEV1 in liters from baseline after 3 months of ruxolitinib therapy is shown in 41 evaluable participants with BOS. Dotted lines mark absolute improvement by 10%. Treatment cohorts are indicated by color. Each bar represents an individual participant.
Early treatment effect of ruxolitinib in BOS after HCT. Change in FEV1 in liters from baseline after 3 months of ruxolitinib therapy is shown in 41 evaluable participants with BOS. Dotted lines mark absolute improvement by 10%. Treatment cohorts are indicated by color. Each bar represents an individual participant.
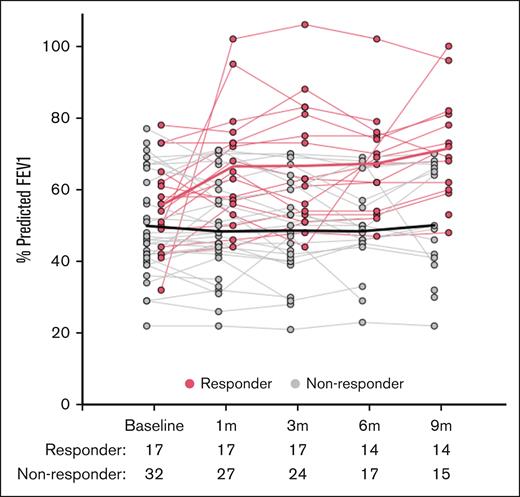
When evaluating response according to NIH consensus criteria, the best lung-specific ORR through cycle 10 for the entire treatment population was 34.7% (CR, 16.3%; PR, 18.4%). Of note, 4 participants who came off the trial before having a posttreatment %FEV1 evaluation were considered as nonresponders. The trajectory of all %FEV1 evaluations collected on the trial according to best lung NIH response (responder vs nonresponder) is shown in Figure 2 (%FEV1 trajectories according to treatment cohort are shown in supplemental Figure 2). Responders had a higher percent predicted FEV1 than nonresponders over time (P = .0001). The median time to first response (PR or CR) according to NIH response criteria was 7.7 months (range, 0.8-8.5). There was a clear correlation between best response and the baseline NIH lung score. The best ORR was 40% (CR, 26.7%; PR, 13.3%) for baseline NIH lung score 1; 38.5% (CR, 11.5%; PR, 26.9%) for baseline NIH lung score 2; and 12.5% (CR, 12.5%; PR, 0%) for NIH lung score 3 (Table 2). No associations between best response and treatment cohorts (A vs B) or BOS diagnostic criteria (NIH vs atypical) were identified. Outside of the lung, the best ORR for the 3 most involved organs were eyes (n = 25; ORR, 24%), skin (n = 16; ORR, 62.5%), and mouth (n = 15; ORR, 26.7%).
Longitudinal changes in %FEV1 measurements collected while on ruxolitinib therapy. Graphical representation of longitudinal demonstrates the %FEV1 measurements for participants who were responders (PR or CR by NIH criteria, n = 17) in red and nonresponders (n = 32) in gray. Fitted lines for responders and nonresponders generated using locally weighted smoothing (LOESS technique) to visually present the relationship between %FEV1 and response over time. LOESS, locally estimated scatterplot smoothing.
Longitudinal changes in %FEV1 measurements collected while on ruxolitinib therapy. Graphical representation of longitudinal demonstrates the %FEV1 measurements for participants who were responders (PR or CR by NIH criteria, n = 17) in red and nonresponders (n = 32) in gray. Fitted lines for responders and nonresponders generated using locally weighted smoothing (LOESS technique) to visually present the relationship between %FEV1 and response over time. LOESS, locally estimated scatterplot smoothing.
