Key Points
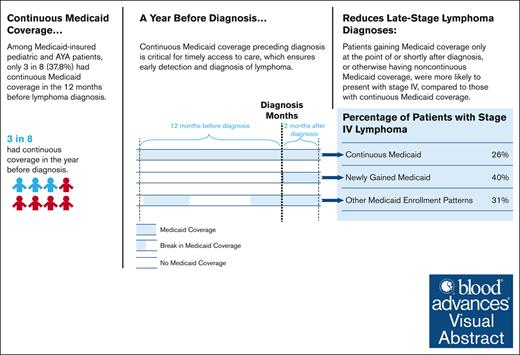
Only 3 of 8 Medicaid-insured children and AYAs with lymphoma were continuously enrolled in Medicaid before lymphoma diagnosis.
Among children/AYAs with lymphoma, continuous Medicaid coverage before diagnosis was associated with lower likelihood of late-stage disease.
Visual Abstract
Lymphoma is the third leading cause of cancer among children and adolescents/young adults (AYAs) in the United States, with later-stage diagnoses often being linked to worse outcomes. Continuous health insurance coverage is crucial for facilitating early cancer detection and diagnosis. Among Medicaid-insured children and AYAs diagnosed with lymphoma, this study examines whether the timing of Medicaid enrollment and coverage continuity are associated with stage at diagnosis. Using the Surveillance, Epidemiology, and End Results–Medicaid data, we identified children and AYAs (aged 0-39 years) newly diagnosed with lymphoma between 2007 and 2013 in 12 states that were linked to the administrative Medicaid data. Medicaid enrollment patterns were categorized into continuous Medicaid (preceding and through diagnosis), newly gained Medicaid (at or shortly after diagnosis), and other Medicaid enrollment patterns. Late-stage disease was defined as Ann Arbor stage IV (vs stage I-III). Multiple logistic regressions were estimated, with marginal effects (MEs) reported. Of 3524 patients identified, 37.8% had continuous Medicaid, followed by newly gained Medicaid (35.2%) and other Medicaid enrollment patterns (27.0%). Compared with patients continuously enrolled in Medicaid, those with newly gained Medicaid and with other Medicaid enrollment patterns were 54% (ME, 13.9 percentage points [ppt]; 95% confidence interval [CI], 8.5-19.2; P < .001) and 18% (ME, 4.6 ppt; 95% CI, 2.2-7.0; P < .001) more likely to present with stage IV lymphoma, respectively. Overall, having continuous Medicaid coverage before diagnosis was associated with a lower likelihood of late-stage lymphoma at diagnosis; however, only 3 in 8 Medicaid-insured children and AYAs with lymphoma were continuously enrolled in Medicaid before their diagnosis.
Introduction
Lymphoma is the third leading cause of cancer among children and adolescents/young adults (AYAs) in the United States,1 accounting for 10% of all pediatric cancers and 23% of adolescents’ cancers.2 In 2019, an estimated 11 771 children and adolescents aged <20 years in the United States were newly diagnosed with lymphoma, with 94 154 lymphoma cases among AYAs aged 15 to 39 years.3 Despite the increasing 5-year survival rate,4 disparities in health outcomes still exist between uninsured and insured children and AYAs with lymphoma.5 However, the associations of the timing of insurance enrollment and coverage continuity with lymphoma outcomes are unknown for this young population.
Medicaid is the largest insurance program for low-income or disabled Americans, including >2 million people with a cancer history and 1 in 3 children newly diagnosed with cancer.6 Continuous Medicaid coverage provides timely access to care, which can help detect the early onset of cancer signs and symptoms through primary care and referral to oncology care, leading to diagnosis of cancer at an earlier stage.7,8 However, many eligible young patients are not enrolled in Medicaid until the point of, or shortly after, a cancer diagnosis. This delay may result in cancer detection at a later stage with more severe disease, a need for more aggressive therapy, and ultimately, poorer lymphoma outcomes, including more complications and subacute morbidity and increased risk of mortality.8
Most prior research examining Medicaid coverage continuity has focused on solid tumors in individual states, limiting the generalizability of findings.9 Furthermore, the association of insurance coverage continuity leading up to diagnosis with disease stage at diagnosis remains unclear for Medicaid-insured children and AYAs with lymphoma. We aim to address this gap by examining whether the timing of gaining Medicaid coverage and the continuity of coverage, from the year before to shortly after diagnosis, are associated with disease stage in a large, multistate cohort of Medicaid-insured children and AYAs diagnosed with lymphoma.
Methods
Data
This study was a secondary analysis of the Surveillance, Epidemiology, and End Results (SEER) cancer registry data that have been linked with the Medicaid administrative enrollment data in 12 US states by the Centers for Medicare & Medicaid Services and the National Cancer Institute (ie, SEER-Medicaid data).10 The SEER registries included rich data on sociodemographic characteristics, cancer-related factors, and vital status for all incident cancer cases in the 12 states.11
The Medicaid enrollment data provided information on monthly enrollment status between 2006 and 2013.10 Consistent with prior research,7 we used this information to measure patients’ Medicaid enrollment patterns over a “15-month assessment window,” defined as the 12 months (or time since birth for those aged <12 months) preceding the first diagnosis of lymphoma through 2 months following the month of diagnosis. We chose the 12 months preceding diagnosis, hypothesizing that this period is critical for continuous coverage of care, symptom recognition, and cancer detection.12,13 The assessment window was extended up to 2 months after the month of diagnosis because patients who gain Medicaid coverage because of their cancer diagnosis are likely applying for and being accepted by Medicaid within the first 2 months following diagnosis.7,14-16
Prior SEER-Medicaid reports showed that ∼15% to 17% of all patients with incident cancer reported by the SEER registries were able to be matched to Medicaid each year using a deterministic matching process; linkage details have been reported elsewhere.11,17 A match score ranging from 1 to 12 reflects the strength of the match, with score 12 indicating complete match and score 1 indicating weakest match.11,17 Consistent with prior research,17 we conducted a sensitivity analysis that excluded patients with a match score <5 from the sample of our primary analysis, and the results were consistent (supplemental Table 1).
The study was approved by the Emory University Institutional Review Board.
Study sample
From the SEER-Medicaid data, we included all children and AYAs (aged 0-39 years) who were newly diagnosed with Hodgkin or non-Hodgkin lymphomas (supplemental Table 2) between 2006 and 2013 from SEER registries in the 12 participating states (California, Connecticut, Georgia, Hawaii, Iowa, Kentucky, Louisiana, Michigan [Detroit], New Jersey, New Mexico, Utah, Washington [Seattle]). We then excluded patients (1) with unknown diagnosis month, (2) deceased before or at diagnosis, (3) diagnosed before January 2007 or after October 2013 (who thus did not have complete Medicaid enrollment data for the 15-month assessment window), or (4) with unknown stage at diagnosis (supplemental Figure 1). Our primary analysis further restricted the analytic sample to patients who were linked with the Medicaid data (ie, Medicaid-insured patients; supplemental Figure 2).
Medicaid enrollment patterns
For patients meeting the inclusion criteria described above, Medicaid enrollment patterns were categorized into (1) continuous Medicaid (enrolled in Medicaid for ≥12 months preceding and through the month of diagnosis), (2) newly gained Medicaid (gained Medicaid only in the month prior to, during the month of, or ≤2 months after the month of diagnosis), or (3) other Medicaid enrollment patterns (enrolled in Medicaid for some time during the total 15-month window but not in the first 2 categories; detailed in supplemental Table 3).
We also conducted a secondary analysis that further included SEER patients who were either not linked to Medicaid data or linked outside of the 15-month window. Although we lacked data on the continuity of non-Medicaid insurance coverage over time, we simply explored the available information on insurance status at diagnosis and its association with stage IV disease in patients not insured by Medicaid. For these patients, we used the insurance status recorded in the SEER registry data at the time of diagnosis, including private insurance, Tricare, Military/Veterans Affairs, Medicare, insurance not otherwise specified, and unknown insurance status or uninsured at diagnosis.
Outcome measures
Late-stage lymphoma was based on SEER use of the Ann Arbor staging classification and defined as stage IV (vs I-III). The Ann Arbor staging system is used by the SEER registries to classify the anatomic stage of both Hodgkin lymphoma and non-Hodgkin lymphoma.18 In a sensitivity analysis, we examined stage III to IV (vs I-II) at diagnosis, and the results were consistent (supplemental Tables 4 and 5).
Statistical analysis
We conducted bivariate analyses, comparing patient characteristics by Medicaid enrollment patterns. Next, the association between Medicaid enrollment patterns and the likelihood of late-stage diagnoses was estimated by multivariable logistic regression models. These models adjusted for sex, race/ethnicity, age at diagnosis, rurality of residence, neighborhood-level socioeconomic status (SES), year of diagnosis, and SEER cancer registries, with standard errors clustered by SEER cancer registries. Neighborhood-level SES was measured by the census tract–level Yost index, a composite score calculated from 7 factors (median household income, median house value, median rent, percentage of residents below 150% of the poverty line, education index, percentage of working class, and percentage of unemployed population).19,20 Subgroup analyses were conducted, stratifying models by lymphoma subtypes (Hodgkin and non-Hodgkin lymphomas), age groups (0-14, 15-25, and 26-39 years, according to the National Cancer Institute’s definition of AYA),21 sex, race/ethnicity, and quintiles of the Yost index to examine how the association of Medicaid enrollment patterns with lymphoma stage varied across diseases and sociodemographic subgroups, respectively.
Consistent with previous research,22,23 marginal effects (MEs) were reported and interpreted as the absolute percentage-point difference in the model-adjusted percentage of patients with late-stage diagnoses when comparing those with a specific Medicaid enrollment pattern (eg, newly gained Medicaid) with the reference group (eg, continuous Medicaid).23 We further converted MEs to relative increases for each outcome by dividing the ME by the model-adjusted percentage of late-stage diagnoses estimated for the reference group (p0), for ease of interpretation. Statistical significance was set at .05, and all tests were 2-sided. Analyses were performed using SAS version 9.4 (SAS Institute Inc) and Stata version 17.0 (StataCorp, College Station, TX).
Results
Sample characteristics
Our primary analysis included 3524 Medicaid-insured patients. Of these, 37.8% (n = 1333) had continuous Medicaid, followed by newly gained Medicaid (n = 1241 [35.2%]) and other Medicaid enrollment patterns (n = 950 [27.0%]; Table 1). Approximately half of the sample were male (n = 1899 [53.9%]), aged 26 to 39 years (n = 1769 [50.2%]), and diagnosed with non-Hodgkin lymphoma (n = 1869 [53.0%]). Non-Hispanic White patients accounted for 44.4% (n = 1564), followed by Hispanic (24.9% [n = 876]) and non-Hispanic Black patients (24.4% [n = 861]). Overall, 32.2% (n = 1133) presented with stage IV lymphoma at diagnosis.
Sample characteristics of Medicaid-insured children and AYAs diagnosed with lymphoma, by Medicaid enrollment patterns
| Characteristics . | Total . | Medicaid-insured groups . | ||
|---|---|---|---|---|
| Continuous Medicaid . | Newly gained Medicaid . | Other Medicaid enrollment patterns . | ||
| N = 3524 . | n = 1333 . | n = 1241 . | n = 950 . | |
| n . | Row % . | Row % . | Row % . | |
| Sex | ||||
| Male | 1899 | 33.2 | 44.4 | 22.3 |
| Female | 1625 | 43.2 | 24.4 | 32.4 |
| Race/ethnicity | ||||
| Non-Hispanic White | 1564 | 34.3 | 39.1 | 26.6 |
| Non-Hispanic Black | 861 | 41.2 | 32.5 | 26.3 |
| Hispanic | 876 | 41.4 | 30.4 | 28.2 |
| Non-Hispanic other | 212 | 34.0 | 38.2 | 27.8 |
| Unknown race/ethnicity | 11 | |||
| Age at diagnosis, y | ||||
| 0-14 | 465 | 61.7 | 17.2 | 21.1 |
| 15-25 | 1290 | 36.7 | 32.7 | 30.5 |
| 26-39 | 1769 | 32.3 | 41.8 | 25.9 |
| Rurality | ||||
| Metropolitan areas | 3028 | 38.1 | 35.4 | 26.5 |
| Nonmetropolitan (including rural) areas | 496 | 35.9 | 34.3 | 29.8 |
| Neighborhood SES quintiles∗ | ||||
| Q1: lowest SES | 1068 | 43.6 | 30.2 | 26.1 |