Best lung-specific organ response while on ruxolitinib, according to lung score at enrollment
| NIH lung score at baseline . | Number of evaluable participants . | CR rate∗ . | PR rate∗ . | Best ORR∗ . |
|---|---|---|---|---|
| 1 | 15 | 26.7% (n = 4) | 13.3% (n = 2) | 40% (n = 6) |
| 2 | 26 | 11.5% (n = 3) | 26.9% (n = 7) | 38.5% (n = 10) |
| 3 | 8 | 12.5% (n = 1) | 0% (n = 0) | 12.5% (n = 1) |
| Total | 49 | 16.3% (n = 8) | 18.4% (n = 9) | 34.7% (n = 17) |
| NIH lung score at baseline . | Number of evaluable participants . | CR rate∗ . | PR rate∗ . | Best ORR∗ . |
|---|---|---|---|---|
| 1 | 15 | 26.7% (n = 4) | 13.3% (n = 2) | 40% (n = 6) |
| 2 | 26 | 11.5% (n = 3) | 26.9% (n = 7) | 38.5% (n = 10) |
| 3 | 8 | 12.5% (n = 1) | 0% (n = 0) | 12.5% (n = 1) |
| Total | 49 | 16.3% (n = 8) | 18.4% (n = 9) | 34.7% (n = 17) |
Response according to 2014 NIH consensus criteria using percent predicted FEV1 evaluations alone.
Corticosteroid dosing and concurrent immunosuppressive agents
Within the entire treatment population, 34 participants were being treated with corticosteroids at enrollment, at a median prednisone dose of 25 mg daily (range, 5-80). The median reduction was 50% (median prednisone dose 10 mg daily, range 0-40; 26 evaluable participants) when comparing the prednisone dose after 3 months of ruxolitinib therapy to enrollment (supplemental Figure 2). This improvement in corticosteroid dosing was sustained at later time points (median prednisone dose after 6 months of therapy in 20 evaluable participants, 10 mg daily, 37% reduction from baseline; median prednisone dose after 9 months of therapy in 18 evaluable participants, 10 mg daily, 58% reduction from baseline). The improvement in corticosteroid dosing was primarily observed in newly diagnosed participants (cohort A, median reduction in prednisone by 67% after 9 months of therapy), whereas participants with established disease (cohort B) experienced no significant change in corticosteroid dosing (no change after 9 months of therapy). For participants with new BOS, the median initial daily dose of corticosteroids was lower in those who achieved a response (according to NIH criteria) than those who failed to respond (responders, 10 mg; nonresponders, 40 mg; P = .03); however, there was no difference in dosing at later time points. Additionally, there was no correlation between the median number of concurrent chronic GVHD agents at baseline and response (responders, 1; nonresponders, 1; P = .36).
PROs
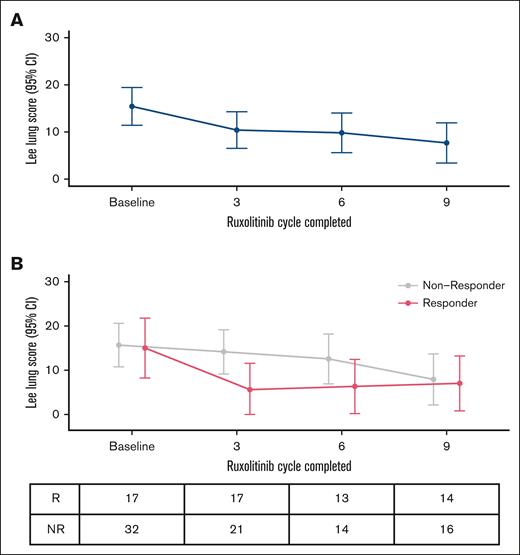
At baseline, the mean LSS lung subscale score was 15.4 (standard deviation [SD] = 14.2; n = 49), and the mean overall LSS score was 22.4 (SD = 11.2; n = 49) for the entire treatment population. Figure 3A illustrates the trajectory of LSS lung subscale scores. For the entire cohort, participants reported a statistically significant improvement in lung-specific symptoms after the completion of cycle 3 (Δ = –5.0; mean = 10.4; 95% CI, 6.5-14.2; P = .008) and cycle 6 (Δ = –5.6; mean = 9.8; 95% CI, 5.6-14.0; P = .01) and clinically meaningful improvement after cycle 9 (Δ = –7.8; mean = 7.7; 95% CI, 3.4-11.9; P = .003) compared with baseline. Overall LSS scores similarly improved after cycle 3 (Δ = –3.3; mean = 19.1; 95% CI, 15.7-22.5; P = .01) compared with baseline; however, overall LSS scores at subsequent time points did not differ from baseline scores.