| Q2 | 808 | 35.6 | 36.0 | 28.3 |
| Q3 | 645 | 35.5 | 36.3 | 28.2 |
| Q4 | 576 | 36.6 | 36.3 | 27.1 |
| Q5: highest SES | 375 | 32.3 | 43.5 | 24.3 |
| Unknown SES | 52 | |||
| Subtype of lymphoma | ||||
| Hodgkin lymphoma | 1655 | 40.4 | 30.3 | 29.3 |
| Non-Hodgkin lymphoma | 1869 | 35.5 | 39.6 | 24.9 |
| Diagnosis year | ||||
| 2007 | 475 | 39.4 | 32.2 | 28.4 |
| 2008 | 515 | 37.3 | 37.5 | 25.2 |
| 2009 | 539 | 37.1 | 38.2 | 24.7 |
| 2010 | 523 | 37.7 | 35.0 | 27.3 |
| 2011 | 531 | 36.7 | 37.1 | 26.2 |
| 2012 | 527 | 38.9 | 35.1 | 26.0 |
| 2013 | 414 | 37.9 | 30.0 | 32.1 |
| SEER registry | ||||
| California | 1227 | 40.2 | 34.0 | 25.8 |
| Connecticut | 178 | 49.4 | 23.0 | 27.5 |
| Georgia | 402 | 30.6 | 39.6 | 29.9 |
| Hawaii | 52 | 44.2 | 36.5 | 19.2 |
| Iowa | 157 | 36.3 | 35.7 | 28.0 |
| Kentucky | 210 | 32.4 | 37.1 | 30.5 |
| Louisiana | 349 | 33.2 | 43.8 | 22.9 |
| Detroit (metropolitan) | 222 | 39.6 | 36.5 | 23.9 |
| New Jersey | 282 | 42.2 | 30.9 | 27.0 |
| New Mexico | 110 | 43.6 | 20.9 | 35.5 |
| Utah | 105 | 34.3 | 41.0 | 24.8 |
| Seattle (Puget Sound) | 230 | 32.2 | 36.5 | 31.3 |
| Characteristics . | Total . | Medicaid-insured groups . | ||
|---|---|---|---|---|
| Continuous Medicaid . | Newly gained Medicaid . | Other Medicaid enrollment patterns . | ||
| N = 3524 . | n = 1333 . | n = 1241 . | n = 950 . | |
| n . | Row % . | Row % . | Row % . | |
| Sex | ||||
| Male | 1899 | 33.2 | 44.4 | 22.3 |
| Female | 1625 | 43.2 | 24.4 | 32.4 |
| Race/ethnicity | ||||
| Non-Hispanic White | 1564 | 34.3 | 39.1 | 26.6 |
| Non-Hispanic Black | 861 | 41.2 | 32.5 | 26.3 |
| Hispanic | 876 | 41.4 | 30.4 | 28.2 |
| Non-Hispanic other | 212 | 34.0 | 38.2 | 27.8 |
| Unknown race/ethnicity | 11 | |||
| Age at diagnosis, y | ||||
| 0-14 | 465 | 61.7 | 17.2 | 21.1 |
| 15-25 | 1290 | 36.7 | 32.7 | 30.5 |
| 26-39 | 1769 | 32.3 | 41.8 | 25.9 |
| Rurality | ||||
| Metropolitan areas | 3028 | 38.1 | 35.4 | 26.5 |
| Nonmetropolitan (including rural) areas | 496 | 35.9 | 34.3 | 29.8 |
| Neighborhood SES quintiles∗ | ||||
| Q1: lowest SES | 1068 | 43.6 | 30.2 | 26.1 |
| Q2 | 808 | 35.6 | 36.0 | 28.3 |
| Q3 | 645 | 35.5 | 36.3 | 28.2 |
| Q4 | 576 | 36.6 | 36.3 | 27.1 |
| Q5: highest SES | 375 | 32.3 | 43.5 | 24.3 |
| Unknown SES | 52 | |||
| Subtype of lymphoma | ||||
| Hodgkin lymphoma | 1655 | 40.4 | 30.3 | 29.3 |
| Non-Hodgkin lymphoma | 1869 | 35.5 | 39.6 | 24.9 |
| Diagnosis year | ||||
| 2007 | 475 | 39.4 | 32.2 | 28.4 |
| 2008 | 515 | 37.3 | 37.5 | 25.2 |
| 2009 | 539 | 37.1 | 38.2 | 24.7 |
| 2010 | 523 | 37.7 | 35.0 | 27.3 |
| 2011 | 531 | 36.7 | 37.1 | 26.2 |
| 2012 | 527 | 38.9 | 35.1 | 26.0 |
| 2013 | 414 | 37.9 | 30.0 | 32.1 |
| SEER registry | ||||
| California | 1227 | 40.2 | 34.0 | 25.8 |
| Connecticut | 178 | 49.4 | 23.0 | 27.5 |
| Georgia | 402 | 30.6 | 39.6 | 29.9 |
| Hawaii | 52 | 44.2 | 36.5 | 19.2 |
| Iowa | 157 | 36.3 | 35.7 | 28.0 |
| Kentucky | 210 | 32.4 | 37.1 | 30.5 |
| Louisiana | 349 | 33.2 | 43.8 | 22.9 |
| Detroit (metropolitan) | 222 | 39.6 | 36.5 | 23.9 |
| New Jersey | 282 | 42.2 | 30.9 | 27.0 |
| New Mexico | 110 | 43.6 | 20.9 | 35.5 |
| Utah | 105 | 34.3 | 41.0 | 24.8 |
| Seattle (Puget Sound) | 230 | 32.2 | 36.5 | 31.3 |
Authors’ analysis of the 2006 to 2013 SEER-Medicaid data.
Neighborhood SES was measured by the census tract–level Yost index, which is a composite score of 7 factors (median household income, median house value, median rent, percent below 150% of the poverty line, education index, percent working class, and percent unemployed), all calculated at the census tract level.
Medicaid enrollment patterns varied by patient sociodemographic characteristics (Table 1). The proportion of patients with continuous Medicaid was higher, whereas the proportion with newly gained Medicaid was lower, among non-Hispanic Black and Hispanic patients relative to non-Hispanic White peers. Compared with children aged ≤14 years, a lower proportion of AYA patients (15-39 years) had continuous Medicaid (32.3%-36.7% vs 61.7%), whereas a higher proportion of AYAs had newly gained Medicaid (32.7%-41.8% vs 17.2%) or had other Medicaid enrollment patterns (25.9%-30.5% vs 21.1%). Additionally, areas with the lowest SES had a higher proportion of patients with continuous Medicaid (43.6% vs 32.3%) and a lower proportion of patients with newly gained Medicaid (30.2% vs 43.5%) compared with areas with the highest SES.
Association between Medicaid enrollment patterns and late-stage diagnoses
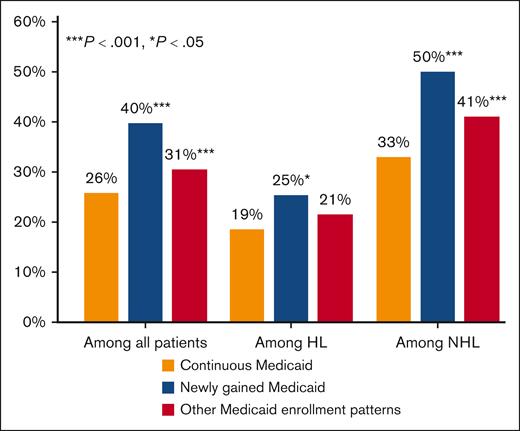
In our primary analysis (N = 3524), bivariate comparisons showed that the proportion of patients diagnosed with stage IV lymphoma was highest among those with newly gained Medicaid (41.0%), followed by those with other Medicaid enrollment patterns (29.5%), and lowest among those with continuous Medicaid (25.8%; Table 2). In adjusted analyses, compared with patients with continuous Medicaid, those with newly gained Medicaid and with other Medicaid enrollment patterns experienced an increase of 13.9 percentage points (ppt) (95% confidence interval [CI], 8.5-19.2; P < .001) and 4.6 ppt (95% CI, 2.2-7.0; P < .001), respectively, in the likelihood of presenting with a stage IV lymphoma diagnosis (Table 2; Figure 1). These estimates correspond to a 54% (13.9 ppt/26.0% [ie, ME/p0]) and 18% relative increase among patients with newly gained Medicaid and other Medicaid enrollment patterns, respectively, compared with continuous Medicaid enrollees.
Association between Medicaid enrollment patterns and the likelihood of stage IV diagnoses among Medicaid-insured children and AYAs diagnosed with lymphoma
| Characteristics . | Total (N = 3524) . | Among Hodgkin lymphoma (n = 1655) . | Among non-Hodgkin lymphoma (n = 1869) . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted percentage (row %) . | ME∗ . | 95% CI . | P value . | Unadjusted percentage (row %) . | ME∗ . | 95% CI . | P value . | Unadjusted percentage (row %) . | ME∗ . | 95% CI . | P value . | |
| Insurance continuity | ||||||||||||
| Continuous Medicaid | 25.8 | Ref. | 18.5 | Ref. | 33.1 | Ref. | ||||||
| Newly gained Medicaid | 41.0 | 13.9 | (8.5 to 19.2) | <.001 | 27.5 | 6.9 | (0.5 to 13.3) | .03 | 50.1 | 17.5 | (12.0 to 23.1) | <.001 |
| Other Medicaid enrollment patterns | 29.5 | 4.6 | (2.2 to 7.0) | <.001 | 19.4 | 2.9 | (–1.8 to 7.5) | .23 | 40.0 | 7.9 | (4.1 to 11.7) | <.001 |
| Sex | ||||||||||||
| Male | 37.0 | Ref. | 26.0 | Ref. | 44.4 | Ref. | ||||||
| Female | 26.5 | −7.1 | (−9.3 to −4.8) | <.001 | 17.6 | −6.6 | (−8.9 to −4.4) | <.001 | 37.1 | −3.3 | (−6.5 to −0.1) | .04 |
| Race/ethnicity | ||||||||||||
| Non-Hispanic White | 31.0 | Ref. | 19.7 | Ref. | 42.3 | Ref. | ||||||
| Non-Hispanic Black | 36.6 | 6.0 | (2.2 to 9.7) | .002 | 29.1 | 10.9 | (7.5 to 14.3) | <.001 | 42.7 | 0.4 | (−4.6 to 5.4) | .87 |
| Hispanic | 30.3 | −1.6 | (−4.6 to 1.5) | .32 | 18.2 | −0.3 | (−4.6 to 4.0) | .89 | 40.6 | −5.2 | (−10.6 to 0.2) | .06 |
| Non-Hispanic other or unknown race/ethnicity† | 30.5 | −1.4 | (−8.3 to 5.5) | .70 | 19.5 | 1.0 | (−7.1 to 9.2) | .80 | 36.9 | −7.3 | (−16.1 to 1.4) | .10 |
| Age at diagnosis, y | ||||||||||||
| 0-14 | 34.0 | Ref. | 21.7 | Ref. | 42.0 | Ref. | ||||||
| 15-25 | 28.6 | −6.4 | (−11.2 to −1.6) | .009 | 18.3 | −3.5 | (−7.1 to 0.2) | .06 | 44.3 | −1.7 | (−8.6 to 5.2) | .63 |
| 26-39 | 34.3 | −3.0 | (−8.8 to 2.9) | .32 | 25.1 | 1.6 | (−2.0 to 5.1) | .39 | 40.2 | −6.2 | (−14.2 to 1.7) | .13 |
| Rurality of residence | ||||||||||||
| Metropolitan areas | 32.6 | Ref. | 22.1 | Ref. | 41.6 | Ref. | ||||||
| Nonmetropolitan (including rural) areas | 29.2 | −2.6 | (−8.7 to 3.4) | .40 | 18.6 | −1.6 | (−9.1 to 5.9) | .68 | 41.2 | −0.6 | (−7.3 to 6.2) | .87 |
| Neighborhood SES quintiles | ||||||||||||
| Q5: highest SES | 37.9 | Ref. | 31.3 | Ref. | 42.9 | Ref. | ||||||
| Q4 | 29.7 | −7.1 | (−11.9 to −2.3) | .004 | 20.4 | −11.2 | (−18.0 to −4.4) | .001 | 37.9 | −3.4 | (−9.4 to 2.7) | .27 |
| Q3 | 32.3 | −4.8 | (−13.1 to 3.4) | .25 | 18.2 | −14.6 | (−25.1 to −4.1) | .006 | 46.4 | 5.2 | (−2.3 to 12.6) | .17 |
| Q2 | 32.6 | −4.6 | (−9.4 to 0.2) | .06 | 22.6 | −9.6 | (−16.0 to −3.3) | .003 | 41.0 | −0.2 | (−6.4 to 6.1) | .96 |
| Q1: lowest SES | 31.5 | −6.1 | (−10.0 to −2.3) | .002 | 20.2 | −14.2 | (−21.7 to −6.6) | <.001 | 41.5 | 2.2 | (−3.7 to 8.1) | .47 |
| Unknown SES | 25.0 | −11.6 | (−26.8 to 3.6) | .14 | 21.7 | −11.7 | (−33.8 to 10.4) | .30 | 27.6 | −11.5 | (−27.8 to 4.7) | .16 |
| Diagnosis, y | ||||||||||||
| 2007 | 25.7 | Ref. | 14.5 | Ref. | 37.2 | Ref. | ||||||
| 2008 | 33.4 | 6.3 | (1.5 to 11.2) | .01 | 22.2 | 7.0 | (2.5 to 11.5) | .002 | 42.1 | 4.1 | (−3.6 to 11.8) | .29 |
| 2009 | 33.2 | 6.2 | (0.4 to 11.9) | .04 | 24.2 | 8.8 | (3.5 to 14.0) | .001 | 40.9 | 2.3 | (−6.0 to 10.5) | .59 |
| 2010 | 31.4 | 4.7 | (−1.7 to 11.0) | .15 | 19.4 | 4.8 | (−1.4 to 10.9) | .13 | 42.2 | 3.9 | (−5.3 to 13.1) | .41 |
| 2011 | 30.5 | 3.8 | (−3.9 to 11.5) | .33 | 22.5 | 7.0 | (−0.8 to 14.8) | .08 | 37.6 | 0.2 | (−12.9 to 13.4) | .97 |
| 2012 | 35.3 | 7.7 | (2.2 to 13.3) | .006 | 24.9 | 8.6 | (4.4 to 12.9) | <.001 | 45.2 | 6.7 | (−3.5 to 16.8) | .20 |
| 2013 | 35.8 | 8.6 | (3.6 to 13.6) | <.001 | 23.0 | 8.0 | (0.4 to 15.6) | .04 | 46.3 | 8.1 | (−0.8 to 16.9) | .07 |
| p0‡ | 26.0% | 18.5% | 32.7% | |||||||||
| Characteristics . | Total (N = 3524) . | Among Hodgkin lymphoma (n = 1655) . | Among non-Hodgkin lymphoma (n = 1869) . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted percentage (row %) . | ME∗ . | 95% CI . | P value . | Unadjusted percentage (row %) . | ME∗ . | 95% CI . | P value . | Unadjusted percentage (row %) . | ME∗ . | 95% CI . | P value . | |