Serial changes in patient-report BOS symptoms. (A) Trajectory of LSS lung subscale scores over time. (B) Trajectory of LSS lung subscale scores stratified by best response to ruxolitinib (R vs NR, according to NIH criteria). NR, nonresponder; R, responder.
Serial changes in patient-report BOS symptoms. (A) Trajectory of LSS lung subscale scores over time. (B) Trajectory of LSS lung subscale scores stratified by best response to ruxolitinib (R vs NR, according to NIH criteria). NR, nonresponder; R, responder.
Figure 3B illustrates the trajectory of LSS lung subscale scores stratified by best response to ruxolitinib, as defined by NIH response at any time. Compared with nonresponders, responders to ruxolitinib had clinically and statistically significant improvements in LSS lung symptoms after the completion of cycle 3 (mean responder = 5.5 [95% CI, 0-11.5] vs mean nonresponder = 14.2 [95% CI, 9.2-19.1]; P = .03). Of note, there were no statistical differences after cycle 6 (responder, 6.3 [CI, 0.2-12.4] vs nonresponder, 12.6 [CI, 6.9-18.2]; P = .21) nor cycle 9 (responder, 7.0 [CI, 0.8-13.2] vs nonresponder, 7.9 [CI, 2.2-13.7]; P = .96).
Exploratory evaluation of blood biomarkers
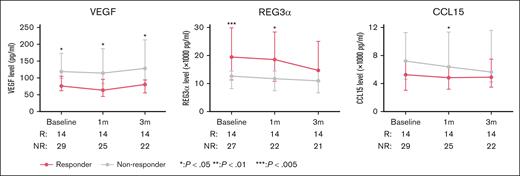
We explored serial levels of 22 biomarkers at 3 time points: baseline, after 1 month of ruxolitinib (before cycle 2), and after 3 months of ruxolitinib (before cycle 4). First, we observed concomitant elevations between select biomarkers at all 3 time points. At baseline, we observed a strong correlation in the levels between TNFα and IL-4, IL-6, IL-17, INF-γ, and CXCL11 (correlation coefficient, r = 0.58-0.7) and between IL-4 and IL-17 and INF-γ (r = 0.72 and 0.79, respectively; supplemental Figure 4A). A similar correlation was observed among these biomarkers at the 2 subsequent time points during ruxolitinib treatment (supplemental Figures 4B-C). Next, we explored associations between select biomarkers with disease cohort, BOS severity, and response to ruxolitinib. CD138 (cohort effect, P = .027) and CCL15 (cohort effect, P = .0052) demonstrated higher median levels for participants in cohort A than cohort B (supplemental Table 1). When examining associations with BOS severity, CXCL9 and CXCL11 demonstrated higher median levels for participants with mild or moderate disease than those with severe disease (supplemental Table 2). Finally, lower median levels of VEGF (response effect, P = .0026) and CCL15 (response effect, P = .044), and higher levels of REG3α (response effect, P = .007) were observed for participants who achieved a clinical response by NIH criteria than nonresponders (Figure 4; supplemental Table 3), although the significance was not maintained at all time points. This association was mainly observed by the participants in cohort A (supplemental Table 3).
Evaluation of biomarkers panels in participants with BOS over the first 3 months of ruxolitinib therapy. Median levels of 3 biomarkers to demonstrate a response effect (VEGF, REG3α, and CCL15) at baseline, after 1 month, and after 3 months of ruxolitinib in responders and nonresponders, with response defined according to NIH criteria.
Evaluation of biomarkers panels in participants with BOS over the first 3 months of ruxolitinib therapy. Median levels of 3 biomarkers to demonstrate a response effect (VEGF, REG3α, and CCL15) at baseline, after 1 month, and after 3 months of ruxolitinib in responders and nonresponders, with response defined according to NIH criteria.