| Insurance continuity | ||||||||||||
| Continuous Medicaid | 25.8 | Ref. | 18.5 | Ref. | 33.1 | Ref. | ||||||
| Newly gained Medicaid | 41.0 | 13.9 | (8.5 to 19.2) | <.001 | 27.5 | 6.9 | (0.5 to 13.3) | .03 | 50.1 | 17.5 | (12.0 to 23.1) | <.001 |
| Other Medicaid enrollment patterns | 29.5 | 4.6 | (2.2 to 7.0) | <.001 | 19.4 | 2.9 | (–1.8 to 7.5) | .23 | 40.0 | 7.9 | (4.1 to 11.7) | <.001 |
| Sex | ||||||||||||
| Male | 37.0 | Ref. | 26.0 | Ref. | 44.4 | Ref. | ||||||
| Female | 26.5 | −7.1 | (−9.3 to −4.8) | <.001 | 17.6 | −6.6 | (−8.9 to −4.4) | <.001 | 37.1 | −3.3 | (−6.5 to −0.1) | .04 |
| Race/ethnicity | ||||||||||||
| Non-Hispanic White | 31.0 | Ref. | 19.7 | Ref. | 42.3 | Ref. | ||||||
| Non-Hispanic Black | 36.6 | 6.0 | (2.2 to 9.7) | .002 | 29.1 | 10.9 | (7.5 to 14.3) | <.001 | 42.7 | 0.4 | (−4.6 to 5.4) | .87 |
| Hispanic | 30.3 | −1.6 | (−4.6 to 1.5) | .32 | 18.2 | −0.3 | (−4.6 to 4.0) | .89 | 40.6 | −5.2 | (−10.6 to 0.2) | .06 |
| Non-Hispanic other or unknown race/ethnicity† | 30.5 | −1.4 | (−8.3 to 5.5) | .70 | 19.5 | 1.0 | (−7.1 to 9.2) | .80 | 36.9 | −7.3 | (−16.1 to 1.4) | .10 |
| Age at diagnosis, y | ||||||||||||
| 0-14 | 34.0 | Ref. | 21.7 | Ref. | 42.0 | Ref. | ||||||
| 15-25 | 28.6 | −6.4 | (−11.2 to −1.6) | .009 | 18.3 | −3.5 | (−7.1 to 0.2) | .06 | 44.3 | −1.7 | (−8.6 to 5.2) | .63 |
| 26-39 | 34.3 | −3.0 | (−8.8 to 2.9) | .32 | 25.1 | 1.6 | (−2.0 to 5.1) | .39 | 40.2 | −6.2 | (−14.2 to 1.7) | .13 |
| Rurality of residence | ||||||||||||
| Metropolitan areas | 32.6 | Ref. | 22.1 | Ref. | 41.6 | Ref. | ||||||
| Nonmetropolitan (including rural) areas | 29.2 | −2.6 | (−8.7 to 3.4) | .40 | 18.6 | −1.6 | (−9.1 to 5.9) | .68 | 41.2 | −0.6 | (−7.3 to 6.2) | .87 |
| Neighborhood SES quintiles | ||||||||||||
| Q5: highest SES | 37.9 | Ref. | 31.3 | Ref. | 42.9 | Ref. | ||||||
| Q4 | 29.7 | −7.1 | (−11.9 to −2.3) | .004 | 20.4 | −11.2 | (−18.0 to −4.4) | .001 | 37.9 | −3.4 | (−9.4 to 2.7) | .27 |
| Q3 | 32.3 | −4.8 | (−13.1 to 3.4) | .25 | 18.2 | −14.6 | (−25.1 to −4.1) | .006 | 46.4 | 5.2 | (−2.3 to 12.6) | .17 |
| Q2 | 32.6 | −4.6 | (−9.4 to 0.2) | .06 | 22.6 | −9.6 | (−16.0 to −3.3) | .003 | 41.0 | −0.2 | (−6.4 to 6.1) | .96 |
| Q1: lowest SES | 31.5 | −6.1 | (−10.0 to −2.3) | .002 | 20.2 | −14.2 | (−21.7 to −6.6) | <.001 | 41.5 | 2.2 | (−3.7 to 8.1) | .47 |
| Unknown SES | 25.0 | −11.6 | (−26.8 to 3.6) | .14 | 21.7 | −11.7 | (−33.8 to 10.4) | .30 | 27.6 | −11.5 | (−27.8 to 4.7) | .16 |
| Diagnosis, y | ||||||||||||
| 2007 | 25.7 | Ref. | 14.5 | Ref. | 37.2 | Ref. | ||||||
| 2008 | 33.4 | 6.3 | (1.5 to 11.2) | .01 | 22.2 | 7.0 | (2.5 to 11.5) | .002 | 42.1 | 4.1 | (−3.6 to 11.8) | .29 |
| 2009 | 33.2 | 6.2 | (0.4 to 11.9) | .04 | 24.2 | 8.8 | (3.5 to 14.0) | .001 | 40.9 | 2.3 | (−6.0 to 10.5) | .59 |
| 2010 | 31.4 | 4.7 | (−1.7 to 11.0) | .15 | 19.4 | 4.8 | (−1.4 to 10.9) | .13 | 42.2 | 3.9 | (−5.3 to 13.1) | .41 |
| 2011 | 30.5 | 3.8 | (−3.9 to 11.5) | .33 | 22.5 | 7.0 | (−0.8 to 14.8) | .08 | 37.6 | 0.2 | (−12.9 to 13.4) | .97 |
| 2012 | 35.3 | 7.7 | (2.2 to 13.3) | .006 | 24.9 | 8.6 | (4.4 to 12.9) | <.001 | 45.2 | 6.7 | (−3.5 to 16.8) | .20 |
| 2013 | 35.8 | 8.6 | (3.6 to 13.6) | <.001 | 23.0 | 8.0 | (0.4 to 15.6) | .04 | 46.3 | 8.1 | (−0.8 to 16.9) | .07 |
| p0‡ | 26.0% | 18.5% | 32.7% | |||||||||
Authors’ analysis of the 2006 to 2013 SEER-Medicaid data. All regression models also controlled for SEER cancer registries. Standard errors were clustered by SEER cancer registries.
Ref., reference group.
ME and the associated 95% CI were generated using the delta method with the “margins” command of Stata software. MEs were interpreted as the absolute percentage-point difference in the model-adjusted proportion of late-stage diagnoses between patients with a specific characteristic (eg, newly gained Medicaid) and their reference group (eg, those with continuous Medicaid; p0).
Include 11 patients with unknown race/ethnicity.
Model-predicted percentage with stage IV diagnoses among patients with continuous Medicaid (ie, the reference group for Medicaid enrollment status), with other covariates held at their observed values.
Model-predicted percentages with stage IV diagnoses among Medicaid-insured children and AYAs diagnosed with lymphoma, overall and by Medicaid enrollment patterns. Authors’ analysis of the 2006 to 2013 SEER-Medicaid data. HL, Hodgkin lymphoma; NHL, non-Hodgkin lymphoma.
Model-predicted percentages with stage IV diagnoses among Medicaid-insured children and AYAs diagnosed with lymphoma, overall and by Medicaid enrollment patterns. Authors’ analysis of the 2006 to 2013 SEER-Medicaid data. HL, Hodgkin lymphoma; NHL, non-Hodgkin lymphoma.
These patterns persisted across most subgroups stratified by lymphoma subtypes (Table 2) and key demographic factors (Table 3). Notably, the association between newly gained (vs continuous) Medicaid and the likelihood of stage IV lymphoma diagnoses was larger in magnitude among AYA patients (15-25 age group [ME, 15.4 ppt; 95% CI, 10.5-20.3; P < .001]; 26-39 age group [ME, 12.2 ppt; 95% CI, 4.8-19.5; P = .001]) than among pediatric patients aged 0 to 14 years (ME, 7.7 ppt; 95% CI, −4.3 to 19.6; P = .20).
Subgroup analyses on the association between Medicaid enrollment patterns and the likelihood of stage IV diagnoses among Medicaid-insured children and AYAs diagnosed with lymphoma, by key sociodemographic characteristics
| Characteristics . | Likelihood of stage IV diagnoses . | ||
|---|---|---|---|
| ME . | 95% CI . | P value . | |
| By age group | |||
| Among 0-14 (n = 465) | |||
| Continuous Medicaid | Ref. | ||
| Newly gained Medicaid | 7.7 | (–4.3 to 19.6) | .21 |
| Other Medicaid enrollment patterns | 12.1 | (–0.2 to 24.5) | .05 |
| Among 15-25 (n = 1290) | |||
| Continuous Medicaid | Ref. | ||
| Newly gained Medicaid | 15.4 | (10.5 to 20.3) | <.001 |
| Other Medicaid enrollment patterns | 1.4 | (–2.8 to 5.5) | .52 |
| Among 26-39 (n = 1769) | |||
| Continuous Medicaid | Ref. | ||
| Newly gained Medicaid | 12.2 | (4.8 to 19.5) | .001 |
| Other Medicaid enrollment patterns | 4.3 | (–2.4 to 10.9) | .21 |
| By sex | |||
| Among male (n = 1899) | |||
| Continuous Medicaid | Ref. | ||
| Newly gained Medicaid | 15.8 | (9.5 to 22.1) | <.001 |
| Other Medicaid enrollment patterns | 7.4 | (3.8 to 11.1) | <.001 |
| Among female (n = 1625) | |||
| Continuous Medicaid | Ref. | ||
| Newly gained Medicaid | 11.4 | (5.0 to 17.8) | <.001 |
| Other Medicaid enrollment patterns | 2.3 | (–1.1 to 5.6) | .18 |
| By race/ethnicity | |||
| Among non-Hispanic White patients (n = 1564) | |||
| Continuous Medicaid | Ref. | ||
| Newly gained Medicaid | 13.5 | (8.3 to 18.8) | <.001 |
| Other Medicaid enrollment patterns | 4.5 | (–0.8 to 9.7) | .10 |
| Among non-Hispanic Black patients (n = 858)∗ | |||
| Continuous Medicaid | Ref. | ||
| Newly gained Medicaid | 18.8 | (7.8 to 29.7) | .001 |
| Other Medicaid enrollment patterns | 6.2 | (–1.4 to 13.8) | .11 |
| Among Hispanic patients (n = 875)† | |||
| Continuous Medicaid | Ref. | ||
| Newly gained Medicaid | 10.5 | (–1.6 to 22.6) | .09 |
| Other Medicaid enrollment patterns | 5.0 | (0.4 to 9.7) | .03 |
| By neighborhood SES quantiles | |||
| Among Q4-5 (high SES) (n = 951) | |||
| Continuous Medicaid | Ref. | ||
| Newly gained Medicaid | 14.7 | (10.6 to 18.8) | <.001 |
| Other Medicaid enrollment patterns | 8.2 | (5.2 to 11.2) | <.001 |
| Among Q1-3 (low SES) (n = 2521) | |||
| Continuous Medicaid | Ref. | ||
| Newly gained Medicaid | 13.7 | (6.1 to 21.4) | <.001 |
| Other Medicaid enrollment patterns | 2.9 | (–0.6 to 6.4) | .10 |
| Characteristics . | Likelihood of stage IV diagnoses . | ||
|---|---|---|---|
| ME . | 95% CI . | P value . | |
| By age group | |||
| Among 0-14 (n = 465) | |||
| Continuous Medicaid | Ref. | ||
| Newly gained Medicaid | 7.7 | (–4.3 to 19.6) | .21 |
| Other Medicaid enrollment patterns | 12.1 | (–0.2 to 24.5) | .05 |
| Among 15-25 (n = 1290) | |||
| Continuous Medicaid | Ref. | ||
| Newly gained Medicaid | 15.4 | (10.5 to 20.3) | <.001 |
| Other Medicaid enrollment patterns | 1.4 | (–2.8 to 5.5) | .52 |
| Among 26-39 (n = 1769) | |||
| Continuous Medicaid | Ref. | ||
| Newly gained Medicaid | 12.2 | (4.8 to 19.5) | .001 |
| Other Medicaid enrollment patterns | 4.3 | (–2.4 to 10.9) | .21 |
| By sex | |||
| Among male (n = 1899) | |||
| Continuous Medicaid | Ref. | ||
| Newly gained Medicaid | 15.8 | (9.5 to 22.1) | <.001 |
| Other Medicaid enrollment patterns | 7.4 | (3.8 to 11.1) | <.001 |
| Among female (n = 1625) | |||
| Continuous Medicaid | Ref. | ||
| Newly gained Medicaid | 11.4 | (5.0 to 17.8) | <.001 |
| Other Medicaid enrollment patterns | 2.3 | (–1.1 to 5.6) | .18 |
| By race/ethnicity | |||
| Among non-Hispanic White patients (n = 1564) | |||
| Continuous Medicaid | Ref. | ||
| Newly gained Medicaid | 13.5 | (8.3 to 18.8) | <.001 |
| Other Medicaid enrollment patterns | 4.5 | (–0.8 to 9.7) | .10 |
| Among non-Hispanic Black patients (n = 858)∗ | |||
| Continuous Medicaid | Ref. | ||
| Newly gained Medicaid | 18.8 | (7.8 to 29.7) | .001 |
| Other Medicaid enrollment patterns | 6.2 | (–1.4 to 13.8) | .11 |
| Among Hispanic patients (n = 875)† | |||
| Continuous Medicaid | Ref. | ||
| Newly gained Medicaid | 10.5 | (–1.6 to 22.6) | .09 |
| Other Medicaid enrollment patterns | 5.0 | (0.4 to 9.7) | .03 |
| By neighborhood SES quantiles | |||
| Among Q4-5 (high SES) (n = 951) | |||
| Continuous Medicaid | Ref. | ||
| Newly gained Medicaid | 14.7 | (10.6 to 18.8) | <.001 |
| Other Medicaid enrollment patterns | 8.2 | (5.2 to 11.2) | <.001 |
| Among Q1-3 (low SES) (n = 2521) | |||
| Continuous Medicaid | Ref. | ||
| Newly gained Medicaid | 13.7 | (6.1 to 21.4) | <.001 |
| Other Medicaid enrollment patterns | 2.9 | (–0.6 to 6.4) | .10 |
Authors’ analysis of the 2006 to 2013 SEER-Medicaid data. All regression models also controlled for age at diagnosis, sex, race/ethnicity, year of diagnosis, rurality of residence, and neighborhood-level SES, as appropriate, as well as SEER cancer registries. Standard errors were clustered by SEER cancer registries.