Discussion
In this multicenter, phase 2 trial, we found that ruxolitinib is associated with clinical responses in BOS. Given the logistical considerations of conducting a trial in a high-risk population, we chose early treatment effect (evaluation at 3 months) as the primary end point. Participants with newly diagnosed disease experienced more dynamic changes in lung function (improvement or worsening of FEV1) than those with established disease, when lung function may have plateaued due to irreversible fibrosis of small airways. However, we observed continuing improvement in lung function beyond the early months, with a best ORR of 34.7% for the entire study population. Clinical responses were more commonly observed in participants with mild or moderate BOS, suggesting that identifying and treating disease before it reaches advanced stages is imperative. Furthermore, the median time to response was >7 months after initiating ruxolitinib, suggesting that the BOS is a disease manifestation that may require longer treatment periods to achieve optimal clinical responses, a finding observed in other BOS analyses.23 We observed a low incidence of severe adverse events during ruxolitinib treatment, including few cytopenias. Eight respiratory infections (upper respiratory infections or pneumonia) occurred, which is a common complication in this population. Additionally, most participants were able to taper corticosteroids while on study treatment. In summary, we believe these results support ruxolitinib as a safe and effective treatment option for BOS after allogeneic HCT.
We believe having a dedicated trial focused on BOS and designed to serially monitor lung function provided a proper setting to assess clinical responses and associated measures of clinical activity. A similar approach has been followed in other BOS-focused trials6,24,25 and has advantages over general chronic GVHD studies. In the REACH3 trial, PFTs and spirometry were not required assessments and thus not performed on a regular basis. As such, lung responses (8.6% for ruxolitinib and 6.1% for best available therapies) were predominantly based on NIH symptom scores and likely underestimated.16 More recently, a secondary analysis of 2 belumosudil prospective trials, which did include regular assessments of FEV1, found a lung-specific ORR of 32%.23 Similarly, 2 prospective clinical trials with axatilimab, a monoclonal antibody that inhibits colony-stimulating factor 1 receptor, have reported lung-specific ORRs of 31% and 47%, respectively, for participants with chronic GVHD failing at least 2 prior therapies.26,27 The current trial also limited enrollment to participants meeting an established diagnostic criteria for BOS, either NIH or atypical. Conversely lung involvement in larger chronic GVHD clinical trials is typically determined by subjective clinical assessment at enrollment and likely reflects either established disease or lung dysfunction incidental to other chronic GVHD manifestations. The BOS diagnostic criteria in this trial may have selected for enrollment a subset of patients with more established lung involvement as compared with general chronic GVHD trials. Nonetheless, in the current era of targeted therapies, NIH response rates are commonly >30%, which is encouraging for BOS and an improvement from historical experiences.
A key observation is that once severe disease (%FEV1 <40%) occurs, clinical responses are less likely. Only 1 participant with severe disease experienced a clinical response in this trial; similarly, no responses occurred with the belumosudil in severe BOS in the aforementioned analysis.23 This finding is intuitive, because severe damage to airway epithelial cells is generally irreversible, but it also highlights important considerations in caring for patients with BOS. The first is the importance of early identification of pulmonary decline to allow for the treatment of BOS before severe disease or presumed irreversible organ damage occurs.28 An initial decrease in FEV1 and forced expiratory flow between 25% and 75% maximum after allogeneic HCT can occur before the diagnosis of BOS, and it typically occurs before the clinical disease is evident.29 More frequent screening of patients with spirometry may help to better identify these patients who would be candidates for potential pre-emptive intervention. Whether initiating treatment earlier in the disease course ultimately changes the long-term outcomes for patients with BOS will require extensive investigation. The second key consideration is identifying treatment goals for patients with severe BOS. Because clinical responses (ie, improvements in FEV1) may be rare, stable disease by PFTs associated with improvement in other clinical features, such as corticosteroid dose or PRO measures, may be important considerations when trying to characterize treatment success in this high-risk population. Thus, we advocate for these patients to continue being eligible for clinical trial participation, whereas acknowledging that their definition of clinical benefit is likely different.
We explored PROs in this trial as key secondary analysis to the clinical response rates. The lung scale of the LSS is most pertinent for evaluating BOS-related symptoms, and we observed a more rapid improvement in lung LSS scores in clinical responders than nonresponders. However, at later time points, lung LSS changes were similar to baseline. We are encouraged by the PRO findings from this trial but acknowledge the room for further advancement. This analysis suggests that additional measures or development of a new tool are warranted for patients with BOS.