Ref., reference group.
Three observations were dropped from the regression model because of the perfect prediction issue (ie, only 1 non-Hispanic Black patient was in the Hawaii registry who had stage IV lymphoma at diagnosis, and none of the 2 non-Hispanic Black patients in the Utah registry had stage IV lymphoma at diagnosis).
One observation was dropped from the regression model because of the perfect prediction issue (ie, only 1 Hispanic patient was in the Kentucky registry who did not have stage IV lymphoma at diagnosis).
In our secondary analysis (N = 15 366), having private insurance was associated with a decrease of 3.9 ppt (95% CI, −6.0 to −1.7; P < .001) in the likelihood of being diagnosed with stage IV lymphoma compared with those with continuous Medicaid (supplemental Table 6). No significant differences were observed between continuous Medicaid and unknown/no insurance coverage, nor between continuous Medicaid and most other insurance groups.
Discussion
Using the linked SEER-Medicaid data, our study revealed that only 3 in 8 (37.8%) Medicaid-insured children and AYAs had continuous Medicaid coverage before and during the initial lymphoma diagnosis. Children and AYAs with newly gained Medicaid or other Medicaid enrollment patterns faced an 18% to 54% higher likelihood of presenting with stage IV lymphoma diagnosis compared with continuous Medicaid enrollees. Additionally, AYA patients were more likely to have newly gained Medicaid at or after lymphoma diagnosis and less likely to have continuous Medicaid coverage relative to younger pediatric patients.
These findings are consistent with previous single-state studies reporting an association between Medicaid enrollment continuity and more advanced cancer stage among AYAs or older adults diagnosed with solid tumors.7,17,24-26 Our study extends these findings by providing, to our knowledge, one of the first multistate, population-based assessments of Medicaid coverage continuity patterns before, during, and shortly after a new diagnosis of lymphoma. Furthermore, by focusing on the full age spectrum from children to AYAs, this is also, to our knowledge, one of the first studies to examine the association between Medicaid coverage continuity and disease stage at diagnosis among vulnerable young patient populations. Importantly, late-stage disease is associated with increased acuity of illness at presentation; a need for more intensive therapies such as radiation therapy, targeting larger fields for certain lymphomas; a risk for disease progression or relapse requiring further therapies such as stem cell transplantation; and a heightened risk of morbidity from therapy-associated long-term effects, which ultimately contribute to poorer overall survival.27,28
Multiple factors may account for the finding that having newly gained Medicaid or other Medicaid enrollment patterns is associated with a greater likelihood of late-stage diagnoses. Although there is no routine screening test for early lymphoma detection, a range of warning signs can be recognized by patients, caregivers, and primary care physicians. Physical examination and symptom evaluation require access to the health care system.7,29 Compared with patients with continuous Medicaid coverage, those who enroll in Medicaid only at cancer diagnosis could have missed opportunities for early evaluation of signs and symptoms and timely referral to oncologists for diagnostic testing and therapy initiation.7 Insurance gaps, even for a short period, may delay access to necessary medical care. Notably, accessible primary care services via continuous insurance are especially relevant for early detection and referral for pediatric and AYA lymphoma. Unlike some adult-onset solid tumor cancers, there are no asymptomatic population-wide screening tests or guidelines for early detection of lymphoma, and thus, disease onset can be acute and even fatal.
The association between continuous Medicaid and reduced late-stage diagnoses was stronger among AYAs than pediatric patients; however, AYAs were less likely to have continuous Medicaid coverage (32.3%-36.7% vs 61.7%). Such disparity may be partially due to the more stringent eligibility and renewal criteria for adult (vs child) Medicaid eligibility.30 This disparity may also be explained by the unique challenges faced by AYAs, including the transition from parent-managed pediatric health care to self-managed adult health care, life transitions (eg, entering the workforce, income fluctuation, and mobility) potentially affecting Medicaid eligibility, and difficulties in navigating the complex Medicaid system during young adulthood.31,32 These challenges ultimately impact not only disease stage at diagnosis but also care access, treatment adherence, and overall well-being.16
Our findings have important implications amid the unwinding of the Medicaid continuous enrollment protections implemented during the COVID-19 pandemic.33 States were incentivized to provide continuous Medicaid enrollment by pausing eligibility redeterminations; however, this provision ended in early 2023, resulting in widespread disenrollment.34 By August 2024, over 25.1 million individuals, including many children and AYAs, lost Medicaid coverage.35 In this context, our analysis provides timely evidence on the importance of seamless Medicaid coverage in reducing late-stage diagnoses, a critical mediator of inferior outcomes of pediatric and AYA blood cancer. Our findings also highlight the urgency of state- and community-level interventions to improve Medicaid coverage continuity for both children and AYAs.
For example, states may consider simplifying eligibility redetermination processes or otherwise reducing administrative barriers to gaining and maintaining Medicaid coverage.34 The policy permitting 12-month (or 24-month) continuous coverage for Medicaid-insured children, as advocated by the Urban Institute and the Centers for Medicare & Medicaid Services, is one potential approach.36,37 States may also adopt or continue Medicaid expansion under the Affordable Care Act (ACA), which has been shown to reduce coverage disruptions and disproportionately benefit young adults, including benefits for earlier-stage diagnoses and survival.38-41 Additionally, community outreach and education programs may help children, AYAs, and their families navigate the Medicaid system, ensuring that eligible individuals, including those newly eligible under the ACA expansion, are indeed enrolled in Medicaid and access needed care. Lastly, the importance of continuous insurance coverage, as emphasized by the National Comprehensive Cancer Network Guidelines for AYA Oncology,42 should also be integrated into the National Comprehensive Cancer Network Clinical Practice Guidelines for Lymphomas.