Data regarding the biomarkers in BOS after allogeneic HCT are limited, and longitudinal data characterizing changes in cytokine levels among patients with chronic GVHD receiving ruxolitinib are lacking. We hypothesized that ruxolitinib therapy could be associated with a decrease in select inflammatory cytokines, such as IFN-γ and IL-6,30 as well as BOS-associated biomarkers such as MMP3,31 in participants achieving clinical responses. However, our exploratory analysis of GVHD and BOS-related biomarkers failed to demonstrate compelling changes that could predict response to treatment. Although ruxolitinib has been reported to modulate VEGF levels in select patients with myeloproliferative disorders,32,33 this has not been reported in the setting of GVHD or BOS, and the clinical significance of the association in this analysis is unknown. The incorporation of correlative analyses into chronic GVHD clinical trials remains of high importance to enhance our understanding of biological effect and clinical response.
There are limitations to this trial that warrant acknowledgment. The trial is moderately sized, but given the relatively low incidence of BOS,34 larger trials would be difficult to conduct. The trial is limited by the lack of a nonrandomized cohort of patients with BOS not receiving ruxolitinib, which would help contextualize the findings of this single-arm study. We were limited in the number of correlative studies that could be performed. We did not include computed tomography imaging or parametric response mapping35 as image-based assessment of baseline disease or treatment response. Similarly, we did not incorporate bronchoscopy or bronchoalveolar lavage in the study assessments. Although our exploratory analysis of blood biomarkers identified correlations between individual markers and decreasing level of select markers over time, no association with clinical response was observed and raises the question whether a measure of biological response from bronchoalveolar lavage or transbronchial biopsy might be more pertinent in BOS. Finally, as a single-arm trial, we were unable to provide a comparison of ruxolitinib with other therapeutic agents. As mentioned earlier, we believe that patients with BOS now have multiple promising agents to treat their disease, but head-to-head comparisons are lacking.
In conclusion, ruxolitinib is associated with clinical responses in BOS, which were mostly observed in mild or moderate disease. Ruxolitinib was associated with few severe adverse events and limited infections in this high-risk population. The results support the use of ruxolitinib in the management of BOS after allogeneic HCT, as well as further investigation into the best timing of intervention and potential combination approaches with other emerging therapies.
Acknowledgment
Clinical trial funding and ruxolitinib were provided by Incyte.
Authorship
Contribution: Z.D. and Y.-B.C. designed the study; Z.D., G.-S.C., B.K.H., S.C., M.H., K.S.S., L.P., C.J.L., T.L.B., C.G., B.M.B., A.E.-J., and Y.-B.C. recruited patients to the study and collected clinical data and clinical specimens; K.G. performed the analysis of blood specimens; Z.D., H.T.K., and R.A.N. analyzed the data; Z.D. and H.T.K. wrote the first draft of the manuscript; and all authors approved the final draft of the manuscript and submission of the manuscript.
Conflict-of-interest disclosure: Z.D. reports receiving research support from Incyte, REGiMMUNE Corp, and Taiho Oncology Inc; and consulting fees from Sanofi, Incyte Corp, MorphoSys AG, Inhibrx, PharmaBiome AG, and Ono Pharmaceutical. B.K.H. has received consulting fees from Sanofi, Incyte, Rigel, Maat Pharma, and ACI Group; and has served on the data safety monitoring board for Angiocrine and on the Adjudication Committee for CSL Behring. R.A.N. has received equity from TimeDoc. Y.-B.C. has received consulting fees from Takeda, Incyte, Vor BioPharma, Pharmacosmos, Editas, and Celularity. The remaining authors declare no competing financial interests.
Correspondence: Zachariah DeFilipp, Massachusetts General Hospital, 55 Fruit St, Boston, MA 02114; email: zdefilipp@mgh.harvard.edu.
References
Author notes
Original data are available on request from the corresponding author, Zachariah DeFilipp (zdefilipp@mgh.harvard.edu).
The full-text version of this article contains a data supplement.