Limitations
Our study has limitations. First, in the absence of insurance claims, the SEER-Medicaid data lack information on patients’ health care utilization, particularly treatment for comorbidity and prediagnosis patterns of care. Second, we could not establish causality given the observational study design. Third, our study focused on the patterns and continuity of Medicaid enrollment. We lacked data on private or other insurance enrollment before, during, or after diagnosis for patients experiencing Medicaid coverage disruptions or who never enrolled in Medicaid. Future work should establish (or leverage the existing) linkages between state cancer registries and all-payer claims data to comprehensively examine the impact of health insurance continuity, including private and other types of insurance, on lymphoma outcomes in children and AYAs. Fourth, our data do not include information on family income or disability status, thus limiting our ability to relate changes in an individual’s underlying eligibility for Medicaid over time to observed patterns of enrollment. Patients may transition to new Medicaid coverage or other Medicaid enrollment patterns for various reasons, such as changes in eligibility due to income fluctuations or loss of coverage due to administrative challenges, even while remaining eligible. Further research is needed to deepen our understanding of the underlying factors driving Medicaid enrollment patterns and their associations with receipt of care and health outcomes.
Additionally, the Ann Arbor staging system, initially developed for Hodgkin lymphoma, has been adapted for non-Hodgkin lymphoma by SEER; however, its prognostic accuracy may be limited for certain non-Hodgkin lymphoma subtypes.43 Our data lacked patient-level SES measures, and the neighborhood-level SES metric may not accurately reflect individual or family-level socioeconomic circumstances. We were unable to include state registries that do not participate in the SEER Program; thus, our findings may not be generalizable to non-SEER regions. Nevertheless, the SEER-Medicaid data are the only available linkage of multistate population-based registries to insurance records from children and AYAs with blood cancer.10 The insurance type at diagnosis recorded in SEER registries may underreport Medicaid coverage.44,45 However, this study mitigates this limitation by leveraging Medicaid administrative data linked to SEER registries, thereby providing robust estimates of the timing and continuity of Medicaid enrollment. Moreover, our data cover the period of 2006 to 2013 and may not reflect insurance patterns in the post-ACA era. Nevertheless, the major provisions of the ACA, particularly the establishment of marketplaces and Medicaid expansion, were introduced in 201446; this study period (2006-2013) ensures that our results are not affected by biases arising from these later policy changes.
Lastly, our secondary analysis showed a difference in late-stage diagnoses between continuous Medicaid enrollees and those with private insurance, which could stem from factors beyond the type of insurance coverage. Medicaid eligibility is based on low-income or disability status; enrollees often face health-related social needs (HRSN) that can hinder access to quality care despite having insurance, including poor health with comorbidities before a cancer diagnosis, low health literacy, logistical barriers to care, and/or difficulty finding Medicaid-accepting providers.47 However, our secondary analysis lacked data to capture these factors. The recent introduction of the International Classification of Diseases, 10th Revision codes for HRSN presents an opportunity for future studies to measure HRSN and investigate differences in lymphoma staging between privately insured patients and those with Medicaid after adjusting for HRSN-related factors.48
Conclusion
This multistate study presents important evidence that among children and AYAs diagnosed with lymphoma and insured by Medicaid, those who maintained continuous Medicaid coverage before diagnosis were significantly less likely to have late-stage diseases compared with those with newly gained Medicaid or with other Medicaid enrollment patterns. The results suggest that stable Medicaid coverage can increase the likelihood of early symptom detection during routine health care encounters, potentially leading to earlier diagnosis and better outcomes. More research is needed to understand the patterns and continuity of Medicaid coverage along the cancer care continuum (including the active treatment phase, posttreatment survivorship, and supportive and palliative care) and the association of coverage patterns with survival outcomes for children and AYA diagnosed with lymphoma.49
Acknowledgments
This study used the linked Surveillance, Epidemiology, and End Results (SEER)–Medicaid database. The authors acknowledge the efforts of the National Cancer Institute; the Centers for Medicare & Medicaid Services; Information Management Services, Inc; and the SEER Program tumor registries in the creation of the SEER-Medicaid database.
The collection of cancer incidence data used in this study was supported by the California Department of Public Health pursuant to California Health and Safety Code Section 103885; Centers for Disease Control and Prevention’s National Program of Cancer Registries (cooperative agreement 1NU58DP007156); and the National Cancer Institute’s SEER Program (contract HHSN261201800032I awarded to the University of California, San Francisco; contract HHSN261201800015I awarded to the University of Southern California; and contract HHSN261201800009I awarded to the Public Health Institute). This work was supported by the Leukemia & Lymphoma Society Equity in Access Research Program grant HSR9015-23 (S.M.C. [multiple principal investigator], S.B., A.C.M., and X.J. [multiple principal investigator]) and grant K01MD018637 (X.J. [principal investigator], A.C.M., and S.M.C.) from the National Institute on Minority Health and Health Disparities.
The interpretation and reporting of the data, ideas, and opinions expressed herein are those of the authors and do not necessarily reflect the official views of the Leukemia & Lymphoma Society, the State of California, Department of Public Health, the National Cancer Institute, the National Institute on Minority Health and Health Disparities, and the Centers for Disease Control and Prevention, or their contractors and subcontractors.
Authorship
Contribution: X.E.Z. contributed to formal analysis, conceptualization, investigation, methodology, software, validation, visualization, and writing (original draft preparation and review and editing); S.M.C. contributed to conceptualization, funding acquisition, resources, investigation, methodology, project administration, validation, visualization, supervision, and writing (review and editing); K.R.Y. contributed to conceptualization, resources, investigation, methodology, validation, visualization, and writing (review and editing); W.S. contributed to conceptualization, resources, investigation, methodology, validation, and writing (review and editing); P.C. contributed to conceptualization, resources, investigation, and writing (review and editing); S.B. contributed to conceptualization, funding acquisition, investigation, methodology, validation, visualization, and writing (review and editing); A.C.M. contributed to conceptualization, funding acquisition, resources, investigation, methodology, and writing (review and editing); J. L. contributed to conceptualization, resources, investigation, methodology, validation, visualization, and writing (review and editing); and X. J. contributed to data curation, conceptualization, funding acquisition, resources, investigation, methodology, project administration, validation, visualization, supervision, and writing (original draft preparation and review and editing).
Conflict-of-interest disclosure: S.M.C. has served on the advisory boards of Seagen Inc and Bristol Myers Squibb. K.R.Y. reports having served on a Flatiron Health and a National Comprehensive Cancer Network working group; all honoraria are donated to her employer, the American Cancer Society. The remaining authors declare no competing financial interests.
Correspondence: Xu Ji, Emory University School of Medicine, 2015 Uppergate Dr, Atlanta, GA 30322; email: xu.ji@emory.edu.
References
Author notes
The authors are unable to make the data publicly available given the Data Use Agreement with the Surveillance, Epidemiology, and End Results (SEER)–Medicaid team. Interested researchers may visit https://healthcaredelivery.cancer.gov/seermedicaid/obtain/ for information on accessing the SEER-Medicaid data.
The full-text version of this article contains a data supplement.